Abstract
Introduction:
Degenerative cortical dementias affect several million people worldwide. Early diagnosis and categorization are essential for initiating appropriate pharmacological and nonpharmacological treatment so that deterioration can be postponed, and disability adjusted life years can be saved both for the patient and for the caregiver. Therefore, an early, simple, noninvasive biomarker will serve as a boon.
Patients and Methods:
Patients who satisfied probable Alzheimer's disease (AD) or frontotemporal dementia (FTD) using international consensus criteria for FTD and National Institute of Neurological Disorders and Stroke-AD and Related Disorders Association criteria for AD were evaluated using single pulse transcranial magnetic stimulation with figure of eight coil and motor evoked potential from right first dorsal interossei. Resting threshold (MT), central motor conduction time (CMCT), and silent period (SP) were evaluated.
Results:
Resting MT and SP are reduced in patients with Alzheimer's disease whereas CMCT is prolonged in patients with FTD and SP is in the lower limit of normal in both conditions.
Conclusion:
The patterns of central motor conduction and MT are distinctly different in patients with early Alzheimer's disease (AD) and FTD.
Keywords: AD-Alzheimer's dementia, central motor conduction time, cortical inhibition, frontotemporal dementia, transcranial magnetic stimulation
INTRODUCTION
Transcranial magnetic stimulation (TMS) is a technique used for study of the central motor pathways and was introduced in the year 1985 by Barker et al. He stated that a magnetic stimulus applied to the primary motor cortex elicits response in those muscles that receive output from that area and is, therefore, helpful in studying the central motor pathways. Surface electrodes are sufficient and hence easy to use. Electromagnetic induction is done by a transducing coil attached to a high voltage (400 V to 3 kV) discharge system producing about 1-1.5 tesla lasting less than a millisecond. The coil is kept tangentially at M1 region to facilitate penetration through the skull; the brain tissue is nonhomogenous and, therefore, there is regional variation in conductivity which principle is exploited to study health and disease.[1,2] The electrical field in the tissues is oriented perpendicular to the magnetic field, and it is in opposite direction to the current in the coil. The resting membrane potential gets depolarized, and the action potential is produced, the induced electrical field and the resultant current flow in the cortex are proportionate to the rate of change of the induced electromotive force. This action potential spreads trans-synaptically to cortical and subcortical areas and corticospinal tracts, and motor nerves causing muscle responses, which can be recorded as motor evoked potential (MEP).[2,3,4] The procedure needs no skin preparation but the point of stimulation needs to be marked, and it is painless.
Single pulse stimulation — Mechanism and principles
The apparatus consists of wire coils attached to a box containing large capacitors attached by electrical cables. These wires are charged by a power source and discharges when it is triggered. It is shaped in a figure of eight so that the junction contains twice the number of windings providing focused stimulation of the cortical target. Single pulse transcranial motor stimulation consists of electrical recording of the muscle twitch in response to cortical stimulation of the motor area and is called as MEP. The amplitude of the MEP is an aggregate measure of the excitation potential of output cells in the motor cortex.[2,4,5] Deeper structures are difficult to study except when they are in the neighborhood of cerebrospinal fluid or special coils, which may reach the deeper structures, are used. The stimulation depolarizes axons running in the plane of the stimulating current. The stimulating current is parallel to the plane of the coil. Therefore, TMS has a preference to tangentially oriented axons whereas pyramidal neurons, which are radially oriented, are activated indirectly. Therefore, it is assumed that the MEP obtained is due to the synaptic transmission of the stimulus. The figure of eight coils also permits differential targeting of the various axons by rotating the coil around the point selected. The electrical pulse delivered in a single pulse is time-locked to the stimulus and provides information about the activation of the motor system with high temporal resolution in milliseconds and gives information about the cortico-motor dynamics.[5,6,7,8]
Motor threshold
Lowest TMS intensity which is capable of eliciting an MEP of 50 mcV in a resting muscle and 200 mcV in an active muscle in at least 5 out of 10 trials. There are several other methods using supramaximal and submaximal stimulation and motor threshold (MT) probably reflects neuronal membrane excitability as it increases by drugs, which block voltage-gated sodium channels and decreased by non N-Methyl-D-aspartate (NMDA) glutamate transmission. MT is highly variable across individuals but remains constant in a given individual. This is used as a way of calibrating and normalizing TMS coil output energy for individual physiologic variability and thus determines both dose and safety limits.[3,4,5,7]
Central motor conduction time
It is the time taken for the nerve impulse to travel through the corticospinal tract, motor neurons, and nerves to reach the muscles from cortical excitation time. The measured latency is a combination of peripheral motor conduction time (PMCT) and the central motor conduction time (CMCT). PMCT is measured by the MEP, which is elicited in response to Spine stimulation. It can also be obtained by estimating the M-wave latency obtained by stimulating the motor nerve, F-wave latency which is produced by the alpha motor neuron and PMCT can be calculated using the formula (F + M − 1)/2.
