Abstract
Background and Aims:
Transfusion of blood and blood products poses several hazards. Antifibrinolytic agents are used to reduce perioperative blood loss. We decided to assess the effect of tranexamic acid (TA) on blood loss and the need for transfusion in head and neck cancer surgery.
Methods:
After Institutional Review Board approval, 240 patients undergoing supramajor head and neck cancer surgeries were prospectively randomised to either TA (10 mg/kg) group or placebo (P) group. After induction, the drug was infused by the anaesthesiologist, who was blinded to allocation, over 20 min. The dose was repeated every 3 h. Perioperative (up to 24 h) blood loss, need for transfusion and fluid therapy was recorded. Thromboelastography (TEG) was performed at fixed intervals in the first 100 patients. Patients were watched for post-operative complications.
Results:
Two hundred and nineteen records were evaluable. We found no difference in intraoperative blood loss (TA - 750 [600–1000] ml vs. P - 780 [150–2600] ml, P = 0.22). Post-operative blood loss was significantly more in the placebo group at 24 h (P - 200 [120–250] ml vs. TA - 250 [50–1050] ml, P = 0.009), but this did not result in higher number of patients needing transfusions (TA - 22/108 and P - 27/111 patients, P = 0.51). TEG revealed faster clot formation and minimal fibrinolysis. Two patients died of causes unrelated to study drug. Incidence of wound complications and deep venous thrombosis was similar.
Conclusion:
In head and neck cancer surgery, TA did not reduce intraoperative blood loss or need for transfusions. Perioperative TEG variables were similar. This may be attributed to pre-existing hypercoagulable state and minimal fibrinolysis in cancer patients.
Keywords: Blood transfusion, cancer surgery, hypercoagulability, tranexamic acid
INTRODUCTION
Administration of blood and blood products carries the risk of post-operative bacterial infection and increased rates of recurrence in various malignancies.[1,2] Lower transfusion trigger, pre-operative autologous blood donation (with or without erythropoietin), intraoperative red blood cell salvage, regional anaesthesia, controlled hypotension and antifibrinolytic agents are all useful means to decrease the need for allogenic transfusions. Tranexamic acid (TA) is a synthetic antifibrinolytic agent that is approximately 7–10 times more potent than aminocaproic acid. It blocks the lysine-binding sites of plasminogen, plasmin and tissue plasminogen activator (tPA), and thus delays fibrinolysis and degradation of blood clot.[3] A recent systematic review of over 10,000 patients undergoing various surgeries suggested that administration of TA reduces the need of receiving transfusion by 38% (relative risk [RR] 0.62, 95% confidence interval [95% CI]: 0.58–0.65; P < 0.001).[4] We wanted to find out whether intraoperative administration of TA reduced blood loss in patients undergoing supramajor head and neck cancer surgery.
Thromboelastography™ (TEG) allows evaluation of kinetics of clot formation and presence and inhibition of fibrinolysis.[5] Hypercoagulability, difficult to detect with routine coagulation tests, can be diagnosed with TEG displaying a short r-time, broad alpha angle and maximum amplitude (MA) >70 mm.
METHODS
This prospective, double-blind, randomised, placebo-controlled trial was conducted after approval from the Institutional Review Board in a Tertiary Referral Cancer Institute in India. We included 240 patients with resectable squamous cell carcinoma of the oral cavity undergoing supramajor surgery viz., composite resection of the mandible along with neck dissection, requiring reconstructive procedures in the form of pedicled flaps. Informed consent was obtained from all patients. Patients with coagulopathy (partial prothrombin time >50 s, or international normalised ratio >1.5, platelets <50 × 109/L), or those who had recent history of (<5 days) acetylsalicylic acid ingestion, patients on anticoagulant therapy (heparin received within 4 h or warfarin received 3 days pre-operatively) or those with peripheral vascular disease, pre-existing renal dysfunction (serum creatinine >1.2 mg/dL), liver dysfunction or known allergy to TA were excluded.
