Abstract
Background:
Over the past two decades, it has been observed that hypertension shows an increasing trend in children and adolescents. Various factors are contributing to this upward trend, and they primarily include changes in lifestyle and dietary habits.
Objectives:
The aim of this study was to evaluate the prevalence of hypertension in school going adolescent children and to study the associated risk factors.
Materials and Methods:
This prospective cross-sectional observational study was conducted over a period of one year on apparently healthy adolescents of randomly selected urban schools of Bhopal district of Madhya Pradesh, Central India. A pretested and prevalidated questionnaire was used to collect the details including present or past history of illness, family history of hypertension, socioeconomic status, and sleep pattern and birth weight of the children. This was followed by anthropometric and blood pressure (BP) measurements and thorough systemic examination.
Results:
Out of 1221 children recruited in the study, 618 were boys, and 603 were girls. 22.7%, body mass index (BMI) of majority (85%) of the students was between 5th and 84th percentile, 5.65% were obese (BMI ≥95th) and 9.18% children were overweight (85th-95th percentile). Systolic and diastolic hypertension (BP >95th percentile) was seen in 61 (4.1%) and 48 (3.9%) participants, respectively. Both systolic and diastolic hypertension was seen in 30 (2.45%) participants. Systolic and diastolic prehypertension (BP 90th to <95th percentile) was seen in 88 (7.3%) and 68 (5.6%) participants, respectively. A highly significant association (P < 0.01) of sex, BMI, systolic BP, family history of hypertension, and birth weight with diastolic BP was seen.
Conclusion:
There is a significant positive correlation of BMI with both systolic and diastolic BP. The family history of hypertension appears to be an important risk factor for the increase in both systolic and diastolic BP. Low birth weight and male sex seem to be risk factors for diastolic hypertension.
Keywords: Adolescents, blood pressure, body mass index, height, hypertension, weight
INTRODUCTION
Increasing trends of hypertension is a worldwide phenomenon. India is a very large and populous and typical developing country. It has been documented that during past few decades, the prevalence of hypertension has increased many folds not only among urban dwellers but also in rural inhabitants in India. Various factors might have contributed to this rising trends and among others, consequences of urbanization such as a change in life style pattern, diet, and stress; increasing population and shrinking employment have also been implicated.[1]
Children and adults, who are obese, have significantly higher blood pressure (BP), than do those who are lean. Childhood obesity is often associated with future development of hypertension.[2] Body mass index (BMI) is generally recognized as the most reliable method to determine increased adiposity.[3] Tracking of BMI in adolescents have high predictive value for adult obesity. The rate of increase in BMI is a good predictor of the adult level of BP, insulin resistance, hyperlipidemia, and other complications associated with obesity.[4]
Hypertension is an important risk factor for future cardiovascular diseases, stroke, nephropathy, and retinal damage. Therefore, regular BP measurement is very important in children and especially adolescents for early detection, intervention, and prevention of complications due to hypertension. The present study was conducted to know the prevalence of hypertension in apparently healthy school going adolescent children and to evaluate the correlation between BMI and other life style factors with hypertension in them.
MATERIALS AND METHODS
The study was conducted over a period of 12-month in the Department of Pediatrics of a Tertiary Care Teaching Hospital of Central India after getting approval from the Institutional Ethics Committee. This was a cross-sectional study done on apparently healthy adolescent children of age group of 14-17 years selected from affluent and nonaffluent school of Bhopal, which were selected by simple randomization method. All the secondary schools located in the urban area of Bhopal registered at the District Education Office were divided into two groups; government and private. From each group, six schools were selected by lottery method; further from the selected group, all the students of 14-17 years of age were enlisted. From these students, approximately equal numbers of male and female children in each age group were further selected by lottery method.
Prior permission was taken from the principals of the respective schools, and a written consent form was distributed to eligible children for parental consent. Children who returned with the positive consent of their parents were recruited in the study. A pretested and prevalidated close ended questionnaire was distributed to the recruited students for family details. This was filled by parents and included details regarding the birth weight of their children, socioeconomic status, family history of hypertension, and sleep pattern. A sample size of 1200 was calculated to be appropriate for the study using estimated prevalence of 8% based on available literature. Formula 4PQ/L2 was applied with an allowable error of 20% of prevalence.