The number “1” in this formula represents 1 ms, which is the turnaround time for the stimulus through the cell body of the spinal motor neuron. It is divided by 2 because it represents the time required for the nerve impulse to reach the spinal cord and come back to target muscles. Another method of calculation is tendon reflex latency time, that is, (T-1)/2. Five to ten responses are recorded superimposed, and the shortest latency is taken, and the CMCT thus obtained may be intracranial or extracranial. Intracranial is from the motor cortex to the brainstem. MEP is elicited with supramaximal stimuli (150%) of the resting MT (T1). Second MEP is elicited from the C7 spinous process (T2), and CMCT can be also calculated as T1-T2. This method is simple and applied in our study.[5,7,8,9,10]
Silent period
A single TMS pulse delivered during voluntary muscle contraction produces a period of electromyogram (EMG) suppression, which is called silent period (SP) and first described by Cantello et al., in 1992. This SP detected by the upper limb muscles were demonstrated to be arising from activation of cortical inhibitory interneurons of the motor cortex and motor association cortex. SP is longer in the hand muscles and shorter in the upper arm and leg.[1,3] SP is related to the intensity of the stimulation and not related to the size of the preceding MEP. It is altered by physiological situations which change the cortical excitability such as hyperventilation, sleep deprivation, drugs of gamma-aminobutyric acid (GABA) nergic nature, fatigue and high frequency repetitive TMS, and also pathological situations of altered cortical excitability. It is measured as follows: as the latency from the onset of EMG arrest following supramaximal stimulus and the later appearance of the EMG burst.[5,10,11]
Transcranial magnetic stimulation in Alzheimer's disease
The parameters that have been studied in the literature are an alteration in the motor cortical excitability and cortical reorganization of motor output. Studies have reported cortical hyperexcitability and subclinical motor cortical reorganization in the early stages. This will explain the absence of motor involvement in early Alzheimer's disease. This reorganization probably takes place via alternative circuits though nucleus basalis of Maynert puts its major cholinergic input to the motor cortex and is one of the most affected brain areas. The motor cortex has self-defensive reorganization possibly dysregulation of inhibitory frontal areas like area 4S, and this could result in TMS changes but no clinical motor changes.[11,12]
It is hypothesized that cortical hyperexcitability is probably a natural compensatory mechanism to improve executive function. The role of excitatory glutaminergic circuits or impairment of cholinergic or GABAergic circuits is not very clear. Resting MT is generally reduced in Alzheimer's disease. This could be either due to increased excitability or impaired inhibition. Probably there is a role for the alternative glutaminergic hypothesis indicating an imbalance between NMDA and non-NMDA neurotransmission. The next hypothesis postulated is the impairment of intracortical inhibitory circuits leading to disinhibition of motor cortex as supported by abnormality of inhibitory mechanisms accessible via TMS such as short intracortical inhibition and short latency afferent inhibition, which are mediated by GABA-A receptors and cholinergic neuron activity, respectively. There are references showing global hyperexcitability in AD and hypoexcitability in aging.[10,11] However, there are variable reports with reference to alteration in MT with reference to patients with AD and normal aging. There is also reported to be reduction in inhibition in Alzheimer's disease but not in controls and frontotemporal dementia (FTD) patients. This is possibly due to decreased cholinergic transmission as it improves with acetylcholinesterase inhibitors.[11] These observations confirm central cholinergic dysfunction in early Alzheimer's disease and not in FTD. With reference to FTD, literature reference is very scarce. Prolonged CMCT and reduced SP is also reported.[8,9,10,12,13]
Utility of transcranial magnetic stimulation in differential diagnosis of dementia
TMS is likely to be beneficial in differentiating cholinergic and noncholinergic forms of dementia. The loss of MEP inhibition is more often seen with Alzheimer's disease and not with FTD and could serve as a tool in differentiating these two forms of dementia.[10,12]
PATIENTS AND METHODS
Patients attending the neurology outpatient unit of our team were included for our study. Patients were evaluated with Hindi Mental Status Examination (HMSE), Clinical Dementia Rating Scale (CDR), as well as DSM-IV criteria. Patients who have HMSE score more than 20 and CDR 0.5-1.5 were included for assessment. They were categorized into either Alzheimer's disease or FTD based on international consensus criteria for FTD and National Institute of Neurological Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria for AD. All of them underwent magnetic resonance imaging with 1.5 tesla machine. Those with mixed features, pyramidal, extrapyramidal or cerebellar signs, advanced dementia, and those with seizures, aneurysm clips were excluded. The normal values were differentiated from abnormal values based on the institute normative data for age and gender matched persons from the right upper limb. These normative data were generated as controls from healthy bystanders for previous studies in TMS for other disorders assessed in our institute with proper ethical guidelines.
