Abstract
A 6-year-old male child born with hypoplastic left heart syndrome (HLHS) was palliated with an extracardiac nonfenestrated Fontan procedure (18-mm Gore-Tex tube graft). He developed low-pressure (mean Fontan pressure 10 mmHg) protein-losing enteropathy 6 months after Fontan palliation. After initially responding to medical therapy and transcatheter pulmonary artery stent implantation, he developed medically refractory protein-losing enteropathy. At this time, his transthoracic echocardiogram showed new restriction across his native atrial septum with an 8 mmHg mean gradient. Cardiac catheterization now showed high-pressure (mean Fontan pressure 18-20 mmHg) protein-losing enteropathy and a new 6 mmHg mean gradient across the atrial septum. To avoid cardiopulmonary bypass, he underwent successful transcatheter relief of atrial septal restriction and creation of a fenestration with rapid clinical and biochemical improvement of his protein-losing enteropathy.
Keywords: Diabolo stent, hypoplastic left heart syndrome (HLHS), atrial septal restriction
INTRODUCTION
Although long-term outcomes of Fontan palliation continue to improve, a number of late complications have been described. Protein-losing enteropathy is a well-known and dreaded complication after Fontan palliation with an incidence rate as high as 3.7%, and a cumulative risk of 13.4% by 10 years.[1,2,3] Treatment options include medical therapy, transcatheter and surgical interventions, and finally heart transplantation.[2] Native atrial septal restriction is a rare but treatable cause of protein-losing enteropathy, and is traditionally addressed by surgical resection of the tissue causing atrial septal restriction.[4] We report the first successful case of transcatheter procedure to relieve new native atrial septum restriction using a Diabolo stent and the creation of a fenestration with rapid clinical and biochemical improvement.
CASE REPORT
A 6-year-old male child with hypoplastic left heart syndrome (HLHS) (mitral and aortic stenosis) underwent staged palliation culminating in an extracardiac nonfenestrated Fontan procedure when he was 3 years old. The pre-Fontan hemodynamics assessment showed a normal Glenn circuit pressure at 10 mmHg. Eight months after his Fontan procedure he was found to have mild intermittent facial edema with no evidence of biochemical signs of protein-losing enteropathy. Cardiac catheterization showed mild angiographic left pulmonary artery stenosis and low pressure in the Fontan circuit (10 mmHg). One year and 2 months after his Fontan procedure, he developed clinical and biochemical signs of protein-losing enteropathy. Repeat catheterization revealed mean Fontan pressure of 12 mmHg that increased to 18 mmHg after 10 mL/kg fluid bolus. There was 1 mm mean gradient across the left pulmonary artery stenosis that was successfully treated with stent implantation. Over the next 18 months, he had clinical and biochemical evidence of protein-losing enteropathy that initially responded to therapy with budesonide. Unfortunately, he was finally refractory to high dose budesonide with side effects from therapy and growth failure. Interestingly, he was noted to have new development of restriction across the native atrial septum with 8-10 mm mean gradient by echocardiogram. Throughout his course, he had adequate systemic ventricular function, no significant systemic atrioventricular valve regurgitation, and an unobstructed aortic arch. After a discussion at a multidisciplinary conference, a decision was made to proceed with the attempt of transcatheter relief of atrial septal restriction and creation of a fenestration to avoid additional stress of cardiopulmonary bypass.
