Abstract
Background and Aims:
Bilateral superficial cervical plexus block (BSCPB) is effective in reducing pain following thyroid surgeries. We studied the effect of dexmedetomidine on duration and quality of analgesia produced by BSCPB with 0.5% ropivacaine in patients undergoing thyroid surgeries.
Methods:
In this prospective double-blinded study, 60 adults undergoing thyroid surgeries were randomised into two equal groups to receive BSCPB, either with 20 ml 0.5% ropivacaine (Group A) or 20 ml 0.5% ropivacaine with 0.5 μg/kg dexmedetomidine (Group B) after induction of anaesthesia. Visual analogue scale (VAS) was used to assess analgesia postoperatively at 0, 2, 4, 6, 12 and 24 h and patient satisfaction at 24 h. Haemodynamics were recorded peri-operatively. Wilcoxon signed rank test and Mann–Whitney U-test were applied for VAS and sedation scores. Unpaired t-test was applied for age, weight, duration of surgery and duration of post-operative analgesia.
Results:
There was significantly longer duration of analgesia in Group B (1696.2 ± 100.2 vs. 967.8 ± 81.6 min; P < 0.001) and higher patient satisfaction at 24 h (7 [7–9] vs. 5 [4–6]; P < 0.001). While VAS score for pain were similar up to 6 h, they were lower in Group B at 12 h (0 [0–1] vs. 2 [1–2]; P < 0.001) and 24 h (2 [2–2] vs. 5 [5–6]; P < 0.001). Haemodynamic stability and sedation scores were similar across the groups. There were no adverse events. However, pain during swallowing persisted in both the groups.
Conclusion:
Combination of 0.5% ropivacaine and dexmedetomidine for BSCPB provided significantly prolonged and better quality of postoperative analgesia and patient satisfaction than with 0.5% ropivacaine alone in patients undergoing thyroidectomy.
Keywords: Dexmedetomidine, post-operative analgesia, superficial cervical plexus block, thyroid surgery
INTRODUCTION
Post-operative analgesia is a vital part of perioperative care. Good post-operative analgesia can positively improve the surgical outcome.[1] Various modalities are in vogue to relieve the surgical pain.[2] Lately, regional blocks are being used more frequently in this context with commendable outcomes.[3]
Superficial cervical plexus block (SCPB) has been found to be very effective in procedures of neck such as thyroid surgeries, clavicular surgery, carotid endarterectomy and tracheostomy.[4,5,6,7] The duration of analgesia following the nerve blocks is a matter of concern as most of the blocks last for only a few hours. Interestingly, resurgence of the use of α2-agonists in combination with local anaesthetics has dramatically improved the duration of action of these blocks.[8]
Dexmedetomidine is the most recent α2-adrenergic agonist introduced into the clinical practice and has found to be useful in different ways.[8] Ropivacaine, a newer local anaesthetic with better safety profile is known to produce prolonged analgesia of nerve blocks.[9]
Little evidence is available supporting the usefulness of dexmedetomidine in bilateral SCPB (BSCPB). Therefore, the current study was conducted to compare the duration and effectiveness of post-thyroidectomy analgesia of BSCPB using 0.5% ropivacaine with a combination of 0.5% ropivacaine and dexmedetomidine.
METHODS
After obtaining approval from the Institutional Ethical Committee (INST. EC/E.C/31/2013-14) and written informed consent from patients, 60 adult patients belonging to ASA physical status I–II scheduled to undergo thyroid surgeries under general anaesthesia were enroled for this prospective, randomised, double-blinded study. The following patients were excluded from the study - those with a known history of sensitivity and contraindications to drugs used, non-euthyroid at the time of surgery, the presence of coagulopathy, infection at the site of the block, neurological deficits, chronic analgesic consumption and psychiatric illness.
After thorough pre-anaesthetic evaluation, the visual analogue scale (VAS) score was explained to the patients. All subjects were kept nil per oral for 8 h preceding surgery. Pre-medication consisted of tablet diazepam (5 mg for the patients weighing <50 kg and 10 mg for patients more than 50 kg) as per the institutional practice and tablet ranitidine 150 mg at 12 h and 2 h before surgery.
Patients were randomised with the help of computer generated random number tables into one of the following groups to receive BSCPB as follows: Group A with 19.5 ml of 0.5% ropivacaine and 0.5 ml normal saline, 10 ml on each side and Group B with dexmedetomidine 0.5 μg/kg made upto 0.5 ml in normal saline added to 19.5 ml 0.5% ropivacaine, 10 ml on each side. The patients were under general anaesthesia while receiving BSCPB, drug was prepared by another anaesthesiologist and post-operative observer was unaware of the drug used. Hence, they were not aware of the allocation of subjects in the study.
