Abstract
Lectins are carbohydrate binding proteins present in seeds of many plants, especially corals and beans, in fungi and bacteria, and in animals. Apart from their hemagglutinating property, a wide range of functions have been attributed to them. Their importance in the area of immunohematology is immense. They are used to detect specific red cell antigens, to activate different types of lymphocytes, in order to resolve problems related to polyagglutination and so on. The introduction of advanced biotechnological tools generates new opportunities to exploit the properties of lectins, which were not used earlier. Stem cell research is a very important area in transplant medicine. Certain lectins detect surface markers of stem cell. Hence, they are used to understand the developmental biology of stem cells. The role of various lectins in the areas of transfusion and transplant medicine is discussed in detail in this review.
Keywords: Blood groups, lectins, mitogenic activity, stem cells
Introduction
Lectins are either proteins or glycoproteins and have nonimmune origin. They combine specifically with carbohydrate molecules or with carbohydrate groups present in complex glycoproteins or glycolipids. Generally, carbohydrates are universally present on the outer surface of the cell membranes; so, lectins can be used as tools to detect antigens on cells on the basis of its surface structure.
Lectins are widely distributed in nature and found in all kinds of organisms such as plants, fungi, animals, bacteria, and viruses.[1] The amount of lectin varies in different organisms. In the plant kingdom, they are mainly seen in the seeds of leguminous plants. Apart from this, they are seen in the fruiting bodies of many fungi.[2] They may be toxic, inflammatory, and resistant to cooking or enzymes. There are about 500 species of plants where the hemagglutinating lectins have been documented. Considering the total number of plants in the plant kingdom, it can be considered that lectins are present in plants as an exception rather than the rule. The hemagglutinating activity of lectins is inhibited by simple sugars (monosaccharide), which represent the binding site for the lectin on the cell surface. Certain plant extracts contain more than one form of lectin (Ulex europeaus and Bandeiraea simplicifolia). Lectins can be inactivated by procedures which denature or break down proteins such as heating, extremes of pH, and treatment with proteolytic enzymes such as papain or trypsin.
Materials and Methods
The present review aims at understanding the importance of uses of various lectins in the field of transfusion medicine. This includes their use in blood grouping, in mitogenic activity, in resolving problems related to polyaaglutination, and it's application in stem cell transplantation.
The data presented in this review have been accessed from various databases such as Web of Science, PubMed Central, Science Direct, and National Center for Biotechnology Information to find out the current status of lectin research and use of lectins mainly in the area of transfusion medicine. “Lectins and red cells” and “lectins and transfusion medicine” were the terms used for collecting the data. Apart from this, standard text books on transfusion medicine and various review articles were also searched for information on lectins. In addition, several reviews and research articles where lectins have been used for various purposes were considered.
History
By the end of the 19th century, it was known that some proteins can agglutinate red blood cells. Earlier, the lectins identified were derived from plants, specifically from seeds of leguminous plants. They were called “phytohemagglutinins” because of their property to agglutinate red blood cells (RBCs). Later on, it was observed that some hemagglutinins selectively agglutinate red blood cells of a particular human blood group within the ABO blood group system. Therefore, in 1954, Boyd and Shapleigh[3] named them “lectins” from the Latin word legere meaning “to choose” or “to select.” This definition was further broadened by Goldstein et al.,[4] which says that lectin is “a sugar-binding protein or glycoprotein of nonimmune origin, which agglutinates cells and/or precipitates glycoconjugates.”
In 1888, Peter Stillmark from Russia isolated highly toxic extracts from seeds of the castor tree (Ricinus communis) and named it “ricin.” He found that this preparation could agglutinate RBCs. In 1891, Hellin isolated another toxic hemagglutinin, “abrin” from jequirity bean (Abrus precatorius). Paul Ehrlich took lot of interest in these two lectins and subsequently used them in his basic immunological studies. In 1919, Sumner isolated lectin from jack bean seeds (Canavalia ensiformis) and purified it for the first time in crystalline form. This was named as Concanavalin A. Subsequently in 1936, it was shown by Sumner that this lectin's hemagglutinating property was inhibited by sucrose demonstrating for the first time the specificity of the lectin. Today, this lectin is widely used for the characterization and purification of sugar-containing molecules and cellular structures.
During the early days, lectins were prepared by “the way of pharmacological isolation” – the phrase coined by Stillmark. This involved following steps: Salt extraction of the crushed or mashed beans, precipitation with magnesium sulfate and ammonium sulfate followed by dialysis. Today, various chromatographic procedures with some modifications are used to increase the yield of lectin.
Subsequently several lectins have been detected and extracted from various sources and during the past two decades, the three-dimensional (3D) structures of various lectins have also been established.[5]
Lectins in Immunohematology
Lectins have been known for the last 126 years. As mentioned earlier, Stillmark was the first scientist to extract lectin from the seeds of Ricinus communis in 1888. That time, he observed that this lectin had hemagglutnating property. Subsequently, lectins were detected in various plants’ seeds extracts [Abrus precatorius (Hellin 1891), Jatropha curcas (Siegel 1893), and Croton tiglium (Elfstrand 1897)] and various invertebrate animals [Helix pomatia—snail (Camus 1899), horse shoe crab (Noguchi 1903)]. All these extracts were known to agglutinate RBCs. However, separation of RBCs on the basis of blood groups was not known at that time. Landsteiner who discovered ABO blood groups in 1900 detected hemagglutinating activity in some nontoxic plant extracts. In 1909, he observed that hemagglutinating activity of certain seed extracts was inhibited by either heat-treated serum or by mucin. The first attempt to find blood group-specific agglutinins in the seed extracts were made by Marcusson-Begun (1926) and Sievers (1927) but they were not successful. The fifth decade of the 20th century witnessed a dramatic improvement of research in the area of plant seed extracts. Several scientists published reports of their extensive surveys on the search for seed extracts showing blood group-specific activity. The reports of Renkonen in Helisinki (1948),[6] Boyd and Reguera in Boston (1949),[7] Bird in India (1955),[8] and Boyd in Egypt (1950)[9] were some important reports describing blood group specificity in some seed extracts.