The anthropometric examination was carried out on recruited participants to measure weight and height. The weight of each participant was taken using an electronic weight machine, having ISO2002 standard and prestige make. Weight was recorded to the nearest 0.1 kg while the student was wearing light clothing without shoes, and standing upright steadily. Height of each participant was measured using stadiometer, with an accuracy of 0.5 cm. Height was measured with the student standing barefoot, heels close together, arms hanging along the body trunk, and palm of hand opened toward the thighs; head was kept in Frankfurt plane, scapula and buttocks remained in close contact with the wall at the same vertical plane with the occiput. BMI of each participant was calculated using following formula:
Quetlet's body mass index = Weight in kilograms/(Height in meter)2
The students were classified according to their BMI centiles, developed by national centre for health statistics (CDC 2000 growth standards) into following categories:
Under weight (<5th percentile),
Normal weight (5th-84th percentile),
Over weight (85th-94th percentile),
Obese (>94th percentile).
BP was recorded by an auscultatory method using standard mercury sphygmomanometer each day between 8.00 am to 11.30 am. It was measured only in the right arm for consistency and comparability with an appropriately sized cuff so as to cover at least 40% of arm circumference, at a point midway between the olecrenan and the acromion. All possible efforts were taken to make participant calm to physiological state while he seated quietly for 5 min with his/her back supported, feet on the floor and right arm supported. After applying cuff snugly over the cubital fossa at heart level, the bladder was inflated 30 mmHg above the point when the radial pulse was not palpable. Systolic BP was recorded by the onset of “tapping” Korotkoff sound (K1) and diastolic BP was recorded by the disappearance of Korotkoff sound (K5).
Each participant had his or her BP measured on the second visit, which was after 7 days. If the difference between two BP measurement was greater than 4 mm of Hg, then a third reading was taken the following week on a third visit. Average systolic and diastolic pressure was then calculated using nearest of two BP values. BP was measured by one of the authors, who was trained to measure BP by appropriate methods, to reduce inter-observer variation. However, the second author rechecked BP in those children who had hypertension. Participants were then classified according to the cut-off points for each age, sex, and height percentiles recommended by the Fourth Report on diagnosis, evaluation, and treatment of high BP in children and adolescents.[5] Participants were classified into following categories:
Normal (<90th percentile),
Prehypertension (90th to <95th percentile),
Hypertension (>95th percentile).
Socioeconomic classification was done using modified Kuppuswamy's socioeconomic status scale with modified family income groups[6] sleep disturbance was assessed using BEARS questionnaire, which provides simple but comprehensive screen for major sleep disorders affecting children between 2 and 18 years.[7] BEARS questionnaire addresses five major sleep domains and has five components which include bed time problems, excessive daytime sleepiness, awakening during the night, regularity and duration of sleep, and sleep-disordered breathing (snoring). Each of these domains has an age appropriate trigger questions and includes responses from both the parents and child as appropriate. This can complete brief screening of sleep history within 5 min. History of hypertension in any of the family member (mother, father or grandparents) was recorded only if evidence was present such as antihypertensive medication history or if the parents provided documented BP recordings.
Statistical analysis
Statistical analysis was done using SPSS version 16.0 for Windows (SPSS Inc., Chicago). To describe the population, Student's t-test was used, and Pearson's correlation was applied to evaluate the correlation between BMI, systolic and diastolic BP. To find the effects of studied variables on systolic and diastolic BP, t-test or analysis of variance one-way was applied and multiple linear regression analysis was carried out to calculate the relationship between these variables.
RESULTS
One thousand and three hundred consent forms were distributed to the students and 1266 returned with positive parental consent. Twenty-seven children having BMI <5th percentile were excluded from the study. In a follow-up visit, 18 students couldn’t be traced out. Therefore, 1221 were adolescents were included in the study, out of these, 618 (50.4%) were boys and 603 were (49.6%) girls. The mean weight and height of boys was 54.42 ± 6.26 kg and 1.60 ± 0.48 m,2 respectively, while that of girls was 53.53 ± 5.34 kg and 1.58 ± 0.31 m,2 respectively. The mean BMI of girls was 21.33 ± 5.82 Kg/m2 and that of boys was 21.13 ± 6.34 kg/m2. No significant difference was seen in BMI between two genders (t = −1.1 and P = 0.267 by Student's t-test). In our study, obesity was observed in 69 (6%) of the participants while 112 (9%) of the participants were overweight. The rest of 1040 (85%) participants had normal BMI.