The patients were allocated to two groups by random numbers generated using a computer programme, and blocks of 10 were generated. The patient, the anaesthesiologist and the person assessing the blood loss were blinded to the assignment. The study drug, either tranexamic acid (TA) 10 mg/kg in 100 ml of normal saline (TA group) or placebo (100 ml normal saline) was prepared by one investigator as per the randomisation and handed over to the attending anaesthesiologist who was blinded to the group assignment. Patients were stratified a priori according to plan of reconstruction (pedicled pectoralis major myocutaneous flap or pectoralis major myocutaneous and deltopectoral flap).
The technique of induction, maintenance and reversal of anaesthesia was left to the discretion of the attending anaesthesiologist. The study drug, prepared in the calculated dose, or placebo was infused over 20 min after induction of anaesthesia before the surgical incision was taken. In the event of prolonged surgeries, the infusion was repeated every 3 h.
Intraoperative blood loss was calculated by gravimetry, the blood collected in the suction bottle and visual assessment of blood loss in the surgical field. Maximum allowable blood loss (MABL) was calculated with a transfusion trigger of 8 g% haemoglobin (Hb) using the formula: MABL = ([Hb − minimum Hb]/Hb) × (weight in kg) × (ml of blood per kg body weight). The blood loss was replaced with Ringer's lactate or a colloid until it exceeded the calculated MABL. Then, it was replaced with either whole blood or packed red cells. The anaesthesiologist could override the trigger if the patient developed haemodynamic instability (heart rate >120 beats/min, or a systolic blood pressure decrease by >20% of pre-operative value) despite adequate volume replacement. Assessment of volume status and the amount of fluid to be infused was left to the judgement of the attending anaesthesiologist.
The primary endpoint was reduction in blood loss, while the secondary endpoint was the number of patients needing transfusion. We noted demographics, comorbidities, pre-operative and post-operative Hb concentration on day 1 and platelet count. We performed TEG in the first 50 patients in each group at five intervals: Pre-operatively, 1 h after the first dose, 1 h after the second dose, immediately post-operatively on transfer to recovery room and on the morning of the first post-operative day. Post-operative blood loss was assessed from the blood collected in the suction drain bottles over the first 24 h. The type and duration of surgery, experience of the surgeon and mean arterial blood pressure during surgery were also recorded. We also noted the urine output in the first 24 h and serum creatinine on post-operative day 1 and 3. The patients were monitored for development of skin flap and reconstruction flap necrosis (both graded as edge necrosis, flap loss either more or <50%), oro-cutaneous fistulas, symptomatic deep vein thrombosis, need for surgical re-exploration and other incidental complications until discharge from the hospital.
From our previous data, the mean blood loss in patients undergoing composite resections and reconstruction was 750 ml (standard deviation [SD] 100). For the trial to have 80% power to detect a reduction of 40% in the average blood loss, at α = 0.05, (PS programme, Copyright© 1997, Vanderbilt University Medical Center - http://www.mc.vanderbilt.edu/prevmed/ps/) we calculated that 100 patients in each group were needed. To compensate for protocol violation such as failure to administer drug, change of surgical plan and non-availability of case record forms, we enrolled 240 patients.
Statistical analysis was performed on intention to treat basis using Student's t-test and Chi-square test. Serial measurements were analysed by paired t-test (for two observations) and by repeated measures ANOVA (for more than two observations) and a P < 0.05 was considered statistically significant.