After shifting the patients to the operation theatre, standard monitors including a neuromuscular monitor (TOF-Watch® SX, Organon (Irland) Ltd. Swords, Dublin, Irland) were attached. Baseline values of heart rate, blood pressure and oxygen saturation were noted. Standard general anaesthesia protocol was followed. Injection fentanyl 2 μg/kg was administered over 30 s followed by pre-oxygenation with 100% oxygen for 3 min. Then, injection propofol 2 mg/kg was given for induction of anaesthesia. Neuromuscular blockade was provided with injection vecuronium 0.1 mg/kg. After three minutes of mask ventilation, patients were intubated using an appropriate sized armoured endotracheal tube and positive pressure ventilation initiated. Anaesthesia was maintained with O2:N2O at 33%:66% and isoflurane. BSCPB was performed using landmark technique before the onset of surgery with 3-point sub-fascial technique after negative aspiration for blood every time.
At the end of surgery, after signs of recovery from neuromuscular blockade, residual paralysis was reversed with injection neostigmine and injection glycopyrrolate. Once the patient was fully awake with TOF ratio of one, endotracheal tube cuff was deflated and the patient was extubated. The patient was shifted to PACU when haemodynamically stable and responsive.
Non-invasive blood pressure, heart rate and SpO2 were noted at baseline and every 30 min intra-operatively, once immediately after shifting to PACU and then at 2, 4, 6 and 24 h post-operatively. Oxygen was delivered at 4 L/min for 1 h via Hudson's mask.
The severity of pain experienced by the patient was assessed at 0, 2, 4, 6, and 24 h post-operatively using a VAS scale.
Post-operative sedation was assessed at 2 h after surgery using Brussels sedation score as follows: (1) Unarousable, (2) responds to pain stimulation, (3) responds to auditory stimulation, (4) awake and calm and (5) agitated.[10]
Injection tramadol 50 mg IV was used as rescue analgesic when VAS score was >3 in both the groups and time of administration was noted.[11] Other effects such as nausea, vomiting, dysrhythmia and urinary retention were noted and treated appropriately.
VAS scores were taken at 24 h post-operatively to assess the patient satisfaction.
Sample size of 30 patients per group was obtained based on the projected improvement in the quality of analgesia of atleast 40% with BSCPB, with 80% power.
The collected data were computed using Microsoft excel 2007 and analysed by SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Wilcoxon signed rank test and Mann–Whitney U-test were applied for VAS and sedation scores. Unpaired t-test was applied for age, weight, duration of surgery and duration of post-operative analgesia. The value P < 0.05 was considered significant, while P < 0.01, highly significant and P < 0.001, very highly significant.
RESULTS
Both groups were comparable in terms of demography and surgical characteristics [Table 1]. VAS scores for pain at 0, 2, 4 and 6 h were comparable, whereas at 12 h they were better in dexmedetomidine group, though statistically not significant. At 24 h, VAS scores were significantly higher in ropivacaine alone group [Table 2].
Table 1.
Patient and surgical characteristics
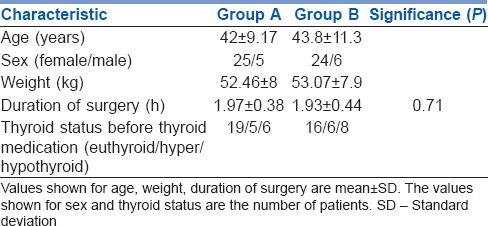
Table 2.
Postoperative outcomes
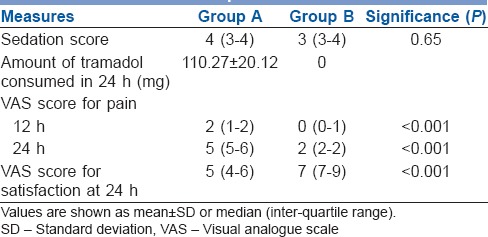
The duration of analgesia was nearly double when dexmedetomidine was added to ropivacaine [Figure 1]. Sedation levels in the post-operative period among both the groups were comparable [Table 2]. No subjects were given additional analgesic intra-operatively.
Figure 1.
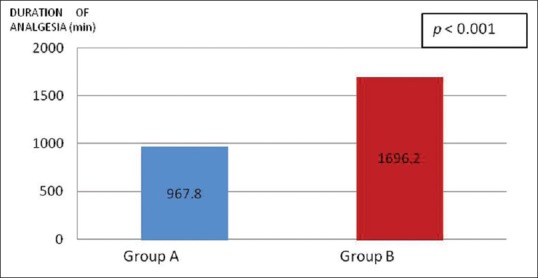
Comparison of the means of duration of post-operative analgesia between the groups
Difference in haemodynamic changes were neither clinically nor statistically significant [Figure 2]. There was no difference in the incidence of other side effects related to dexmedetomidine as they did not occur.