In our study, mean systolic BP (SBP) of a male was 115.81 ± 12.72 mmHg, and that of a female was 115.27 ± 8.25 mmHg. There was no significant difference in SBP of male and female (t = 1.57 and P = 0.129 by Student's t-test). On the other hand, diastolic BP (DBP) was significantly higher in male 70.13 ± 5.61 mmHg in comparison to that of female 69.45 ± 4.79 mmHg (t = 2.294 and P < 0.05 by Student's t-test). Both SBP and DBP were normal in 1049 (85.9%) participants while 30 (2.45%) participants had both systolic and diastolic hypertension. Sixty-one (4.9%) and 88 (7.3%) of the cases had systolic hypertension and prehypertension, respectively, as against 48 (3.9%) and 68 (5.6%) participants having diastolic hypertension and prehypertension, respectively. There was a significant correlation between SBP and DBP (Pearson’ correlation coefficient [r = 0.633, P < 0.01]).
In the study population, only 272 (22.7%) children were of upper socioeconomic class, but a majority of them that is, 565 (46.3%) participants belonged to middle socioeconomic class. The rest 384 (31%) were of lower socioeconomic class. There was no significant difference in weight, height, and BMI in adolescents of different socioeconomic classes. We also found no significant difference in both SBP and DBP between different socioeconomic classes. There was a gradual increase in both SBP and DBP with increasing BMI percentiles as shown in Table 1. We found significant correlation of BMI with SBP and DBP (Pearson's correlation coefficient - [r = 0.701, P < 0.01] and [r = 0.664, P < 0.01] for SBP and DBP, respectively).
Table 1.
Mean blood pressure in various BMI percentiles

The mean SBP and DBP of children with normal sleep were 115 and 69.18 mmHg, respectively, as against 119.10 and 73.84 mmHg, respectively, in children with disturbed sleep. Both SBP and DBP were significantly higher in those with a family history of hypertension. But only DBP was found to be higher in those with disturbed sleep and low birth weight. Step-wise multiple linear regression analysis of the variables shows a significant association (P < 0.01) of BMI, DBP, and family history of hypertension with SBP. A highly significant association (P < 0.01) of sex, BMI, SBP, sleep disturbance, family history of hypertension, and birth weight with DBP was seen as shown in Tables 2 and 3.
Table 2.
Factors determining SBP in participants by multiple linear regression analysis
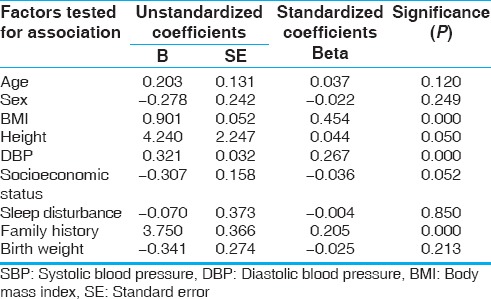
Table 3.
Factors determining DBP in participants by multiple linear regression analysis
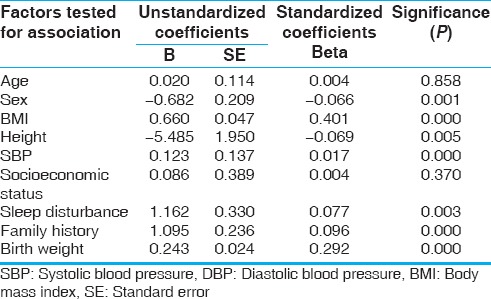
DISCUSSION
In the study, we found that majority of the participants that is, 1040 students (85.18%) had normal BMI while 69 (5.65%) adolescents were obese, and 112 (9.17%) participants were overweight. Similarly results were seen in studies done among school going children by Kapil et al.,[8] Khadilkar et al.,[9] and Marwaha et al.[10] and reported obesity was 7.5%, 5.7% and 5.3% of the study population, respectively. However, the prevalence of overweight was much higher than that of our study (18.4%, 19.9%, and 16.75%, respectively). In developed countries such as the USA, the prevalence of obesity and overweight in school children has been reported to be as high as 22% and 28%, respectively.[11] This difference might due to be the different dietary and lifestyle factors of the participants.