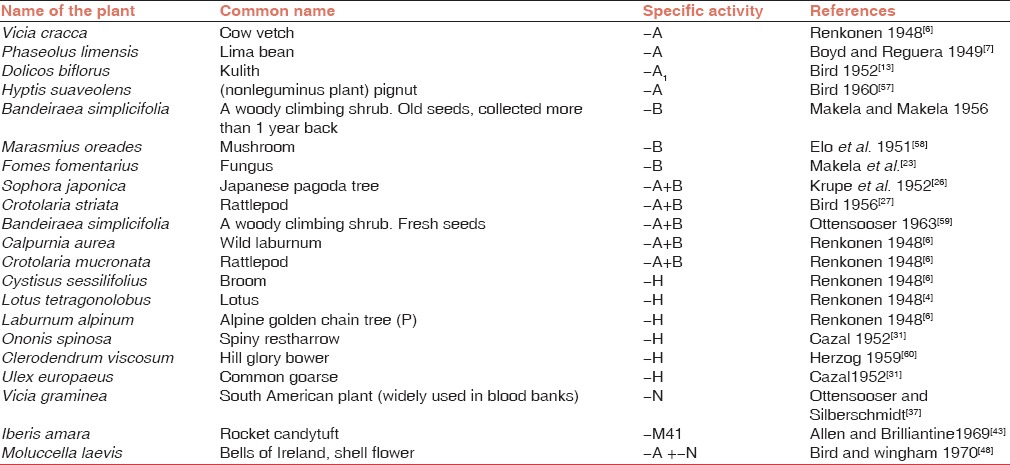
Two different types of extracts were identified. Certain seed extracts showed hemagglutinating activity against all RBCs irrespective of specific blood groups while another group of seed extracts showed blood group-specific activity, which were named “lectins.” Many lectins having activity against different blood groups are listed below [Table 1].
Table 1.
Blood group specific lectins
Determinants of ABO, MN, and P Blood Group Systems
Blood group antigens are either various sugars or proteins and they are attached to various components in the red cell membrane. The antigenic determinants of ABH blood groups are specified by terminal sugar residues added to a common oligosaccharide chain (precursor substance). This is done by a series of reactions in which specific enzymes catalyze the addition of specific sugar molecules. In the case of MN blood group system, the antigenic specificity lies in a glycophorin molecule, which is a sialoglycoprotein. It also carries sugar molecules. Meanwhile, specificity of antigens of P blood group system lies in globo-series of glycosphingolipids.[10,11]
Lewis antigens are biochemically related to the ABH blood group system. Two main antigens of the system, viz., Lea and Leb, are formed by the action of the transferase encoded by FUT3 gene. When a subterminal α 1-4 linked fucose is added to the N-acetylglucosamine of type I chain precursor, Lea antigen is formed while Leb antigen is formed when α-1-4-linked fucose is added to N-acetylgalactosamine of type I H antigen.[10]
Lectins bind specifically to the sugar residues, which project from red cell membrane causing RBCs to clump. This binding is as specific as antigenic recognition by antibodies. Considering the simplicity and cost of the lectins, many lectins are useful as blood typing reagents. Sometimes, the specificity against more than one blood group antigen is seen in a lectin (e.g., Dolichos biflorus against Tn and Cad antigens). However, by diluting this lectin cross-reactivity can be prevented.
Anti-A
The presence of anti-A in Vicia cracca was the first report with blood group specificity.[6] Subsequently, Koulumies (1949)[12] showed that this seed extract can be used to differentiate between blood groups A1 and A2. Bird (1952)[13] reported the presence of anti-A1 activity in the seeds of Dolichos biflorus and he used to differentiate A1 and A2 as well as A1 B and A2 B cells. Lectin from Glycine max shows stronger reactivity with A1 group RBCs than with A2 group RBCs. The activity is much more than group B RBCs. (A1 > A2 > B). Boyd and Reguera[7] found anti-A activity in Lima bean extract (Phaseolus limensis).
A lectin showing specific anti-A1 activity was found in the seeds of Phaseolus coccineus[14] while a lectin showing specificity for A2 antigen was found in the seeds of Falcata japonica.[15]
Anti-B
Lectins showing anti-B activity are relatively less common than those showing anti-A activity. The anti-B specificity was detected in the “old” seeds (more than 1 year since harvested) of Bandeiraea (now Griffonia) simplicifolia, a leguminous shrub from West Africa. It is important because fresh seeds of this plant show some anti-A activity in addition to anti-B activity.[16] This lectin contains at least three isolectins, viz., GS I, GS II, and GS IV. Of these, GS I mainly shows anti-B activity but it also shows some activity against A, N, and Tn antigens. GS II is specific for Tk antigen while GS IV is considered to react with Leb and Y antigens.[17] Later on, Lescar et al.[18] studied the crystal structure of GS I. They found that this isolectin contains two different subunits A and B, which combine to form five different tetrameric structures made up of different proportions of two subunits. They are A4, A3 B, A2 B2, AB3, and B4 with different binding specificities.[19] The A subunit is specific for αGalNAc but it also recognizes αGal. So it agglutinates both A and B blood group RBCs. The B subunit is specific for only αGal end groups and so it agglutinates only B group RBCs. Therefore, GS I B4 is used for the detection of αGal residues in biological material, e.g., tissue from human breast carcinomas.[20] The GS I A4 has a strong affinity for the Forssman antigen (αGalNAc 1-3 GalNAc) and the Tn (αGaINAc- Ser/Thr) antigen.[21]
Anti-B activity was also detected in the seed coats of Evonymus species,[22] in the fungi Fomes formentarius,[23] and Clavutinopsis fusiformis[2] and in sea weed Ptilota plumose.[24] Anti-B activity was detected in the roe of various species of fish, especially from the salmon and herring families. Since they are D-galactose-specific, they also show some activity against P, P1, and Pk antigens, which also have the galactosyl determinants.[25]
Anti-A+B
Several seed extracts agglutinate both A and B blood group RBCs but not O group RBCs; Sophora japonica,[26] Crotolaria striata,[27] fresh seeds of Bandeiraea simplicifolia and Calpurnia aurea[28] are some examples. Lectin extracted from the fruiting bodies of the mushroom Hygrophorus hypothejus also agglutinates both A and B blood group RBCs.[29] Lectin isolated from Jerusalem sage, Phlomis fruticosa, a flowering plant of the Lamiaceae family seen mainly in the Mediterranean countries is another example of this category.[30]
Anti-H
Anti-H was first detected by Renkonen[6] in the extracts of the seeds of Cystisus sessilifolius, Laburnum alpinum, and Lotus tetragonolobus. H specificity was exhibited by several lectins but very few of them are being used as regular reagents. Among these, the lectin extracted from Ulex europaeus seeds is most widely used in the area of transfusion medicine.[31] It is being used for identifying secretor status from the salivas of O blood group individuals. Similarly, it is also used to differentiate Bombay group individuals from normal O group individuals. RBCs from Bombay blood group individuals lack H antigen while those from A, B, O, and AB blood group individuals have H antigen on their surface. Therefore, anti-H lectin gives negative reaction with RBCs from “Bombay” phenotype individuals while it shows agglutination with variable strength with RBCs from individuals with other blood groups. The strength of agglutination decreases as follows: O> A2 B > A2 > B > A1 > A1 B. Other seed extracts revealing anti-H activity have been tabulated in Table 1. Apart from this, anti-H like activity was seen in the extracts of Pleurocybells porriagens, Naematoloma sublateritium, and Pholiota squarrosa, which are various species of fungi.[2]
It has been observed that two different types of lectins are present in Ulex europeaus seed extract.[32] They are named as Ulex I and Ulex II. Ulex I is inhibited by L-fucose but Ulex II is not inhibited by L-fucose but it is inhibited by di-N-acetylchitobiose, a sugar with an N-acetylglucosaminyl residue.[33] It likely that Ulex II reacts with subterminal N-acetylglucosaminyl residue in the H structure but only in the presence of terminal L-fucose. Based on this pattern, anti-H lectins can be divided into two classes.
