Abstract
Arterial desaturation may occur after the Kawashima procedure and, in the absence of venovenous collaterals is usually due to pulmonary arteriovenous malformations. Stenting of the pulmonary arteries, oxygen therapy, and pulmonary vasodilators such as sildenafil have not been able to resolve the arterial desaturation and the only way to do this has been Fontan completion. The time course of the formation of these malformations after the Kawashima and the progression of cyanosis and its resolution after the Fontan has only been demonstrated in case reports and small case series. We pool the available data to model arterial saturations in patients with pulmonary arteriovenous malformations after the Kawashima procedure.
Keywords: Cyanosis, Glenn, heterotaxy, inferior caval vein, inferior vena cava, interrupted, isomerism, Kawashima, saturations
INTRODUCTION
Patients with atrial isomerism, also known as “heterotaxy,” can have a constellation of congenital malformations. Isomerism can be segregated into those with right or left isomerism. Those with right isomerism will often have complex cardiac malformations that may require univentricular palliation, total anomalous pulmonary venous connection, two trilobed lungs with right sided bronchus bilaterally, the absence of a spleen, and intestinal malrotation. Those with left isomerism will often have less complex cardiac malformations, interruption of the inferior caval vein, two bilobed lungs with left sided bronchus bilaterally, and multiple spleens.[1,2,3]
These malformations go beyond anatomic curiosity and have functional consequences as well. It is straightforward to understand how complex cardiac malformations can have hemodynamic and circulatory consequences. The other findings, however, can also have functional consequences such as splenic malformations. Those with multiple spleens and even those with a solitary, normally located spleen in the setting of isomerism may have functional asplenia.[4]
Those with complex cardiac malformations requiring univentricular palliation may require a Norwood procedure, shunt placement, or pulmonary artery banding in the 1st week of life. Eventually, these patients will undergo the Glenn and Fontan procedures. The Glenn, in which the superior caval vein is directly anastomosed to the pulmonary artery, may be slightly different in those with isomerism.[5] Those with isomerism may have bilateral superior caval veins, requiring bilateral Glenn anastomoses. In addition, there may be an interruption of the inferior caval vein with azygos continuation in which the azygos vein then drains into the superior caval vein. Thus, those with interruption of the left inferior caval vein will have nearly all of their systemic venous return directly entering the pulmonary arteries after the time of the Glenn. In this setting, the Glenn is also known as the Kawashima procedure. While the Fontan procedure, done at a later time, normally consists of directly anastomosing the inferior caval vein to the pulmonary arteries, in those with interruption of the inferior caval vein will only have hepatic veins left to anastomose at this time.[6]
Shortly after the Kawashima procedure, arterial saturations increase into the mid 80% and low 90% and over time increase the mid 90%. Some children may experience a progressive decrease in arterial saturations months after the Kawashima. In the absence of venovenous collaterals, this desaturation is often due to the development of pulmonary arteriovenous malformations.[7] Occurring in up to 32% of those after the Kawashima, these are thought to develop due to the absence of a yet to be isolated “hepatic factor” from the lungs since the hepatic vessels still return to the heart with their venous return directly entering the systemic circulation.[8,9,10]
There are limited data regarding the time course of desaturation after the Kawashima procedure in those with pulmonary arteriovenous malformations. We present a pooled analysis of existing data regarding arterial desaturation due to pulmonary arteriovenous malformations after the Kawashima procedure.
METHODS
A systematic review of the literature was performed to identify accounts of desaturation after the Kawashima procedure due to pulmonary arteriovenous malformations in which arterial saturation at various time points were provided. No previous protocol for this review was utilized. When available, data were collected regarding age at the time of the Kawashima, saturations in the 1st week after the Kawashima, months after the Kawashima when arterial desaturation was noted, arterial saturation at this time, months after Kawashima when the Fontan was done, and arterial saturation in follow-up after the Fontan.