Figure 2.
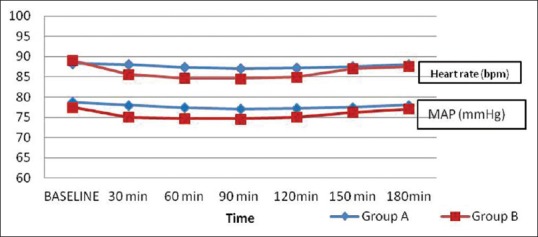
Mean intra-operative arterial pressure and heart rate
DISCUSSION
Peripheral nerve blocks are known to provide analgesia for prolonged periods.[12] The usefulness of BSCPB has not been established beyond suspicion. Some studies have shown it to be less helpful.[13,14] However, our results suggest that it was successful in alleviating post-operative pain following thyroid surgery. The most plausible explanation could be the difference in the technique employed in performing the block: Subcutaneous infiltration against sub-fascial injection and two-point versus three-point injection. In the current study, a three-point technique was used, where local anaesthetic drug was injected sub-fascially, deep to the posterior border of sternocleidomastoid muscle, at the midpoint and along its length above and below from the insertion point. The block is technically very simple with a high success rate, as there was no failure among 60 patients studied.
The post-operative pain scores and rescue analgesic consumed were significantly lower after BSCPB with an α2-agonist (dexmedetomidine) as adjuvant (P < 0.001) after thyroidectomy. The same was demonstrated using levobupivacaine in BSCPB (saline:levobupivacaine = 82.1:410.1 min; P < 0.001) and also with clonidine as an adjuvant to bupivacaine in BSCPB (P < 0.002).[15,16]
Animal studies show that the prolongation of the duration of analgesia of nerve block by dexmedetomidine was due to the blockade of hyperpolarisation-activated cation current in rats.[17] Dexmedetomidine reduces the peak amplitude of compound action potential's reversibility in a concentration dependent manner, thereby prolonging the duration of blockade.[18]
The addition of dexmedetomidine has prolonged the duration of analgesia by nearly 75% in the current study. In a volunteer study, peri-neural dexmedetomidine with 0.75% ropivacaine prolonged block of ulnar nerve by 60%, while systemic administration of 20 μg dexmedetomidine resulted in only 10% prolongation of the same, suggesting the peripheral effect of dexmedetomidine when added to the local anaesthetic.[19] Furthermore, there was no difference in the amount of sedation between groups, indicating the absence of significant central action. Similarly, dexmedetomidine and clonidine provided post-operative analgesia of longer duration and better quality when added to levobupivacaine and bupivacaine, respectively, for brachial plexus block and BSCPB.[9,20]
There was neither statistically nor clinically significant changes in intra-operative mean arterial blood pressure and heart rate between the groups in the current study. There was no need for intervention aimed at bradycardia or hypotension. Addition of dexmedetomidine in a dose of 0.5 μg/kg did not cause sedation. However, significant reduction (P < 0.001) in haemodynamic parameters has been reported by Swami et al., when dexmedetomidine was used in a dose of 1 μg/kg.[21] Hence, a dose of 0.5 μg/kg was decided upon to avoid the haemodynamic and other side effects. The findings justify restricting dose of dexmedetomidine to 0.5 μg/kg for regional blocks.
Patient satisfaction in Group B was superior (P < 0.001) as the quality of analgesia was better when dexmedetomidine was used as an adjuvant.
Patients in both the groups complained of discomfort or throat pain in the immediate post-operative period which increased in intensity during deglutition. Later, it was evident only on deglutition, which lasted for few hours. Our observation indicates that when patients were not concerned about incision pain, they paid more attention to the pain on deglutition. Unfortunately, BSCPB was ineffective in combating throat pain. However, the pain lasted only for a few hours after extubation (3–5 h) and patients did not demand additional analgesics. This outcome corroborates with the earlier findings that post-intubation discomfort lasted only for a short duration and was of mild intensity not requiring additional analgesics.[22]
There are a few limitations in this study. We did not make an effort to quantify the pain arising out of throat irritation secondary to endotracheal intubation. Furthermore, two doses of dexmedetomidine in combination with local anaesthetic could have been studied, viz, 0.5 and 1 μg/kg to compare the effect on prolongation of post-operative analgesia and adverse effects, thereby to be able to conclude about the most relevant dosage. Likewise, inclusion of a control group with BSCPB performed with normal saline would have established role of BSCPB in managing post-operative pain after thyroid surgeries beyond doubt.