RESULTS
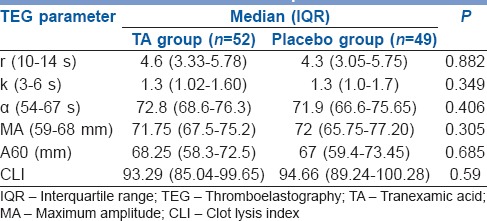
Two hundred and nineteen of 240 records were evaluable. Patients in the TA and control groups were similar in sex, age, weight, comorbidities and baseline investigations including the coagulation parameters obtained with TEG. The type of reconstructive surgery and duration of surgery was also similar [Table 1]. Anaesthetic technique including the use of narcotic analgesics and haemodynamic parameters revealed no differences in two groups. The intraoperative blood loss and total blood loss (a total of intraoperative blood loss and post-operative blood loss) in the first 24 h in perioperative period was similar in both groups (750 ml in TA vs. 780 ml in control group, P = 0.22, 1000 ml in TA group and 1100 ml placebo group, respectively). The difference in post-operative blood loss reached statistical significance (TA 250 ml vs. 320 ml in the control group, P = 0.009), but did not seem to be clinically significant and did not result in an increase in need for blood transfusion. Of 108 patients, 22 needed blood transfusion in TA group while in the placebo group, 27 of 111 needed transfusion (P = 0.51). No patients were transfused in the post-operative period. Intraoperative fluid replacement, crystalloids and colloids were similar in both groups [Table 2]. The TEG showed hypercoagulable profile at baseline, i.e., shorter than normal r-time, k-time and wide α angle indicating faster acceleration (kinetics) of fibrin build up and cross-linking in both groups. At all points when TEG was performed the MA was higher than the normal range, and there was absence of significant fibrinolysis in both groups indicated by high clot lysis index at 60 min [Table 3].
Table 1.
Baseline characteristics, type of reconstruction and duration of surgery
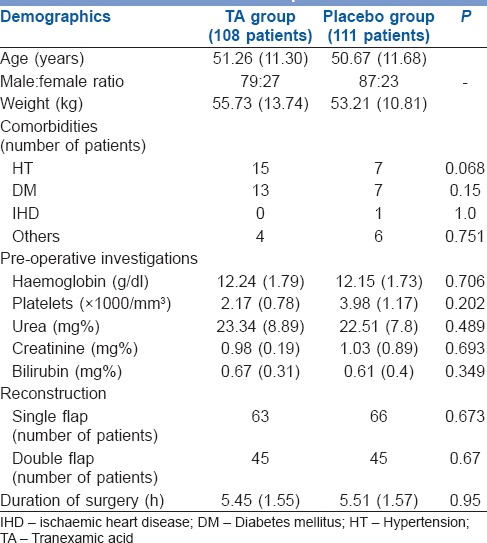
Table 2.
Blood loss, fluids and blood replacement
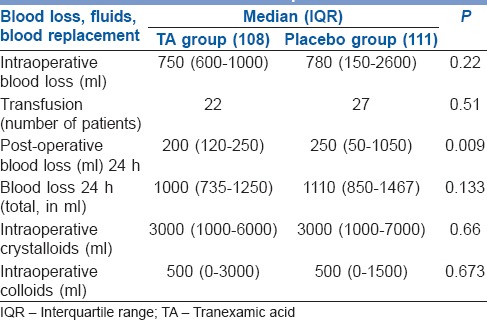
Table 3.
Baseline TEG parameters
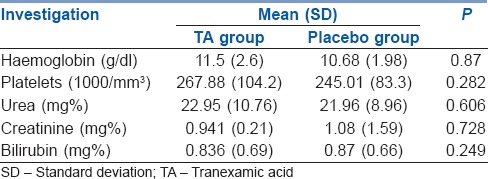
Post-operative investigations revealed no differences in two groups and no renal or hepatic dysfunction in the TA group [Table 4]. Two patients died in the post-operative period, one had a hypoxic cardiac arrest on the second post-operative day in the ward due to a blocked tracheostomy tube (TA group), while the other patient had an unexplained asystolic cardiac arrest (placebo group). Other post-operative complications were similar in both groups viz., two patients in each group had skin flap necrosis while three each had >50% necrosis of reconstruction flap (P = NS). One patient (placebo group) needed re-exploration of the wound for bleeding on the day of surgery. The incidence of oro-cutaneous fistula was also similar seen in two patients in TA group and three patients in placebo group. There were no thromboembolic complications in either group.
Table 4.