Search and identification strategy
Reports were identified using electronic databases, specifically PubMed, EMBASE, and Ovid. They were queried using the following search terms in various combinations: “Heterotaxy,” “left isomerism,” “right isomerism,” “Glenn,” “Kawashima,” “cyanosis,” “desaturation,” and “pulmonary arteriovenous malformation.” No specific restriction was placed on a year of publication, but only manuscripts in English were eligible for inclusion. The cited references from accounts identified by the electronic search were then hand-searched for additional relevant manuscripts.
Assessment of study quality and bias
Since candidate papers were either case reports or case series the National Heart, Lung, and Blood Institute Quality Assessment Tool for Case Series Studies was utilized to assess quality. Bias analysis could not be conducted as the included studies were case reports and case series.
Data extraction
Data regarding arterial saturations in those with pulmonary arteriovenous malformations after the Kawashima procedure were extracted. Patients were included if all the previously identified data was extractable.
Data analysis
Numeric data are presented as median with ranges. Extracted data were graphed using months after the Kawashima procedure as the x-coordinate and the arterial saturation as the y-coordinate. Polynomial curve fitting techniques were used to create an overall model for arterial saturations based on months after the Kawashima. This analysis was performed using CurveExpert software, version 1.4. (Madison, AL, USA).
RESULTS
The initial search yielded 493 unique manuscripts. After reviewing the titles and abstracts, we obtained full-text manuscripts for 19 studies. Of these 19 studies, 9 were included in the final analysis. All the included studies were either case studies or case reports.[7,8,9,11,12,13,14,15,16] No manuscripts were excluded from this analysis due to concerns with study quality based on assessment using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Case Series Studies. A total of 41 patients were included in the analysis.
Median age at the time of the Kawashima was 10 months (range of 8-132 months). Arterial desaturation was noted at a median of 32 months after the Kawashima (range of 8-36 months). Median time of Fontan was 40 months after the Kawashima (range of 15-69 months). Latest follow-up was 44 months after the Kawashima (range of 23-81 months).
Arterial saturations were best modeled by the following 3rd degree polynomial equation: 86.4 — (1.06 × M) + (0.0395 × M2) — (0.000332 × M3) where M is the number of months after the Kawashima. This equation is able to model arterial saturations until 59 months after the Kawashima in patients with pulmonary arteriovenous malformations. From 59 to 81 months after, the Kawashima procedure saturations plateau at 93%. No comment can be made regarding the applicability of this beyond 81 months after the Kawashima [Figure 1].
Figure 1.
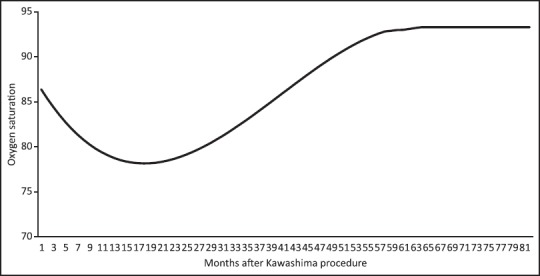
Graph arterial demonstrating saturations in patients with pulmonary arteriovenous malformations after the Kawashima
The lowest saturation that was documented was 63%, and this patient had improvement after the Fontan with saturations reaching the low 90%.
DISCUSSION
Cardiac malformations in the setting of isomerism may pose unique considerations when univentricular palliation must be undertaken. Specifically, those with interruption of the inferior caval vein must undergo a Kawashima procedure. This procedure is not unlike a Glenn from a purely surgical standpoint but the anastomosis of the superior caval vein to the pulmonary artery now leads to venous return from both the superior and inferior caval veins to directly flow into the pulmonary artery since the inferior caval vein empties into the azygos vein which then empties into the superior caval vein. The only venous return directly to the heart after the Kawashima are the hepatic veins.[6]
Those who undergo the class Glenn procedure may develop pulmonary arteriovenous malformations, likely secondary to lack of a yet to be isolated “hepatic factor.”[17,18,19] Those who undergo a Kawashima are more likely to develop pulmonary arteriovenous malformations with up to 32% of patients developing such pulmonary arteriovenous malformations.[8,9] These malformations lead to progressive arterial desaturation that can only be reversed by Fontan completion that leads to hepatic venous flow to directly enter the pulmonary circulation, allowing the lungs to once again be exposed to “hepatic factor.” This was elegantly demonstrated by Praus et al. who tried multiple interventions such as sildenafil and oxygen therapy and found that only the Fontan led to resolution of arterial desaturation.[20]
Data regarding the time course of the decline in arterial saturations after the Kawashima due to pulmonary arteriovenous malformations is not fully delineated as much data comes from case reports and small case series. We pooled data from such previous studies to better demonstrate the course taken by arterial saturations in this unique setting and demonstrate the resolution of arterial desaturation after the Fontan. Saturations tend to be in the mid to high 80% immediately following the Kawashima. Patients who develop pulmonary arteriovenous malformations tend to do so in the first 30 months with saturations that generally decrease into the high 70%. Once the Fontan is undertaken there is a prompt increase in arterial saturations with saturation reaching 90% within a month of the operation in a majority of patients.