Raj et al.[12] examined the time trend in childhood obesity and prevalence of hypertension in 24842 students of 5-15 years of age during 2003-2004 and 20263 students during 2005-2006. The proportion of overweight children increased from 4.94% of total students in 2003 to 6.5% in 2005. They found systolic or diastolic incidental hypertension in 17.34% of overweight children versus 10.1% of the remaining students. The majority of the participants had normal systolic (87.8%) and normal diastolic BP (90.6%). systolic hypertension and prehypertension were present in 4.9% and 7.3% cases, respectively, as against 3.9% and 5.6% participants having diastolic hypertension and prehypertension, respectively. In a study conducted by Buch et al., the prevalence of hypertension was 6.48% that is in agreement with our study results.[13]
Childhood obesity and its consequences have been attracting more attention in the medical field because of the increasing prevalence worldwide and long-term effects of childhood obesity in later years of life. He et al.[14] in their nationwide case-control study have observed that an increase of 1 BMI unit was associated with 0.56 and 0.54 mmHg increase in SBP and DBP, respectively, for obese children. In nonobese children, this increase in SBP and DBP was 1.22 and 1.20 mmHg, respectively. Furthermore, an increase in the adjusted BMI was associated with an increase in SBP and DBP in both obese and nonobese children. In our study also, we found a gradual increase in both systolic and diastolic BP with increasing BMI.
Friedemann et al.[15] conducted a meta-analysis to find the association of BMI, sex, and cardiovascular disease risk parameters in school aged children in highly developed countries and found worsening of risk parameters for cardiovascular disease in overweight and obese participants. Compared with normal weight children, systolic BP was higher by 4.54 mmHg (99% CI 2.44 to 6.64; n = 12,169, eight studies) in overweight children and by 7.49 mmHg (99% CI 3.36 to 11.62; n = 8074, 15 studies) in obese children.
In our study, a significant association of disturbed sleep with hypertension was also found. Similar associations have been described by Leung et al.[16] However, we have used BEARS questionnaire to assess the sleep disorder which is a screening tool only. We did not classify the type of disorder by polysomnography such as obstructive sleep apnea syndrome which was found to have an association with hypertension. Sleep-disordered breathing is characterized by intermittent episodes of partial or complete obstruction of the upper airway during sleep which disrupt the normal ventilation and sleep architecture and is typically associated with snoring and daytime sleepiness. Sympathetic overactivity occurs with sleep-disordered breathing resulting in diverse autonomic, humoral, neurohumoral, and hemodynamic responses.[17] Sleep-disordered breathing may be related to endothelial dysfunction with the production of more endothelin (producing vasoconstriction) than nitric oxide (causing vasorelaxation).[18]
The mean systolic and diastolic BP was significantly higher in children with low birth weight. A large number of studies have shown that birth weight, an indicator of fetal growth in utero, is inversely related with systolic BP in young children and adults. In a prospective study conducted by Li et al.,[19] it was found that birth weight was inversely related with systolic BP in prepubertal children, which continued through late puberty.
Our study was a prospective cross-sectional study that was sufficiently powered and included children from different socioeconomic status. However, few study limitations need special mention here. First, our study population might not be a truly representative sample from the district e.g., only urban schools were included and representation from rural schools was not there. Second, we just screened the children for sleep disorders and did not diagnose and classify their types by polysomnography. Furthermore, the association between sleep disorder and hypertension should have been established by 24 h ambulatory BP monitoring.