Transcranial magnetic stimulation procedure
All participants were seated comfortably in a chair. TMS was performed using a figure of eight magstim 200 stimulator discharging a maximum output of 2.2 tesla. A single pulse stimulation of the left motor cortex was done at optimum scalp position. Surface muscle response was recorded using belly tendon method. The active electrode was placed over the first dorsal interossei belly (FDI) and a reference electrode over the metacarpophalangeal joint of the right index finger. The figure of eight coil handle was positioned at an angle 45° pointing backward. The stimulus intensity was gradually increased in 5% increments until a satisfactory MEP of at least 50 μV was obtained. The stimulation was repeated at least 10 times at intervals of 3 s. The resting MT was calculated as follows: The minimum stimulus intensity that evokes at least five MEP of a minimum of 50 μV in the relaxed FDI. CMCT was calculated as follows from the relaxed FDI. MEP elicited with supramaximal stimuli that is, 150% of resting MT from the motor cortex (T1). Second MEP was elicited from C7 spinous process (T2) CMCT is equal to T1-T2. SP was studied in the partially contracted FDI on the right side. About 150% intensity stimulus was delivered. SP was identified as follows: The period of EMG arrest to the appearance of EMG.
RESULTS
Demographic details
There were 17 patients with AD and 8 patients with FTD. Data were analyzed after entering data into Statistical Package for Social Sciences (SPSS) version 15 software. Their mean age was 61.8 ± 8.5 years and ages ranged from 44 to 74 years. The male:female ratio was 16:9. Comparison across the groups was done using Student's t-test. Mean HMSE score for the entire group was 23.3 ± 1.07 and for patients with AD and FTD were 22.47 ± 1.23 and 25.12 ± 1.35 respectively and was significantly different across the two groups (P = 0.001). MT values were significantly reduced in the AD group (37 ± 7%) as compared to the FTD (54.38 ± 14%) [Table 1].
Table 1.
TMS parameters in cortical dementias
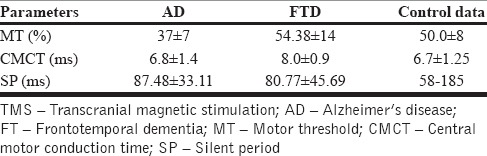
Patients with AD also had lesser cortical inhibition as evidenced by lower MT as compared to FTD group (P = 0.013) whereas CMCT is prolonged in FTD compared to AD (P = 0.048). A trend of consistently lower SP was also seen among the FTD patients when compared to patients with AD.
DISCUSSION
This study reveals the following. Resting MT is reduced in Alzheimer's disease and normal in FTD, central motor conduction is slightly increased in FTD and normal in Alzheimer's disease, SP is reduced in both groups. Reduced resting MT and SP in AD suggest increased cortical excitability and reduced inhibition [Figures 1 and 2]. This might suggest a role for asymptomatic changes in GABAergic and cholinergic systems. In FTD patients, central motor conduction is prolonged, and SP is reduced suggesting early subclinical involvement of motor pathways, as well as reduced inhibition. The common TMS parameter between FTD and AD seems to be the reduced SP. This might indicate a common chemical factor existing between these two diseases may be the underlying mechanism for the reduced SP, which can be postulated as NMDA transmission though a trend for shorter SP was observed among patients with FTD. This study is perhaps the first of its kind in India utilizing the value of TMS as a tool for studying cortical dementias. Our findings suggest that TMS can be considered as a complementary and useful tool in detecting and differentially diagnosing cholinergic deficient and noncholinergic deficient dementias in the early stage itself. There is possibility that early asymptomatic changes in the GABAergic, cholinergic systems are taking place in AD. These changes are absent in FTD. There may be comparable changes in NMDA-mediated excitotoxicity in both groups. This explains the usefulness of cholinesterase inhibitors in Alzheimer's disease and its ineffectiveness in FTD, as well as the utility of NMDA blockers in both groups especially in patients with FTD. Possible role of GABA agonists in the management of Alzheimer's disease in early stages deserves to be evaluated. The presence of abnormality in motor conduction assessment in the absence of clinical motor impairment might point to the cortical reorganization as postulated in the literature.