This data can be helpful to those caring for patients having undergone the Kawashima, who develop pulmonary arteriovenous malformations. Fontan completion in these patients is what ultimately needs to be done to resolve the arterial desaturation can safely be done within the first 35 months after the Kawashima. Even patients with arterial saturations in the mid 60% can have improvement in arterial saturations into the low 90%.
Strengths of this analysis include the fact that the number of patients included in this study is at least double that of the largest case series included. In addition, this study offers understanding of the progression of arterial desaturation due to pulmonary arteriovenous malformations after the Kawashima. This analysis also demonstrates the amount of time over which improvements are noted in arterial saturations after the Fontan is completed. Limitations of this study are the low number of patients. As a result of the low number of patients analysis of specific patient characteristics was not feasible either. In addition, lack of specific details of included cases only allowed for a limited number of endpoints to be extracted. Some studies did not offer any patient level data and only offered study level data.
CONCLUSION
In the absence of venovenous collaterals, progressive arterial desaturation after the Kawashima is likely due to pulmonary arteriovenous malformations which seem to become evident around 30 months after the Kawashima. Fontan completion with incorporation of the hepatic venous return is the only method by which to reverse this arterial desaturation and can be done successfully with arterial saturations reaching the low 90% within a few weeks, even in patients who may have had a nadir near 60 % at the time of greatest desaturation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jacobs JP, Anderson RH, Weinberg PM, Walters HL, 3rd, Tchervenkov CI, Del Duca D, et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(Suppl 2):1–28. doi: 10.1017/S1047951107001138. [DOI] [PubMed] [Google Scholar]
- 2.Uemura H, Ho SY, Devine WA, Anderson RH. Analysis of visceral heterotaxy according to splenic status, appendage morphology, or both. Am J Cardiol. 1995;76:846–9. doi: 10.1016/s0002-9149(99)80243-4. [DOI] [PubMed] [Google Scholar]
- 3.Uemura H, Ho SY, Devine WA, Kilpatrick LL, Anderson RH. Atrial appendages and venoatrial connections in hearts from patients with visceral heterotaxy. Ann Thorac Surg. 1995;60:561–9. doi: 10.1016/0003-4975(95)00538-V. [DOI] [PubMed] [Google Scholar]
- 4.Nagel BH, Williams H, Stewart L, Paul J, Stümper O. Splenic state in surviving patients with visceral heterotaxy. Cardiol Young. 2005;15:469–73. doi: 10.1017/S1047951105211320. [DOI] [PubMed] [Google Scholar]
- 5.Glenn WW. Circulatory bypass of the right side of the heart. IV. Shunt between superior vena cava and distal right pulmonary artery; report of clinical application. N Engl J Med. 1958;259:117–20. doi: 10.1056/NEJM195807172590304. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima Y, Kitamura S, Matsuda H, Shimazaki Y, Nakano S, Hirose H. Total cavopulmonary shunt operation in complex cardiac anomalies. A new operation. J Thorac Cardiovasc Surg. 1984;87:74–81. [PubMed] [Google Scholar]