Ulex I: Those that are inhibited by L-fucose, e.g., Lotus tetragonolobus.
Ulex II: Those which are inhibited by N-acetylglucosmine derivatives, e.g., Cystisus sessilifolius and Laburnum alpinum. This fucose-specific lectin was studied in detail by Loris et al.[34] They found that its complexes with N-acetylglucosamine, galactose, and fucosyl galactose showed a promiscuous binding site. The hydrogen-binding network in these complexes is considered as suboptimal and is compensated by extensive hydrophobic interactions in αGal/αGalNAc-binding subsite. So, this is the first lectin with a promiscuous binding site and stresses the importance of hydrophobic interactions in protein carbohydrate complexes.
Anti-H reagent extracted from the seeds of Momordica dioica Roxb.ex wild revealed normal serological properties after performing standard tests.[35] This lectin showed the strongest reaction with group O RBCs and negative reactions with RBCs of 25 Bombay phenotype individuals. However, hemagglutination inhibition studies conducted using specific sugar molecules suggested that along with H antigen, this lectin recognized unsubstituted terminal beta-linked galactose units.
The lectin purified from Erythrina corallodendron seeds interacts with the H antigen in association with the I antigen exhibiting H/HI specificity.[36]
Anti-M and anti-N
Ottenssoser and Silberschmidt[37] observed for the first time that the seed extract of a vetch, Vicia graminea showed agglutination of RBCs with N antigen more strongly than those lacking it. It enhances the reaction with trypsin treated red cells.[38] However, sialidase does not affect M or N antigen-binding sites to Vicia graminea.[39] The determinant recognized by Vicia graminea lectin is often called as Nvg to distinguish it from normal N. In spite of this, it is widely used in blood banking practice. Some other sources of anti-N are seed extracts of Bauhinia purpurea,[40] Bauhinia variegata,[41] and the extract from the leaves of Vicia unijuga.[42] Allen and Brilliantine[43] isolated lectin with anti-M specificity from two species of the genus Iberis, viz., Iberis amara and Iberis umbletta.
Cross Reacting Lectins
Sometimes, single lectin cross-reacts with two different antigens. Lectins with specificities for both A and B antigens are under this category. Lectins isolated from Euonymus europeus[44] and from Scardinius eryhrophtalmus[45] showed activity against B and H antigens while lectin prepared from Styela plecata[46] exhibited activity against A and H antigens. Sophora japonica lectin is a well-known anti-A+B lectin but apart from this, it shows activity against I antigen.[47] The most remarkable cross-reacting lectin is the one extracted from Molucella laevis. This is the only lectin that shows specificity against two antigens from two distinct blood group systems, viz., A and N antigens.[48] Later on, Alperin et al.[49] isolated this lectin and found that it was made up of three subunits. Of these, a 26kDa subunit exhibited both anti-A and anti-N activities.
Certain seed extracts contain separable specific lectins against different determinants. Many of them are against cryptoantigens; so these lectins are used in the kit to detect antigens present on polyagglutinable RBCs. Some of their examples are as follows: Salvia horminum (anti-Tn + anti-Cad) and Vicia hyrcanica (anti-T + anti-Tk). The details are discussed under polyagglutination.
Lectins Detecting Rare Blood Groups
Lectins generally combine with simple sugar molecules, which are usually present in the terminal position in the carbohydrate chains. The specificity depends not only on the terminal sugar molecules but also on the type of overall subterminal structure, on the number and distribution of receptor sites, and on the steric hindrance caused by surrounding molecules. Some lectins pick up antigens expressing very rare blood groups. In such cases instead of using expensive antisera, the use of lectins becomes a cheap technique to detect rare antigens.
MNS is a very complex blood group system and consists of 46 different antigens.[50] The common antigens such as M, N, or S are polymorphic in nature. The remaining antigens are variants of this system, the majority of which reveal a low prevalence and occur due to deficiency of glycophorin A and/or glycophorin B. Dantu (MNS 25) and Sta (Stones, MNS 15) are MNS variants with low prevalence. They are associated with delta alpha hybrid sialoglycoproteins;[51] when ficin pretreated RBCs were tested with lectin from Vicia graminea, which is specific for N antigen (anti-NVg), strong positive results were obtained with St(a+) and Dantu cells only. This indigenous test is very cheap and can be used as an alternative test for screening these low frequency antigens. This avoids the use of scarce and costly anti-St (a) and anti-Dantu antisera.
En (a) is another variant of the MNS system (MNS 28) with high prevalence. When a first case of rare En (a-) pregnant English lady with the presence of anti-Ena was described by Darnborough et al.,[52] it was predicted that her unusual blood grouping reactions were due to some factor affecting the red cell structure possibly by modifying the cell envelope. Hence, the name anti-Ena (for envelope) was proposed. Subsequently, it was found that unusual serological characteristics seen with En (a-) RBCs were due to reduced sialic acid content. Lectin prepared from the seeds of Maclura aurantiaca binds specifically to sialogycoproteins of RBCs[53] and reacts very weakly with En(a-) cells, confirming the defect related to sialic acid content of RBCs. Similarly, lectin receptor sites on RBCs of old En (a-) cells for Limulus polyphemus, Canavalia ensiformis, Triticum vulgaris, and Bauhinia purpurea are significantly lower. So these lectins are not useful for screening of En (a-) cells.[54,55] On the other hand, En (a-) cells react more strongly with certain lectins than with En (a+) cells.[56] These include certain lectins which have specificity for some blood group antigens. They are Sophora japonica (anti A+B), Glycine soja (anti A+B), Bauhinia purpurea (anti-N), Dolichos biflorus (anti-A1), Phaseolus lunatus (anti-A), and Arachis hypogea (anti-T). After removal of activity against the antigens mentioned in brackets, these lectins are useful to distinguish En (a-) cells from En (a+) cells. Hemagglutination by the lectin Maclura aurantiaca is considerably reduced in En (a-) cells;[54] so this lectin is also useful to pick up Ena (-) cells.
Routine Use of Lectins in Blood Banking
As mentioned earlier, although blood group specificity is detected in several lectins from “legume” seeds, all these lectins are not routinely used in day-to-day blood banking.
Anti-A1 lectin obtained from Dolichos biflorus seeds are routinely used in blood banks to differentiate between A1 and A2 RBCs. This is especially important when the recipient is showing the presence of anti-A1 antibody active at physiological temperature. In such cases, he should be cross-matched with RBCs of A2 blood group. This lectin also agglutinates Tn- and Cad-positive RBCs. So this is also useful in detecting antigens involved in polyaggutination.