Post-operative (day 1) investigations
DISCUSSION
In this randomised, double-blind, placebo-controlled study, intravenous administration of TA did not reduce blood loss in patients undergoing supramajor surgery for oral cancers. The need for blood transfusions was also not reduced. Post-operative blood loss was lower in the patients receiving TA but this difference was neither clinically significant and nor did it cause increased requirement for transfusion in the placebo group. TA administration was safe as there was no surgical complication, organ dysfunction or thromboembolic episode.
Coagulation and fibrinolysis are both activated by surgical trauma.[6] Inhibiting fibrinolysis reduces blood loss by increasing clot strength. During major surgery, exposure of tissues to injury causes release of enzymes, mainly tPA, thereby activating the fibrinolytic system.[7] The fibrinolytic response is most pronounced in the intraoperative and early post-operative period. Ekbäck et al. found increased levels of tPA, plasmin-antiplasmin complex and thrombin-antithrombin complex, indicating activation of coagulation. Hyperfibrinolytic phase, indicated by increased levels of D-dimers was seen in the placebo group from 4 h onwards intraoperatively.[8] The D-dimers levels returned to baseline on the first post-operative day. In contrast, the D-dimers levels were much lower throughout in patients given TA indicating inhibition of fibrinolysis. Benoni et al. measured levels of thrombin fragments (1 + 2), D-dimers plasminogen, α2 antiplasmin, tPA and plasminogen activator inhibitor (PAI-1) in blood from wound as well as peripheral venous blood.[9] They found significant activation of coagulation and fibrinolysis in both samples, much more in the blood than the wound. D-dimer levels were lower in TA group indicating inhibition of fibrinolysis. In both these studies, blood loss was lower in patients receiving TA. In patients undergoing an orthotopic liver transplant, there was significant fibrinolytic activity, i.e., high levels of D-dimer and fibrin degradation products, in the normal saline group in contrast to patients receiving TA. Inhibition of fibrinolysis by TA was evident from higher clot lysis index than patients given placebo.[10] However, the need for transfusion was similar in both groups.
TA has been shown to reduce blood loss in a variety of surgical procedures such as coronary revascularisation, orthotopic liver transplantation, scoliosis correction surgery, other orthopaedic procedures and caesarean sections.[8,11,12,13,14,15,16] A recent meta-analysis of over 1100 patients also demonstrated the efficacy of antifibrinolytic agents in reducing blood loss in patients undergoing hip athroplasty and total knee replacement.[17] The likelihood of patients needing transfusion was reduced by 52%. TA was the most efficacious (RR 0.47 [95% CI: 0.40–0.55]). The incidence of venous thromboembolism with antifibrinolytic agents was similar to placebo. In trauma patients, TA reduced all-cause mortality as well as the risk of mortality due to bleeding.[18] It has been suggested that TA should be added for routine use in treatment of trauma patients.[19]
Reducing the need for transfusion in cancer patients may be particularly important as the literature suggests increased recurrence rates in head and neck, colorectal, oesophageal and hepatocellular malignancies after blood transfusion.[20,21,22,23,24] Blood transfusion was associated with earlier recurrence in patients with advanced ovarian cancer undergoing cytoreductive surgery.[25] The literature on the use of TA in cancer patients to reduce blood loss is scarce, with varied results. In 200 patients undergoing retropubic radical prostatectomy, TA led to a 21% absolute reduction (95% CI: 7–34%) in transfusion rate.[26] The median no of units transfused was also reduced (0 [interquartile range (IQR): 0–1] vs. 1 [IQR: 0–1.5]; P = 0.004) in patients who received TA. The blood loss was higher in placebo group (1103 ml [SD 500.8] vs. 1335 ml [SD 686.5], [95% CI: 29.7–370.7; P = 0.02]). In a small case series with historical controls, Bednar et al. found that mean estimated blood loss was not reduced by TA in patients undergoing surgical treatment for metastatic tumours of the spine.[27] In patients undergoing various procedures for head and neck cancers, TA administration did not reduce the drainage duration.[28] In patients undergoing hepatectomy for hepatic tumours, perioperative administration of TA was shown to reduce blood loss (300 ml [30–2100] vs. 600 ml [40–3410]).[29]