- 7.Kutty S, Frommelt MA, Danford DA, Tweddell JS. Medium-term outcomes of Kawashima and completion Fontan palliation in single-ventricle heart disease with heterotaxy and interrupted inferior vena cava. Ann Thorac Surg. 2010;90:1609–13. doi: 10.1016/j.athoracsur.2010.06.114. [DOI] [PubMed] [Google Scholar]
- 8.Baskett RJ, Ross DB, Warren AE, Sharratt GP, Murphy DA. Hepatic vein to the azygous vein anastomosis for pulmonary arteriovenous fistulae. Ann Thorac Surg. 1999;68:232–3. doi: 10.1016/s0003-4975(99)00494-4. [DOI] [PubMed] [Google Scholar]
- 9.Knight WB, Mee RB. A cure for pulmonary arteriovenous fistulas? Ann Thorac Surg. 1995;59:999–1001. doi: 10.1016/0003-4975(94)00735-p. [DOI] [PubMed] [Google Scholar]
- 10.Justino H, Benson LN, Freedom RM. Development of unilateral pulmonary arteriovenous malformations due to unequal distribution of hepatic venous flow. Circulation. 2001;103:E39–40. doi: 10.1161/01.cir.103.8.e39. [DOI] [PubMed] [Google Scholar]
- 11.McElhinney DB, Kreutzer J, Lang P, Mayer JE, Jr, del Nido PJ, Lock JE. Incorporation of the hepatic veins into the cavopulmonary circulation in patients with heterotaxy and pulmonary arteriovenous malformations after a Kawashima procedure. Ann Thorac Surg. 2005;80:1597–603. doi: 10.1016/j.athoracsur.2005.05.101. [DOI] [PubMed] [Google Scholar]
- 12.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. Pulmonary arteriovenous malformations in children after the Kawashima operation. Ann Thorac Surg. 2005;80:1592–6. doi: 10.1016/j.athoracsur.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara T, Yokoyama T. Pulmonary arteriovenous malformation in patients with total cavopulmonary shunt: What role does lack of hepatic venous blood flow to the lungs play? Pediatr Cardiol. 2001;22:343–6. doi: 10.1007/s002460010243. [DOI] [PubMed] [Google Scholar]
- 14.Aidala E, Chiappa E, Cascarano MT, Valori A, Abbruzzese PA. Partial hepatic vein diversion in pulmonary arteriovenous malformations in congenital heart disease. Ann Thorac Surg. 2004;78:1089–90. doi: 10.1016/S0003-4975(03)01441-3. [DOI] [PubMed] [Google Scholar]
- 15.Larsen SH, Emmertsen K, Bjerre J, Hjortdal VE. Progressive cyanosis following Kawashima operation: Slow resolution after redirection of hepatic veins. J Cardiothorac Surg. 2013;8:67. doi: 10.1186/1749-8090-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah MJ, Rychik J, Fogel MA, Murphy JD, Jacobs ML. Pulmonary AV malformations after superior cavopulmonary connection: Resolution after inclusion of hepatic veins in the pulmonary circulation. Ann Thorac Surg. 1997;63:960–3. doi: 10.1016/s0003-4975(96)00961-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Bae EJ, Cho DJ, Park IS, Kim YM, Kim WH, et al. Development of pulmonary arteriovenous fistulas after bidirectional cavopulmonary shunt. Ann Thorac Surg. 2000;70:1918–22. doi: 10.1016/s0003-4975(00)02164-0. [DOI] [PubMed] [Google Scholar]
- 18.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg. 2003;76:1759–66. doi: 10.1016/s0003-4975(03)00450-8. [DOI] [PubMed] [Google Scholar]
- 19.Freedom RM, Yoo SJ, Perrin D. The biological “scrabble” of pulmonary arteriovenous malformations: Considerations in the setting of cavopulmonary surgery. Cardiol Young. 2004;14:417–37. doi: 10.1017/S1047951104004111. [DOI] [PubMed] [Google Scholar]
- 20.Praus A, Fakler U, Balling G, Schreiber C, Ewert P, Hess J. Only hepatic venous blood closes intrapulmonary shunts after cavopulmonary connection. Int J Cardiol. 2014;172:477–9. doi: 10.1016/j.ijcard.2013.12.316. [DOI] [PubMed] [Google Scholar]