Anti-H is used to detect a very rare phenotype, viz., “Bombay” phenotype. All the RBCs accept those from “Bombay” phenotype show positive reaction with this lectin.
Lectins in Polyagglutination
Although polyagglutination is a rare phenomenon in blood banking, today it is considered as a potential pitfall in correct ABO grouping because it hinders the rapid allocation of accurately cross-matched blood to the patient. Lectins are very useful in detecting the cryptoantigens involved in polyagglutination. This is a phenomenon where the RBCs are agglutinated by all the human sera. Cryptoantigens are generally hidden or masked by membrane carbohydrates but they are exposed by the action of various microbial enzymes or by incomplete biosynthesis of membrane carbohydrates or by somatic mutation of an abnormal clone of erythrocyte precursor cells. The first two well-characterized RBC cryptoantigens were T antigen that is a part of carbohydrate portion of the MN glycoproteins, and is exposed by an enzyme sialidase. Tk antigen is situated deep in the ABH Lewis biosynthetic pathway and is exposed by endo-β galactosidase from Bacteroides fragilis.[61] Ness et al.[62] described a case of Tn polyagglutination where the patient developed acute myelomonocytic leulemia after 2 years.
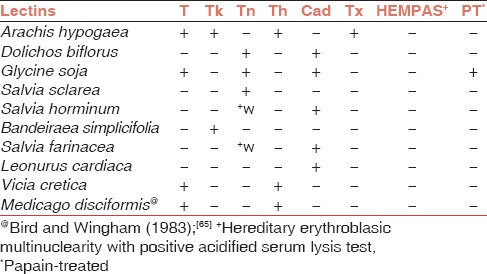
Polyagglutination is divided into two categories. Acquired polyagglutination, which includes T, Tk, Th and Tx antigens, and acquired B condition while polyagglutination as an inherited character occurs due to mutation, which is responsible for poor synthesis of sialic acid residues on glycophorins A and B. Tn, HEMPAS, Cad, and Sda (Super Sid) antigens are involved in it. Cad antigen was described as a private antigen in a Mauritian family.[63] Sid antigen shows considerable individual variation in the strength of the antigen. Subsequently, based on the reactions of Cad and Sid antigens with Dolichos biflorus Sanger et al.[64] concluded that Cad represents strong expression of Sid antigens. Since polyagglutinability is inherited, it is a lifelong process and not transient like acquired polyagglutination. The following lectins [Table 2] are used to differentiate between these antigens. One should keep in mind that clinical and other laboratory reactions also should be considered while identifying the cryptoantigen.
Table 2.
The reactions of various lectins with different erythrocyte cryptoantigens involved in polyagglutination
Lectins in the Era of Monoclonal Antibodies
Glycan is a generic term for any sugar molecule or assembly of molecules of different sugars. Lectins and monoclonal antibodies are widely used in glycan analysis because their specificities help us to discriminate a variety of glycan structures present on the cell surfaces. However, each of them has distinct advantages. Plant lectins are prepared indigenously and are less expensive. Apart from their use in blood typing, they are used for cell separation and identification and selection of mutated cells with altered glycosylation, inducing mitogenesis of cells for tumor cell-killing, and such other reasons.[66] On the other hand, production of monoclonal antibodies using hybridoma technology is an expensive procedure. In spite of this, sometimes these antibodies are specifically required to detect certain determinants that are otherwise not detected by lectins. For example, to detect SLea antigen (SialylLea), which is a prognostic tumor marker,[67] no lectins are available but it can be picked up by specific monoclonal antibodies. On the other hand, the general determinants such as α 2-6-linked sialic acid (Sambucus nigra lectin) and α 1-2-linked fucose (Aleuria aurantia lectin) have lectin specificity but cannot be picked up by any monoclonal antibodies. Kolberg et al.[68] produced three murine immunoglobin G1 (Ig G1) monoclonal antibodies against the glucose/mannose-specific two chain mitogen from the seeds of Lathyrus oloratus (sweet pea). Their antigenic specificities were tested against subunits of five two-chain lectins and six one-chain lectins. Two of these antibodies bound to different epitopes on the lectin molecules. The third monoclonal antibody reacted with another epitope from the heavy subunits. Ito et al.[20] selected 12 different blood group specific lectins and used these along with monoclonal anti-A and anti-B for detecting the corresponding antigens in selected human tissues. They observed that certain A group-specific lectins bind with A antigens in the mucous cells of salivary glands from blood group A or AB secretor as well as from nonsecretor individuals, whereas lectins from Dolichos biflorus, Griffonia simplicifolia, Sophora japonica, and Vicia villosa can react with A antigen from secretor individuals only suggesting either the heterogeneity of A antigen or difference in the specificity of A group lectin from various seeds. When B antigen from Brunner's gland was checked, B antigen-specific lectin did not show any reaction while monoclonal anti-B reacted with this B antigen.
Mitogenic Stimulation
Peter Nowell in 1960[69] observed that the lectin from red kidney bean (Phaseolus vulgaris) is mitogenic, i.e., it stimulates lymphocytes to undergo mitosis. This discovery had a revolutionary impact on immunology because till that time it was thought that lymphocyte could neither divide nor differentiate further. The second mitogenic lectin discovered was from Phytolacca americana (Pokeberry plant). Since then, several lectins with mitogenic activity were discovered [Table 3]. The mitogenic power of many lectins such as Crotalaria juncea, Hura crepticans, and Vicia sativa is very strong. On the other hand, the mitogenic power of lectins such as Glycine max and Datura stramonium is weak. Intermediate mitogenic activity has been exhibited by lectins from Erythrina indica or Phaseolus lunatus. Lectin isolated from pokeweed was one of the earliest lectins with mitogenic stimulation.[70] Later on, Kino et al.[71] isolated three lectins (PL-A, PL-B, and PL-C) from its root, which showed mitogenic activity.
Table 3.