Cancer patients are hypercoagulable due to the production of various procoagulant activities, such as tissue factor and cancer procoagulant.[30] Cancer cells also increase fibrinolytic activity as tPA and urokinase-type plasminogen activator and PAI-1 are expressed on their surface. We wanted to document effective inhibition of fibrinolysis with TA, and, therefore, we performed TEG in first 100 patients. Modrau et al. found a distinct difference in coagulation profile when comparing patients with benign and malignant colorectal lesions.[31] Patients with malignant lesions were hypercoagulable and also showed fibrinolysis inhibition. Our patients were hypercoagulable to start with (shortened r and k-time and wide α angle), and there was also decreased fibrinolytic activity in both groups (high MA and >80% clot lysis index at 60 min). This explains why TA administration did not lead to a reduction in blood loss. It is unlikely that the dose of TA used by us was inadequate to achieve inhibition of fibrinolysis as it was similar to doses used in other studies that demonstrated reduced blood loss.[32,33,34] An even smaller dose of TA (2 mg/kg/h infusion) reduced fibrinolysis in patients undergoing orthotopic liver transplantation.[17] In a dose response study, the D-dimer concentration was reduced with the smallest dose (2.5 mg/kg) of TA as compared to placebo but the reduction of blood loss became significant from the doses upwards of 10 mg/kg.[35]
Our patients did not experience any episodes of symptomatic venous thromboembolism. This may be because the fibrinolytic activity is more pronounced in the wound than in the peripheral blood in patients undergoing surgery.[9] The site of action of TA is, therefore, more likely to be limited to the surgical field than being generalised. Therefore, TA may be safely used in these patients.
CONCLUSIONS
TA (10 mg/kg) did not reduce blood loss and need for transfusion of red cells in patients undergoing head and neck cancer surgeries under general anaesthesia.
Financial support and sponsorship
Intramural grant from Tata Memorial Hospital, Dr. E. Borges Road, Parel, Mumbai - 400 012, Maharashtra, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med. 2006;355:1303–5. doi: 10.1056/NEJMp068178. [DOI] [PubMed] [Google Scholar]
- 2.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 4.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth. 1992;69:307–13. doi: 10.1093/bja/69.3.307. [DOI] [PubMed] [Google Scholar]
- 6.Dahl OE. The role of the pulmonary circulation in the regulation of coagulation and fibrinolysis in relation to major surgery. J Cardiothorac Vasc Anesth. 1997;11:322–8. doi: 10.1016/s1053-0770(97)90102-6. [DOI] [PubMed] [Google Scholar]
- 7.Kluft C, Verheijen JH, Jie AF, Rijken DC, Preston FE, Sue-Ling HM, et al. The postoperative fibrinolytic shutdown: A rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45:605–10. doi: 10.3109/00365518509155267. [DOI] [PubMed] [Google Scholar]
- 8.Ekbäck G, Axelsson K, Ryttberg L, Edlund B, Kjellberg J, Weckström J, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91:1124–30. doi: 10.1097/00000539-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 1997;85:195–206. doi: 10.1016/s0049-3848(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaspar M, Ramsay MA, Nguyen AT, Cogswell M, Hurst G, Ramsay KJ. Continuous small-dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesth Analg. 1997;85:281–5. doi: 10.1097/00000539-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kojima T, Gando S, Morimoto Y, Mashio H, Goda Y, Kawahigashi H, et al. Systematic elucidation of effects of tranexamic acid on fibrinolysis and bleeding during and after cardiopulmonary bypass surgery. Thromb Res. 2001;104:301–7. doi: 10.1016/s0049-3848(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 12.Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85:1043–8. doi: 10.1097/00000542-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93:82–7. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Veien M, Sørensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: A randomized, controlled study. Acta Anaesthesiol Scand. 2002;46:1206–11. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 15.Kakar PN, Gupta N, Govil P, Shah V. Efficacy and safety of tranexamic acid in control of bleeding following TKR: A randomized clinical trial. Indian J Anaesth. 2009;53:667–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: A prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28:233–40. doi: 10.1055/s-0030-1268238. [DOI] [PubMed] [Google Scholar]