His weight on the day of cardiac catheterization was 19.6 kg. The procedure was performed under general anesthesia and showed elevated mean Fontan pressure of 18-20 mmHg without any stenosis across the Fontan pathway including the previously placed left pulmonary artery stent. A 6-mm mean gradient was recorded across the atrial septum (measured by retrograde catheter from the femoral artery across the systemic atrioventricular valve into the atrium). At this point, we considered three options to relieve native atrial septum restriction: Retrograde arterial via the systemic atrioventricular valve, transconduit Brockenbrough puncture from the femoral vein/transhepatic or from the pulmonary artery to the atrial chamber, and finally hybrid approach. Based on our experience, when the systemic ventricle has right ventricular morphology, access to the atrium across the systemic atrioventricular valve can be easily performed. We decided to balloon size the atrial defect and determine the hemodynamic stability with this approach. A pigtail catheter was advanced retrograde to the right ventricle via the neo-aortic valve with the open end directed to the systemic atrioventricular valve. The pigtail was opened using a Glidewire (Terumo Medical Corporation, Somerset, NJ), which was directed across the systemic atrioventricular valve into the right atrium and the pigtail was exchanged for a Glide catheter (Terumo Medical Corporation, Somerset, NJ). The catheter was then advanced across the atrial septum into the left upper pulmonary vein. This was exchanged over a SV5 guidewire and a 12-mm Tyshak II angioplasty catheter (Braun Interventional System Inc., Bethlehem, PA) was advanced over the wire and centered across the atrial septum under transesophageal and fluoroscopic control [Figure 1]. There was no hemodynamic perturbation with the wire and angioplasty catheter in place. Balloon sizing revealed a 7-mm waist. However, in order to place a Diabolo stent, the size of the arterial sheath was felt to be prohibitive. Next, the Brockenbrough puncture across the Gore-Tex tube graft was attempted; however, we were unable to advance the introducer after multiple attempts (including reshaping the needle) and in fact during one of the attempts the needle tip fractured without embolization. Transhepatic approach was considered but not used because of the relative contraindication secondary to ascites. Based on the relative ease of puncture between the native floor of the neo-left pulmonary artery and the adjacent atrial roof, this approach was felt to be optimal. A pulmonary artery angiogram through the right internal jugular vein and simultaneous atrial angiogram through the retrograde atrial catheter confirmed anatomic proximity of the pulmonary artery to the atrium. Brockenbrough puncture from the neo-left pulmonary artery to the atrium was performed under fluoroscopic and transesophageal guidance [Figure 2a]. A 0.035 Amplatzer super stiff wire (Boston Scientific Corporation, Marlborough, MA) was positioned in the right atrium and an 11-Fr Mullins sheath (Medtronic, Minneapolis, MN) was advanced into the right atrium [Figure 2b]. A Diabolo stent was then prepared using a 5-mm Amplatz Gooseneck Snare (ev3 Endovascular Inc., Plymouth, MN) positioned in the middle of a 16-mm BIB balloon (NuMed, Hopkinton, NY). A 2510 Genesis XD stent (Johnson and Johnson Health Care System Inc., Piscataway, NJ) was mounted over the centered snare. Fluoroscopy was used to confirm a proper position of the snare at the middle of the balloon. The distal end of the stent (up to the snare) was unsheathed in the right atrium and the angioplasty catheter was inflated. The entire system while the balloon was still inflated was pulled back to oppose the flared distal end against the atrial septum. Next, the remainder of the stent was unsheathed and the proximal portion was inflated. The stent with the atrial septum was constrained at 5 mm. It however became apparent that the large sheath distorted the anatomy and the stent was actually deployed in the left atrium [Figure 2c]. The snare and balloon were removed and the stent was still over the wire. To reposition the stent, a 25-mm sizing balloon catheter was chosen and this was advanced across the stent and positioned so that most of the balloon was distal to the waist in the stent (toward the septum). The rationale behind this approach was to hold the stent using the balloon and then use it as an introducer to facilitate advancement of the stent. Once inflated in this position at low pressure, the larger distal portion of the balloon dilated the atrial septum and allowed the stent to be advanced without catching on the atrial tissue. Once the stent was nicely seated across the atrial septum the waist was further dilated to 8 mm [Figure 2d]. Repeat hemodynamic assessment showed no residual gradient and significant drop in the Fontan pressure to 14 mmHg. Next, the fenestration was created by deploying another Diabolo stent. A 7 mm × 22 mm I-CAST covered stent (Atrium Medical Corporation, Hudson, NH) was taken off the existing catheter and was remounted on a 12 cm × 3 cm Optapro angioplasty catheter (Cordis Corporation, Bridgewater, NJ) and a 5 mm snare that was positioned at the middle of the balloon. The stent was re-crimped over snare then advanced through the long sheath into the left atrium. The stent was deployed in the same manner described earlier creating a Diabolo configuration across the pulmonary artery to atrial access site [Figure 3a and b]. The covered stent prevented bleeding into the potential space. A repeat hemodynamics was performed and showed a reduction in Fontan pressure to 14 mmHg with systemic saturation in high 80% range. He had an uncomplicated post-procedure course and was discharged home the next day. He was bridged with Lovenox to Coumadin for systemic anticoagulation. He had rapid clinical and biochemical improvement (2 weeks) with increase in albumin levels from 2.2 to 3.2 gm/dL and resolution of ascites, facial edema, and diarrhea.