Some lectins with mitogenic activity
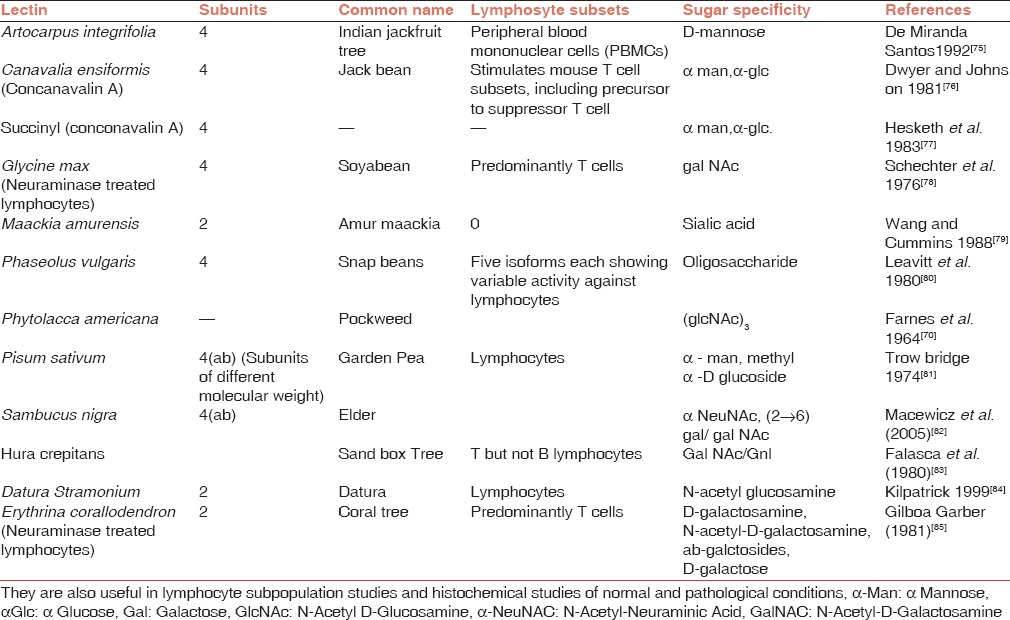
Lectins prepared from jack beans, phytohemagglutinin (PHA), and lentil activate T lymphocytes only[72] while lectin from slime mold Dictyostelium pupureum and chicken tissue stimulate B cells only.[73] Pokeweed mitogen stimulates both T cells and B cells.[72] Wheat germ agglutinin (WGA) also stimulates both T cells and B cells but under selected conditions[74] Lectin from the kidney bean revolutioned the field of mitogenesis.
Can We Improve Upon Lectins?
The yields of natural sources of lectins are very low (0.1-5 mg/L culture medium). Today, this yield has increased to 20 mg/L culture medium by using various advanced techniques.[86] To improve the yield of lectin to a sizable quantity, the lectin genes are expressed in various strains of E.coli.[87,88] However, E.coli also poses some challenges. These include protein misfolding, protein degradation, and low solubility. Small ubiquitin-like modifier (SUMO) fusion technology enhances protein expression and solubility.[89] This technology has been tried to improve lectin yield. Upadhyay et al.[90] prepared fusion of the Allium satuvum leaf agglutinin (ASAL) with SUMO peptide. Certain properties such as dimerization, hemagglutination, and insecticidal properties of the recombinant SUMO-ASAL fusion protein were comparable to native protein. However, its carbohydrate specificity was altered. Therefore, more studies are required to correct the carbohydrate specificity if possible.
Bauhinia purpurea lectin (BPA) contains a long metal-binding loop. A certain area of this loop determines the carbohydrate binding specificity of the lectin. Yamamoto et al.[91] introduced random mutations in the cDNA corresponding to the carbohydrate binding peptide of BPA lectin. These mutants were expressed on the tail of yfoo phages as fusion proteins. Among the recombinant phages, several phage clones were expressing specific sugar binding properties. This immediately raised the possibility of preparing artificial or engineered lectins (cyborg lectins) with desired carbohydrate-binding specificities.
Lectins and Bone Marrow/Stem Cell Transplantation
T cells are a type of lymphocytes, which are involved in cell-mediated immunity. There are different types of T cells having various functions related to immunologic procedures. Helper T cells, when activated, divide rapidly and secrete cytokines that assist in active immune response. They carry MHC class II molecules, which include three major and two minor proteins encoded by HLA genes. Cytotoxic T cells are involved in transplant rejection via graft-versus-host disease (GVHD). These cells are associated with MHC class I molecules, which include three major and three minor proteins encoded by HLA genes. They are present on all nucleated cells. Therefore, for successful transplant across HLA barriers T cells should be removed from the bone marrow before transplant.
The seeds of Glycine max (soyabean) contain 120KDa tetrameric glycoprotein with affinity for GalNAc. It is a product of a single gene locus; however, an insertion of a transposable element blocks its expression.[92] Liener in 1950 initiated pioneering work on this interesting lectin, which was well-reviewed by him in 1974.[93] This lectin is of vast importance in the field of clinical bone marrow transplant.
The soyabean agglutinin (SBA) specifically agglutinates murine B cells but not T cells. However, in humans SBA agglutinates helper T cells, along with B cells and monocytes. A second step of treating unagglutinated fraction with sheep RBCs was employed for enrichment of hematopoietic precursors and complete removal of T cells. The remaining unagglutinated nonrosetting fraction maintained complete hematopoietic function and did not induce GVHD. Using this technique, Reisner et al.[94] performed successful transplant in mice as well as in an infant with acute leukemia[95] and in three patients with severe combined immunodeficiency (SCID).[96] T cell depletion of bone marrow allows transplantation across HLA barriers. Therefore, the abovementioned technique is a cheap and convenient technique to prepare T cell-depleted bone marrow. Using this technique, Slocombe et al.[97] could avoid GVHD in 11 patients with leukemia.
However, Schiff et al.[98] failed to show selective removal of T lymphocytes from the bone marrow using SBA agglutinin while Ben-Yosef et al.[99] could not see any additional beneficial effect in breast cancer patients undergoing SBA purged bone marrow transplant.
The use of sheep RBCs has a risk of transmitting viruses. So, Meijer et al.[100] replaced “the sheep RBC step” from the above technique by using monoclonal antibodies CD2/CD3. But unfortunately, they found increased incidence of Epstein-Barr virus (EBV)-associated lymphoroliferative disorders. In series of 48 children with SCID, it was observed that 100% children (11 cases) survived when they received histocompatible-related donor bone marrow while in the second group (37 cases), 46% children survived when they received T cell-depleted haploidentical bone marrow during transplantation. After comparing various clinical parameters, it has been concluded that early diagnosis, prevention of treatment of opportunistic infections, and enhancement of immune recovery can improve the survival rate of SCID patients treated with T-cell-depleted (TCD) bone marrow transplant.[101]
In a longitudinal study of bone marrow transplant in acute myeloid leukemia cases,[102] T cell Depleted (TCD) bone marrow cells were prepared using soyabean lectin agglutination technique followed by sheep RBC rosette depletion for bone marrow and were compared with unmodified grafts in patients who were conditioned using various drugs; it was observed that GVHD was significantly reduced in TCD than in unmodified graft.
Even though a promising procedure for cure of cancers and other diseases, human stem cell transplant remains a dangerous procedure with many complications such as development of new cancers, GVHD, and autoimmune diseases.
Today the separation of subcell population depends upon use of specific antibodies against various cell surface epitopes. However, lectins can serve as a very good alternate method for cell differentiation using cell lineage-specific glycans. Apart from separation, this can help in understanding the role of glycans in maintenance and proliferation. The main advantage of this method is that lectins can be easily removed from cells using appropriate sugar without damaging the cells. Similarly, cost is an important factor as lectins are not expensive.