- 17.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: A systematic review of randomized trials. Thromb Res. 2009;123:687–96. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2) A randomised, placebo-controlled trial. West Indian Med J. 2010;59:612–24. [PubMed] [Google Scholar]
- 19.Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: A critical review of the literature. J Trauma. 2011;71(1 Suppl):S9–14. doi: 10.1097/TA.0b013e31822114af. [DOI] [PubMed] [Google Scholar]
- 20.Moir MS, Samy RN, Hanasono MM, Terris DJ. Autologous and heterologous blood transfusion in head and neck cancer surgery. Arch Otolaryngol Head Neck Surg. 1999;125:864–8. doi: 10.1001/archotol.125.8.864. [DOI] [PubMed] [Google Scholar]
- 21.Chau JK, Harris JR, Seikaly HR. Transfusion as a predictor of recurrence and survival in head and neck cancer surgery patients. J Otolaryngol Head Neck Surg. 2010;39:516–22. [PubMed] [Google Scholar]
- 22.Amato AC, Pescatori M. Effect of perioperative blood transfusions on recurrence of colorectal cancer: Meta-analysis stratified on risk factors. 1998;41:570–85. doi: 10.1007/BF02235262. [DOI] [PubMed] [Google Scholar]
- 23.Takemura M, Osugi H, Higashino M, Takada N, Lee S, Kinoshita H. Effect of substituting allogenic blood transfusion with autologous blood transfusion on outcomes after radical oesophagectomy for cancer. Ann Thorac Cardiovasc Surg. 2005;11:293–300. [PubMed] [Google Scholar]
- 24.Shiba H, Ishida Y, Wakiyama S, Iida T, Matsumoto M, Sakamoto T, et al. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg. 2009;13:1636–42. doi: 10.1007/s11605-009-0963-y. [DOI] [PubMed] [Google Scholar]
- 25.De Oliveira GS, Jr, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfus Med. 2012;22:97–103. doi: 10.1111/j.1365-3148.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 26.Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: Double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. doi: 10.1136/bmj.d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bednar DA, Bednar VA, Chaudhary A, Farrokhyar F. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976) 2006;31:954–7. doi: 10.1097/01.brs.0000209304.76581.c5. [DOI] [PubMed] [Google Scholar]
- 28.Chen CC, Wang CC, Wang CP, Lin TH, Lin WD, Liu SA. Prospective, randomized, controlled trial of tranexamic acid in patients who undergo head and neck procedures. Otolaryngol Head Neck Surg. 2008;138:762–7. doi: 10.1016/j.otohns.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Wu CC, Ho WM, Cheng SB, Yeh DC, Wen MC, Liu TJ, et al. Perioperative parenteral tranexamic acid in liver tumor resection: A prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243:173–80. doi: 10.1097/01.sla.0000197561.70972.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loreto MF, De Martinis M, Corsi MP, Modesti M, Ginaldi L. Coagulation and cancer: Implications for diagnosis and management. Pathol Oncol Res. 2000;6:301–12. doi: 10.1007/BF03187336. [DOI] [PubMed] [Google Scholar]
- 31.Modrau II, Iversen LL, Thorlacius-Ussing OO. Hemostatic alterations in patients with benign and malignant colorectal disease during major abdominal surgery. Thromb Res. 2001;104:309–15. doi: 10.1016/s0049-3848(01)00373-5. [DOI] [PubMed] [Google Scholar]
- 32.Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107:1479–86. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 33.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–9. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 34.Hynes M, Calder P, Scott G. The use of tranexamic acid to reduce blood loss during total knee arthroplasty. Knee. 2003;10:375–7. doi: 10.1016/s0968-0160(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 35.Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82:383–92. doi: 10.1097/00000542-199502000-00009. [DOI] [PubMed] [Google Scholar]