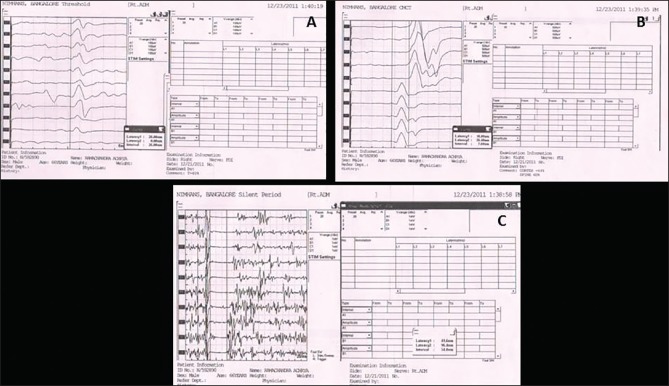
Figure 1.
Transcranial magnetic stimulation graph showing reduced motor threshold in AD
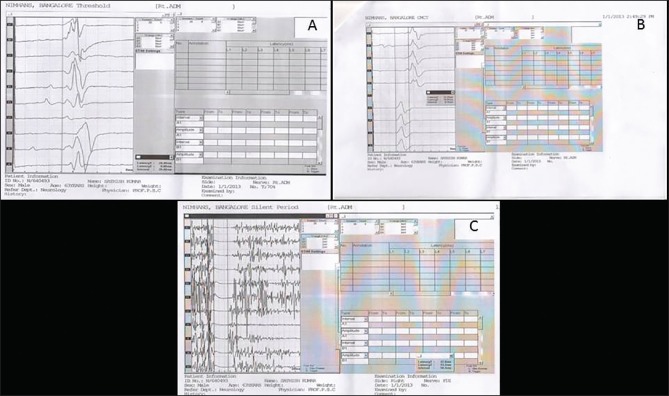
Figure 2.
Transcranial magnetic stimulation graph showing normal motor threshold and prolonged central motor conduction time
CONCLUSION
The above study suggests that single pulse TMS can differentiate early stages of cholinergic deficient dementias like AD from noncholinergic deficient dementias like FTD. This finding is largely in agreement with previous papers focusing on TMS features using single pulse stimulation on AD and FTD. Our study used only single pulse TMS on a relatively very small population. The abnormalities observed in the SP might point to the role of noncholinergic like NMDA receptor mediated neurochemical alterations taking place in neurodegenerative dementias, which might help to open up newer treatment options. When diagnosed early it is known that the quality of life can be maintained to a greater extent in patients with Alzheimer's disease whereas delayed initiation of therapy is not associated with this advantage. The use of cholinesterase inhibitors in noncholinergic deficient dementias produces agitation, insomnia, and hallucinations, and there is no benefit. Onset of these symptoms in these patients if not properly recognized as due to wrong pharmacological option, the treating physician might initiate the patient on antipsychotics, which will herald rapid deterioration by inhibiting natural long-term potentiation involved in repair mechanisms. However, our study consists of relatively less number of patients and anthropometric parameters were not taken into account. Only single pulse stimulation used, spinal magnetic stimulation method was used to determine PMCT. This can cause mild prolongation due to failure to account the latency from anterior horn cell to intervertebral foramina.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2012;123:858–82. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groppa S, Peller M, Siebner HR. Functional assessment of corticospinal conduction with transcranial magnetic stimulation: Basic principles. Klin Neurophysiol. 2010;41:12–22. [Google Scholar]
- 3.Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: Non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593:14–9. doi: 10.1016/0006-8993(92)91256-e. [DOI] [PubMed] [Google Scholar]
- 4.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease: Evidence of impaired glutamatergic neurotransmission? Ann Neurol. 2003;53:824. doi: 10.1002/ana.10600. [DOI] [PubMed] [Google Scholar]
- 5.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Farlow MR. NMDA receptor antagonists. A new therapeutic approach for Alzheimer's disease. Geriatrics. 2004;59:22–7. [PubMed] [Google Scholar]
- 7.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–95. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Voisin T, Reynish E, Portet F, Feldman H, Vellas B. What are the treatment options for patients with severe Alzheimer's disease? CNS Drugs. 2004;18:575–83. doi: 10.2165/00023210-200418090-00003. [DOI] [PubMed] [Google Scholar]
- 9.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:555–9. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Issac TG, Chandra SR, Nagaraju BC. Transcranial magnetic stimulation in patients with early cortical dementia: A pilot study. Ann Indian Acad Neurol. 2013;16:619–22. doi: 10.4103/0972-2327.120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perretti A, Grossi D, Fragassi N, Lanzillo B, Nolano M, Pisacreta AI, et al. Evaluation of the motor cortex by magnetic stimulation in patients with Alzheimer disease. J Neurol Sci. 1996;135:31–7. doi: 10.1016/0022-510x(95)00244-v. [DOI] [PubMed] [Google Scholar]
- 12.Guerra A, Assenza F, Bressi F, Scrascia F, Del Duca M, Ursini F, et al. Transcranial magnetic stimulation studies in Alzheimer's disease. Int J Alzheimers Dis 2011. 2011:263817. doi: 10.4061/2011/263817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]