Mandai et al.[103] used wheat germ agglutinin (WGA) and Erythrina cristagalli agglutinin (ECA) to enrich mouse embryonic stem cells derived from retinal progenitor cells for transplantation therapy. Toyoda et al.[104] in their lectin microarray analysis found that Maackia amurensis, Euonymus europaeus and Phaseolus vulgaris leukoagglutinin lectins can be used to define undifferentiated and differentiated human embryonic stem cells. Dodla et al.[105] used Vicia villosa lectin for isolation of human neural progenitor cells. Wang et al.[106] after their interesting experiments concluded that Ulex europaeus I is a potential tool for isolating viable human pluripotent stem cells (hPSCs) with substantial pleuripotency from heterogeneous cells population. Chen et al.[107] identified a novel cell surface receptor on adult mouse cardiac progenitor cells using Dolichos biflorus lectin. This may help to separate these cells using a simple and cheap test. Mikkola et al.[108] found that Erythrina crystagalli lectin, along with pinacidil, supported the plating efficiency of human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). During the proliferation of these cells, a normal karyotype was seen.
Maintaining ex vivo culture of hematopoietic stem cells is a great challenge as it requires cytokines to maintain primitive cells without inducing proliferation, differentiation, and subsequent loss of repopulating capacity. Colucci et al.[109] extracted a lectin from Dolichos lablab [called Flt3 receptor interacting lectin (FRIL)], which was able to preserve cells for 1 month. Later on, Yao et al.[110] preserved neural progenitor cells for 1 month using the same lectin FRIL.
In human pleuripotent stem cells, each cell lineage has a specific set of surface markers. Well-known pleuripotency markers such as SSEA3 and SSEA4 are glycans. Tateno et al.[111] performed glycome analysis of a large set of human-induced pleuripotent stem cells and human embryonic stem cells using a high density lectin microarray. They found that a recombinant N-terminal somin of a lectin extracted from Burkholderia cenocepacia bound exclusively to all of the undifferentiated hPSCs tested but not to differentiated cells.
Conclusion
The first phytoagglutinin extracted from castor bean was shown to possess hemagglutinating activity. Since then, several plant lectins revealed this activity but blood group specific activity in lectins was detected in the 1950s. Among the several lectins showing blood group specific activity, lectin from Dolichos biflorus (anti-A1) and Ulex europeaus (anti-H) are routinely used in blood banking. A kit designed to detect various cryptoantigens using several specific plant lectins are useful to resolve the problem of polyagglutination. Mitogenic stimutation by lectins revolutionized the area of cytogenetics.
Stem cells are very important due to their remarkable potential to develop into many different cell types in our body. However, the separation processes for the isolation and purification of stem cells and stem-derived cells are a crucial issue. The work initiated using soyabean lectin to prepare T-depleted stem cells helped to use human leukocyte antigen (HLA) haploidentical stem cells for transplantation while today lectin-based microarrays identify several surface markers on stem cells helping to understand the developmental biology.
Thus, lectins have tremendous potential in the field of transfusion medicine and stem cell research. In this era of molecular biology and biotechnology, it is possible to improve upon available lectins for various biological activities in immunohematology and stem cell biology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ingale AG, Hivrale AU. Plant as a plenteous reserve of lectin. Plant Signal Behav. 2013;8:e26595. doi: 10.4161/psb.26595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furukawa K, Ying R, Nakajima T, Matsuki T. Hemagglutinins in fungus extracts and their blood group specificity. Exp Clin Immunogenet. 1995;12:223–31. [PubMed] [Google Scholar]
- 3.Boyd WC, Shapleigh E. Specific precipitating activity of plant agglutinins (Lectins) Science. 1954;119:419. doi: 10.1126/science.119.3091.419. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N. What should be called a lectin? Nature. 1980;285:66. [Google Scholar]
- 5.Sharon N, Lis H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 6.Renkonen KO. Studies on hemagglutinins present in seeds of some representatives of the family of Leguminoseae. Ann Med Exp Fenn. 1948;26:66–72. [Google Scholar]
- 7.Boyd WC, Reguera RM. Hemagglutinating substances for human cells in various plants. J Immunol. 1949;62:333–9. [PubMed] [Google Scholar]
- 8.Bird GW. Hemagglutinins in Indian plants. Army Med Crops J. 1955;2:17–25. [Google Scholar]
- 9.Boyd WC. Hemagglutinating substances for human cells in various Egyptian plants. J Immunol. 1950;65:281–4. [PubMed] [Google Scholar]
- 10.Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: Chemistry, polymorphism, and their developmental stages. Sem Hematol. 1981;18:39–62. [PubMed] [Google Scholar]
- 11.Dahr W. Immunochemistry of Sialoglycoproteins in human red blood cell membranes. In: Vengelen-tyler V, Judd WJ, editors. Recent Advances in Blood Group Biochemistry. Arlington: American Association Blood Banks; 1986. pp. 23–65. [Google Scholar]
- 12.Koulumies R. The subgroups A1, A2 and A1B, A2B and their relation to some hemagglutinins present in seeds of Vicia cracca. Ann Med Exp Fenn. 1949;27:20–4. [Google Scholar]
- 13.Bird GW. Relationship of the blood sub-groups A1, A2 and A1B, A2B to hemagglutinins present in the seeds of Dolicos biflorus. Nature. 1952;170:674. doi: 10.1038/170674a0. [DOI] [PubMed] [Google Scholar]