Figure 1.
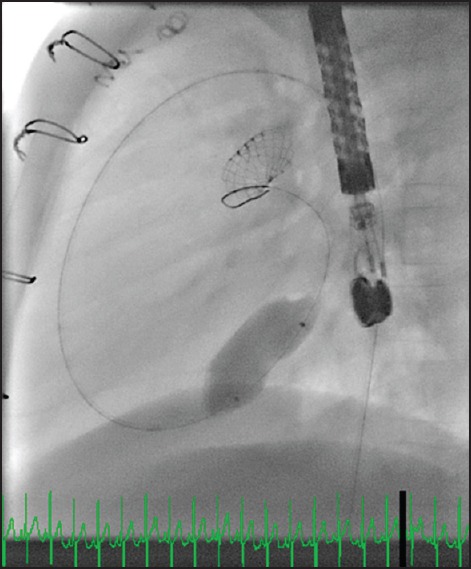
Balloon sizing the atrial septum through a retrograde approach
Figure 2.
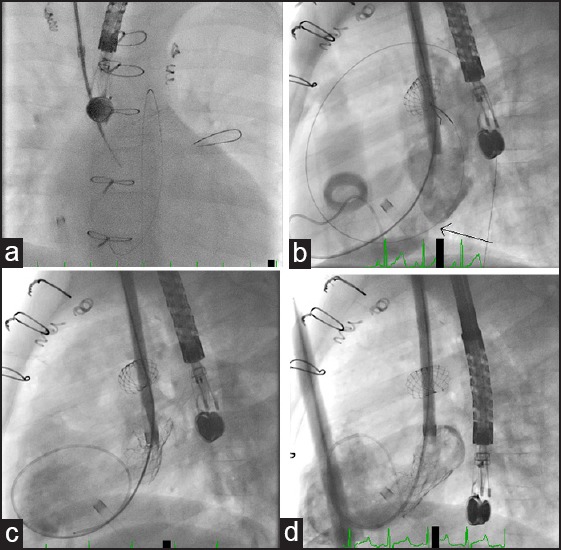
(a) Transseptal puncture through right internal jugular vein from the neo-LPA to LA. (b) LA angiogram through the sheath before stenting, arrow indicates the site of atrial septum. (c) LA angiogram showed the malpositioned Diabolo stent being entirely in the LA. (d) LA angiogram after successful Diabolo stent placement. LPA = Left pulmonary artery, LA = Left atrium
Figure 3.
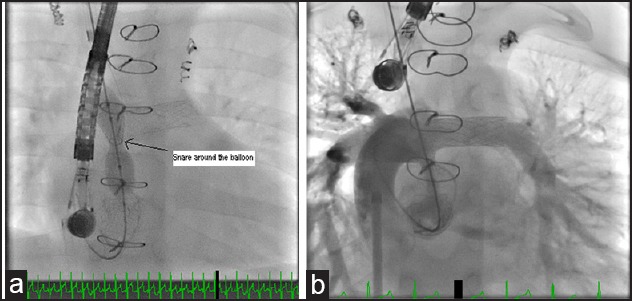
(a) Diabolo stent placement across neo-LPA to LA fenestration, arrow points to the snare site at the middle of the balloon catheter. (b) SVC angiogram after Diabolo stent placement showing a patent stented fenestration with good fenestration position. LPA = Left pulmonary artery, LA = Left atrium
DISCUSSION
Protein-losing enteropathy is a dreaded complication after Fontan palliation. Since the exact pathophysiology of protein-losing enteropathy is not known, the pillars for therapy include optimization of Fontan hemodynamics and empirical medical therapies.[1] Del Nido et al. have shown that in rare circumstances restriction across the native atrial septum can cause obstruction to the pulmonary venous pathway, which in turn increases Fontan pressure and causes or worsens protein-losing enteropathy.[4] Although the cause of protein-losing enteropathy is multifactorial, our patient had new and severe atrial restriction. This has been traditionally addressed by surgical resection of the offending atrial tissue.[4] Ideally, one would want to avoid foreign material within the Fontan circuit or within the systemic circulation. There is increasing use of transcatheter interventions to address residual hemodynamic lesions.[5] The major impetus for this is to avoid sternal reentry, cardiopulmonary bypass, further exposure to blood products since cardiac transplant maybe required. In this case report, we have discussed the different approaches that can be considered when faced with the need for transcatheter intervention to treat restriction of the native atrial septum.