CONCLUSION
In our study, the prevalence of obesity and overweight was 5.65% in 9.17%, respectively, and that of systolic and diastolic hypertension was 4.9% and 3.9%, respectively. There is a significant positive correlation of BMI with both systolic and diastolic BP. The family history of hypertension appears to be an important risk factor for the increase in both systolic and diastolic BP. Low birth weight and male sex seem to be risk factors for diastolic hypertension. Further large-scale studies are needed to confirm this association.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Das SK, Sanyal K, Basu A. Study of urban community survey in India: Growing trend of high prevalence of hypertension in a developing country. Int J Med Sci. 2005;2:70–8. doi: 10.7150/ijms.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubre E, Torro I. Prevalence, persistence and clinical significance of masked hypertension in youth. Am Heart J. 2005;45:493–8. doi: 10.1161/01.HYP.0000160320.39303.ab. [DOI] [PubMed] [Google Scholar]
- 3.Kleigman RM, Behrman RE, Jenson HB, Stanton BF, editors, editors. 18th ed. Elsevier Inc; 2007. Nelson Text Book of Pediatrics; pp. 232–6. [Google Scholar]
- 4.Guo SS, Chumlea WC. Tracking of BMI in children to overweight in adulthood. Am J Clin Nutr. 1999;70:1458–88. doi: 10.1093/ajcn/70.1.145s. [DOI] [PubMed] [Google Scholar]
- 5.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 6.Kumar N, Shekhar C, Kumar P, Kundu AS. Kuppuswamy's socioeconomic status scale-updating for 2007. Indian J Pediatr. 2007;74:1131–2. [PubMed] [Google Scholar]
- 7.Owens JA. Sleep medicine. In: Kleigman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Text Book of Pediatrics. 19th ed. Elsevier Inc; 2011. p. 70. [Google Scholar]
- 8.Kapil U, Singh P, Pathak P, Dwivedi SN, Bhasin S. Prevalence of obesity amongst affluent adolescent school children in Delhi. Indian Pediatr. 2002;39:449–52. [PubMed] [Google Scholar]
- 9.Khadilkar VV, Khadilkar AV. Prevalence of obesity in affluent school boys in Pune. Indian Pediatr. 2004;41:857–8. [PubMed] [Google Scholar]
- 10.Marwaha RK, Tandon N, Singh Y, Aggarwal R, Grewal K, Mani K. A study of growth parameters and prevalence of overweight and obesity in school children from Delhi. Indian Pediatr. 2006;43:943–52. [PubMed] [Google Scholar]
- 11.Mirza NM, Kadow K, Palmer M, Solano H, Rosche C, Yanovski JA. Prevalence of overweight among inner city Hispanic-American children and adolescents. Obes Res. 2004;12:1298–310. doi: 10.1038/oby.2004.164. [DOI] [PubMed] [Google Scholar]
- 12.Raj M, Sundaram KR, Paul M, Deepa AS, Kumar RK. Obesity in Indian children: Time trends and relationship with hypertension. Natl Med J India. 2007;20:288–93. [PubMed] [Google Scholar]
- 13.Buch N, Goyal JP, Kumar N, Parmar I, Shah VB, Charan J. Prevalence of hypertension in school going children of Surat city, Western India. J Cardiovasc Dis Res. 2011;2:228–32. doi: 10.4103/0975-3583.89807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, Ding ZY, Fong DY, Karlberg J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension. 2000;36:165–70. doi: 10.1161/01.hyp.36.2.165. [DOI] [PubMed] [Google Scholar]
- 15.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta-analysis. BMJ. 2012;345:e4759. doi: 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung LC, Ng DK, Lau MW, Chan CH, Kwok KL, Chow PY, et al. Twenty-four-hour ambulatory BP in snoring children with obstructive sleep apnea syndrome. Chest. 2006;130:1009–17. doi: 10.1378/chest.130.4.1009. [DOI] [PubMed] [Google Scholar]
- 17.Quan SF, Gersh BJ. National Center on Sleep Disorders Research; National Heart, Lung, and Blood Institute. Cardiovascular consequences of sleep-disordered breathing: Past, present and future: Report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109:951–7. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 18.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Huang TK, Cruz ML, Goran MI. Birth weight, puberty, and systolic blood pressure in children and adolescents: A longitudinal analysis. J Hum Hypertens. 2006;20:444–50. doi: 10.1038/sj.jhh.1002021. [DOI] [PubMed] [Google Scholar]