- 14.Feria M, Pérez-Santiago A, Cuevas D, Martínez M, Córdoba F. Purification and partial characterization of a new anti-A1 lectin of Phaseolus coccineus collected in Oaxaca, Mexico. Prep Biochem Bitechnol. 1996;26:31–46. doi: 10.1080/10826069608000048. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima T, Kogure T, Furukawa K. Specificity of hemagglutinin of Falcata japonica which react with blood group active N-acetyl-D-galactosamine residues. Exp Clin. 1986;3:187–94. [PubMed] [Google Scholar]
- 16.Makela O, Makela P. Some new blood group specific phytagglutinins; a preliminary report. Ann Med Exp Biol Fenn. 1956;34:402–4. [PubMed] [Google Scholar]
- 17.Spohr U, Hindsgaul O, Lemieux RU. Molecular recognition. II. The binding of the Lewis b and Y human blood group determinants by the lectin IV of Griffoniasimplicifolia. Can J Chem. 1985;63:2644–52. [Google Scholar]
- 18.Lescar J, Loris R, Mitchell E, Gautier C, Chazalet V, Cox V, et al. Isolectins I-A and I-B of Griffonia (Bandeiraea) simplicifolia. Crystal structure of metal-free GS I-B(4) and molecular basis for metal binding and monosaccharide specificity. J Biol Chem. 2002;277:6608–14. doi: 10.1074/jbc.M109867200. [DOI] [PubMed] [Google Scholar]
- 19.Murphy LA, Goldstein IJ. Five alpha-D-galactopyranosyl-binding isolectins from Bandeiraea simplicifolia seeds. J Biol Chem. 1977;252:4739–42. [PubMed] [Google Scholar]
- 20.Ito N, Imai S, Haga S, Nagaike C, Morimura Y, Hatake K. Localization of binding sites of Ulex europaeus I, Helix pomatia and Griffonia simplicifolia I-B4 lectins and analysis of their backbone structures by several glycosidases and poly-N-acetyllactosamine-specific lectins in human breast carcinomas. Histochem Cell Biol. 1996;106:331–9. [PubMed] [Google Scholar]
- 21.Wu AM, Wu JH, Chen YY, Song SC, Kabat EA. Further characterization of the combining sites of Bandeiraea (Griffonia) simplicifolia lectin-I, isolectin A(4) Glycobiology. 1999;9:1161–70. doi: 10.1093/glycob/9.11.1161. [DOI] [PubMed] [Google Scholar]
- 22.Ottensooser F, Sato R, Sato M. A new anti-B lectin. Transfusion. 1968;8:44–6. doi: 10.1111/j.1537-2995.1968.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 23.Makela O, Makela P, Krupe M. Zur spezifitat der anti-B Phythamagglutinine. In: Race RR, Sanger R, editors. Blood Groups in Man. Oxford: Blackwell Scientific Publication; 1968. pp. 63–86. Z Immun Forsch 1959;117:220-9. [Google Scholar]
- 24.Rogers DJ, Blunden G, Evans PR. Ptilota plumosa, a new source of a blood-group B specific lectin. Med Lab Sci. 1977;34:193–200. [PubMed] [Google Scholar]
- 25.Voak D, Todd GM, Pardoe GI. A study of serological behavior and nature of the anti-B-P-Pk activity of Salmonidae roe protectins. Vox Sang. 1974;26:176–88. doi: 10.1111/j.1423-0410.1974.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 26.Krupe M, Christa B. In: Uber ein pflanzliches Hamagglutinin gegen menschliche B-Blutzellen. Race RR, Sanger R, editors. Oxford: Blood Groups in Man. Blackwell Scientific Publication; 1968. pp. 63–86. Naturwissenschaften 1952;39:284-5. [Google Scholar]
- 27.Bird GW. The hemagglutinins of crotalaria striata. Further evidence of similarity of the A and B agglutinogens. Vox Sang. 1956;1:167–71. [Google Scholar]
- 28.Bird GW. Haemagglutinins in Calpurnia aurea. Nature. 1957;180:657. doi: 10.1038/180657a0. [DOI] [PubMed] [Google Scholar]
- 29.Veau B, Guillot J, Damez M, Dusser M, Konska G, Botton B. Purification and characterization of an anti-(A+B) specific lectin from the mushroom Hygrophorus hypothejus. Biochim Biophys Acta. 1999;1428:39–44. doi: 10.1016/s0304-4165(99)00055-0. [DOI] [PubMed] [Google Scholar]
- 30.Bird GW, Wingham J. Agglutinius from Jerusalem sage (Phlomis fruticosa) Experentia. 1970;26:1257–8. doi: 10.1007/BF01898001. [DOI] [PubMed] [Google Scholar]
- 31.Cazal P, Lalaurie M. Specific phyto-agglutinins of the ABO blood groups. Acta Haematol. 1952;8:73–80. doi: 10.1159/000204150. [DOI] [PubMed] [Google Scholar]
- 32.Flory LL. Differences in the H antigen on human buccal cells from secretor and non-secretor individuals. Vox Sang. 1966;11:137–56. doi: 10.1111/j.1423-0410.1966.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto I, Osawa T. Purification and characterization of an anti-H (O) phytohemagglutinin of Ulex europaeus. Biochim Biophys Acta. 1969;194:180–9. doi: 10.1016/0005-2795(69)90193-7. [DOI] [PubMed] [Google Scholar]
- 34.Loris R, De Greve H, Dao-Thi MH, Messens J, Imberty A, Wyns L. Structural basis of carbohydrate recognition by lectin II from Ulex europaeus, a protein with a promiscuous carbohydrate-binding site. J Mol Biol. 2000;301:987–1002. doi: 10.1006/jmbi.2000.4016. [DOI] [PubMed] [Google Scholar]
- 35.Joshi SR, Vasantha K, Robb JS. An unusual anti-H lectin inhibited by milk from individuals with the Bombay phenotype. Immunohematology. 2005;21:1–4. [PubMed] [Google Scholar]
- 36.Sudakevitz D, Gilboa-Garber N, Levene C, Sela R, Bhattacharyya L. Erythrina lectins detect the H/HI blood groups. Zentralbl Bakferiol. 1991;275:343–50. doi: 10.1016/s0934-8840(11)80298-7. [DOI] [PubMed] [Google Scholar]
- 37.Ottensooser F, Silberschmidt K. Hemagglutinin anti-N in plant seeds. Nature. 1953;173:914. [Google Scholar]
- 38.Rolih SD, Issitt PD. Effects of trypsin on the Vicia graminea receptors of glycophorin A and B. Transfusion. 1978;18:637. [Google Scholar]
- 39.Duk M, Lisowska E. Vicia graminea anti-N lectin: Partial characterization of the purified lectin and its binding to erythrocytes. Eur J Biochem. 1981;118:131–6. doi: 10.1111/j.1432-1033.1981.tb05495.x. [DOI] [PubMed] [Google Scholar]
- 40.Boyd WC, Everhart DL, Mcmaster MH. The anti-N lectin of Bauhinia purpurea. J Immunol. 1958;81:414–8. [PubMed] [Google Scholar]
- 41.Fletcher A, Harbour C, Matthews M, de Zwart R, Ford D. Blood grouping with monoclonal anti-N antibodies. Aust J Exp Biol Med Sci. 1986;64:215–22. doi: 10.1038/icb.1986.23. [DOI] [PubMed] [Google Scholar]
- 42.Moon GJ, Wiener AS. A new source of anti-N lectin: Leaves of the Korean Vicia unijuga. Vox Sang. 1974;26:167–70. doi: 10.1111/j.1423-0410.1974.tb02683.x. [DOI] [PubMed] [Google Scholar]
- 43.Allen NK, Brilliantine L. A survey of hemagglutinins in various seeds. J Immunol. 1969;102:1295–9. [PubMed] [Google Scholar]