Although atrial septal interventions including the use of Diabolo stent configuration have been described, all of them are prior to Fontan palliation.[6,7] Once the Fontan pathway is created, especially nonfenestrated, access to the pulmonary venous atrium requires innovative strategies. Multiple authors have discussed options and the technical challenges of performing punctures across extracardiac Gore-Tex conduits. Mehta et al. have recently described innovative access to the pulmonary venous atrium via the floor of the pulmonary artery where it is immediately adjacent to the atrial mass.[8] This method allows one to avoid any artificial tissue and the puncture is performed across native tissue.
Although we were able to perform balloon sizing via a retrograde arterial approach, the size of the arterial sheath precluded deployment of the Diabolo stent from this approach. Transfemoral–transseptal puncture across the extracardiac Fontan was unsuccessful because of inadequate purchase on the conduit and toughness of the tissue resulting in fracture of the needle. Transhepatic approach was relatively contraindicated secondary to ascites. Eventually, we used the internal jugular approach and did our puncture through the pulmonary artery into the left atrium. This approach provided an advantage by avoiding the Gore Tex material as the puncture went through the native tissue. The potential down side of this approach is the higher risk of bleeding in the potential space between the two structures. Other down side of this approach is the lack of good site for wire position (such pulmonary vein) as we approach the atrial septum from the left atrium. In our case, we looped the wire in the right atrium to support stent deployment with no problems. One of the challenges we encountered was distortion of the pliable atrial structures from the larger long sheath such that the sheath tends to tent the structures without actual advancement.
CONCLUSION
Percutaneous intervention for relief of atrial septal restriction is feasible. The long-term outcome remains to be seen. A distinct disadvantage is the need for foreign material and hence anticoagulation.
Financial support and sponsorship
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
Nil.
REFERENCES
- 1.Rychik J. Protein-losing enteropathy after Fontan operation. Congenit Heart Dis. 2007;2:288–300. doi: 10.1111/j.1747-0803.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 2.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115:1063–73. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 3.Feldt RH, Driscoll DJ, Offord KP, Cha RH, Perrault J, Schaff HV, et al. Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 1996;112:672–80. doi: 10.1016/S0022-5223(96)70051-X. [DOI] [PubMed] [Google Scholar]
- 4.Padalino MA, Saiki Y, Tworetzky W, del Nido PJ. Pulmonary venous pathway obstruction from recurrent restriction at atrial septum late after Fontan procedure. J Thorac Cardiovasc Surg. 2004;127:281–3. doi: 10.1016/s0022-5223(03)01296-0. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzer J, Graziano JN, Stapleton G, Rome JJ. Late catheter interventions in hypoplastic left heart syndrome. Cardiol Young. 2011;21(Suppl 2):65–76. doi: 10.1017/S1047951111001612. [DOI] [PubMed] [Google Scholar]
- 6.Stumper O, Gewillig M, Vettukattil J, Budts W, Chessa M, Chaudhari M, et al. Modified technique of stent fenestration of the atrial septum. Heart. 2003;89:1227–30. doi: 10.1136/heart.89.10.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedra CA, Pihkala J, Benson LN, Freedom RM, Nykanen D. Stent implantation to create interatrial communications in patients with complex congenital heart disease. Catheter Cardiovasc Interv. 1999;47:310–4. doi: 10.1002/(sici)1522-726x(199907)47:3<310::aid-ccd11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Mehta C, Jones T, De Giovanni JV. Percutaneous transcatheter communication between the pulmonary artery and atrium following an extra-cardiac Fontan: An alternative approach to fenestration avoiding conduit perforation. Catheter Cardiovasc Interv. 2008;71:936–9. doi: 10.1002/ccd.21453. [DOI] [PubMed] [Google Scholar]