CONCLUSION
Addition of dexmedetomidine to ropivacaine enhanced the duration of post-operative analgesia of BSCPB and patient satisfaction after thyroid surgeries. Consequently, by limiting its dose to 0.5 μg/kg, undesirable haemodynamic effects were avoided. Therefore, a combination of dexmedetomidine with ropivacaine is better than ropivacaine alone for BSCPB.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Crews JC. Multimodal pain management strategies for office-based and ambulatory procedures. JAMA. 2002;288:629–32. doi: 10.1001/jama.288.5.629. [DOI] [PubMed] [Google Scholar]
- 2.Schecter WP, Bongard FS, Gainor BJ, Weltz DL, Horn JK. Pain control in outpatient surgery. J Am Coll Surg. 2002;195:95–104. doi: 10.1016/s1072-7515(02)01148-1. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MS. Managing postoperative pain. Hosp Med. 2001;62:560–3. doi: 10.12968/hosp.2001.62.9.1887. [DOI] [PubMed] [Google Scholar]
- 4.Dieudonne N, Gomola A, Bonnichon P, Ozier YM. Prevention of postoperative pain after thyroid surgery: A double-blind randomized study of bilateral superficial cervical plexus blocks. Anesth Analg. 2001;92:1538–42. doi: 10.1097/00000539-200106000-00038. [DOI] [PubMed] [Google Scholar]
- 5.Choi DS, Atchabahian A, Brown AR. Cervical plexus block provides postoperative analgesia after clavicle surgery. Anesth Analg. 2005;100:1542–3. doi: 10.1213/01.ANE.0000149049.08815.00. [DOI] [PubMed] [Google Scholar]
- 6.Barone M, Diemunsch P, Baldassarre E, Oben WE, Ciarlo M, Wolter J, et al. Carotid endarterectomy with intermediate cervical plexus block. Tex Heart Inst J. 2010;37:297–300. [PMC free article] [PubMed] [Google Scholar]
- 7.Wedel DJ. Nerve blocks. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Elsevier, Churchill Livingstone; 2010. pp. 1664–5. [Google Scholar]
- 8.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res Clin Exp. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClellan KJ, Faulds D. Ropivacaine: An update of its use in regional anaesthesia. Anesth Analg. 2000;60:1065–93. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- 10.Detriche O, Berré J, Massaut J, Vincent JL. The Brussels sedation scale: Use of a simple clinical sedation scale can avoid excessive sedation in patients undergoing mechanical ventilation in the intensive care unit. Br J Anaesth. 1999;83:698–701. doi: 10.1093/bja/83.5.698. [DOI] [PubMed] [Google Scholar]
- 11.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 12.Kamath MR, Mehandale SG, Raveendra US. Comparative study of greater palatine nerve block and intravenous pethidine for postoperative analgesia in children undergoing palatoplasty. Indian J Anaesth. 2009;53:654–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Herbland A, Cantini O, Reynier P, Valat P, Jougon J, Arimone Y, et al. The bilateral superficial cervical plexus block with 0.75% ropivacaine administered before or after surgery does not prevent postoperative pain after total thyroidectomy. Reg Anesth Pain Med. 2006;31:34–9. doi: 10.1016/j.rapm.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Shih ML, Duh QY, Hsieh CB, Liu YC, Lu CH, Wong CS, et al. Bilateral superficial cervical plexus block combined with general anesthesia administered in thyroid operations. World J Surg. 2010;34:2338–43. doi: 10.1007/s00268-010-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffen T, Warschkow R, Brändle M, Tarantino I, Clerici T. Randomized controlled trial of bilateral superficial cervical plexus block versus placebo in thyroid surgery. Br J Surg. 2010;97:1000–6. doi: 10.1002/bjs.7077. [DOI] [PubMed] [Google Scholar]
- 16.Karthikeyan VS, Sistla SC, Badhe AS, Mahalakshmy T, Rajkumar N, Ali SM, et al. Randomized controlled trial on the efficacy of bilateral superficial cervical plexus block in thyroidectomy. Pain Pract. 2013;13:539–46. doi: 10.1111/papr.12022. [DOI] [PubMed] [Google Scholar]
- 17.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha (2) adrenoceptor activation. Br J Pharmacol. 2010;160:1662–76. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 20.Andrieu G, Amrouni H, Robin E, Carnaille B, Wattier JM, Pattou F, et al. Analgesic efficacy of bilateral superficial cervical plexus block administered before thyroid surgery under general anaesthesia. Br J Anaesth. 2007;99:561–6. doi: 10.1093/bja/aem230. [DOI] [PubMed] [Google Scholar]
- 21.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (α2-agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomized double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biro P, Seifert B. Complaints of sore throat after tracheal intubation: A prospective evaluation. Eur J Anaesthesiol. 2005;22:307–11. doi: 10.1017/s0265021505000529. [DOI] [PubMed] [Google Scholar]