- 44.Petryniak J, Pereira ME, Kabat EA. The lectin of Euonymus europeus: Purification, characterization, and an immunochemical study of its combining site. Arch Biochem Biophys. 1977;178:118–34. doi: 10.1016/0003-9861(77)90176-x. [DOI] [PubMed] [Google Scholar]
- 45.Krajhanzl A, Horejsí V, Kocourek J. Studies on lectins. XLII. Isolation, partial characterization and comparison of lectins from the roe of five fish species. Biochim Biophys Acta. 1978;532:215–24. doi: 10.1016/0005-2795(78)90575-5. [DOI] [PubMed] [Google Scholar]
- 46.Vasta GR, Warr GW, Marchalonis JJ. Tunicte lectins: Distribution and specificity. Comp Biochem Physiol. 1982;73:887–900. [Google Scholar]
- 47.Poretz RD, Riss H, Timberlake JW, Chien SM. Purification and properties of the hemagglutinin from Sophora japonica seeds. Biochemistry. 1974;13:250–6. doi: 10.1021/bi00699a004. [DOI] [PubMed] [Google Scholar]
- 48.Bird GW, Wingham J. Agglutinins for antigens of two different blood group systems in the seeds of Moluccella laevis. Vox Sang. 1970;18:235–9. doi: 10.1111/j.1423-0410.1970.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 49.Alperin DM, Latter H, Lis H, Sharon N. Isolation, by affinity chromatography and gel filtration in 8M-urea, of an active subunit from the anti-(blood-group A+N)-specific lectin of Moluccella laevis. Biochem J. 1992;285:1–4. doi: 10.1042/bj2850001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid ME. MNS blood group system: A review. Immunohematology. 2009;25:95–101. [PubMed] [Google Scholar]
- 51.Vengelen-Tyler V, Mogck N. A new test useful in identifying red cells with a (delta-alpha) hybrid sialoglycoprotein. Transfusion. 1986;26:231–3. doi: 10.1046/j.1537-2995.1986.26386209375.x. [DOI] [PubMed] [Google Scholar]
- 52.Darnborough J, Dunsford I, Wallace JA. The Ena antigen and antibody a genetical modification of human red cells affecting their blood grouping reactions. Vox Sang. 1969;17:241–55. doi: 10.1111/j.1423-0410.1969.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 53.Tanner MJ, Anstee DJ. The membrane change in En(a-) human erythrocytes: Absence of the major erythrocyte sialoglycoprotein. Biochem J. 1976;153:271–7. doi: 10.1042/bj1530271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bird GW, Wingham J. The action of seed and other reagents on En(a-) erythrocytes. Vox Sang. 1973;24:48–57. doi: 10.1111/j.1423-0410.1973.tb03856.x. [DOI] [PubMed] [Google Scholar]
- 55.Shinozuka T, Miyata Y, Takei S, Yoshida R, Ogamo A, Nakagawa Y, et al. Changes in En(a-) human red blood cell membranes during in vivo ageing. Mech Ageing Dev. 1996;86:27–37. doi: 10.1016/0047-6374(95)01677-5. [DOI] [PubMed] [Google Scholar]
- 56.Furuhjelm U, Myllylä G, Nevanlinna HR, Nordling S, Pirkola A, Gavin J, et al. The red cell phenotype En(a-) and anti-Ena: Serological and physicochemical aspects. Vox Sang. 1969;17:256–78. doi: 10.1111/j.1423-0410.1969.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 57.Bird GW. Anti-A agglutinins from the seeds of Hyptis suaveolens Poit. Br J Haematol. 1960;6:151–9. doi: 10.1111/j.1365-2141.1960.tb06226.x. [DOI] [PubMed] [Google Scholar]
- 58.Elo J, Estola E, Malmström N. On phytagglutinins present in mushrooms. Ann Med Exp Fenn. 1951;29:297–308. [Google Scholar]
- 59.Ottensooser F, Sato M. Cold lectins. Vox Sang. 1936;8:733–40. doi: 10.1111/j.1423-0410.1963.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 60.Herzog P. Specific phyto-agglutinins from Ononis spinosa roots. Z Immun exp ther. 1959;117:53–9. [PubMed] [Google Scholar]
- 61.Scott ML, Bird GW. Some contributions of the plant kingdom to transfusion medicine - Lectins and plant enzymes. Trans Med Rev. 1992;6:103–15. doi: 10.1016/s0887-7963(92)70160-6. [DOI] [PubMed] [Google Scholar]
- 62.Ness PM, Garratty G, Morel PA, Perkins HA. Tn polyagglutination preceding acute leukemia. Blood. 1979;54:30–4. [PubMed] [Google Scholar]
- 63.Cazal P, Monis M, Caubel J, Brives J. Polyagglutinabilitic hereditaire dominante: Antigene prive (Cad) correspondant a un anti-corps public et a une lectin de Dolichos biflorus. Rev Fr Transfus Immuno Hematol. 1968;11:209–21. doi: 10.1016/s0035-2977(68)80050-1. [DOI] [PubMed] [Google Scholar]
- 64.Sanger R, Gavin J, Tippett P, Teesdale P, Eldon K. Plant agglutinin for another human blood group. Lancet. 1971;1:1130. doi: 10.1016/s0140-6736(71)91865-4. [DOI] [PubMed] [Google Scholar]
- 65.Bird GW, Wingham J. "New" lectins for the identification of erythrocyte cryptantigens and the classification of erythrocyte polyagglutinability: Medicago disciformis and Medicago turbinata. J Clin Pathol. 1983;36:195–6. doi: 10.1136/jcp.36.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cummings RD, Etzler ME. Antibodies and lectins in glycan analysis. In: Varki A, Cummings RD, Esko JD, editors. Essentials of Glycobiology. 2nd ed. New York: Cold Springer Harbor Lab Press; 2009. [Google Scholar]
- 67.Nakamori S, Furukawa H, Hiratsuka M, Iwanaga T, Imaoka S, Ishikawa O, et al. Expression of certain carbohydrate antigen Sialyl Le(a): A new functional prognostic factor in gastric cancer. J Clin Oncol. 1997;15:816–25. doi: 10.1200/JCO.1997.15.2.816. [DOI] [PubMed] [Google Scholar]
- 68.Kolberg J, Michaelsen TE, Wedege E, Heier HE. Reactivity with legume lectins of three monoclonal antibodies made against the Lathyrus odoratus lectin. Biol Chem Hoppe Seyler. 1988;369:365–70. doi: 10.1515/bchm3.1988.369.1.365. [DOI] [PubMed] [Google Scholar]
- 69.Nowell PC. Phytohemaglutinin: An initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–6. [PubMed] [Google Scholar]
- 70.Farnes P, Barker BE, Brownhill LE, Fanger H. Mitogenic activity in Phytolacca americana (pokeweed) Lancet. 1964;2:1100–1. doi: 10.1016/s0140-6736(64)92616-9. [DOI] [PubMed] [Google Scholar]
- 71.Kino M, Yamaguchi K, Umekawa H, Funatsu G. Purification and characterization of three mitogenic lectins from the roots of pokeweed (Phytolacca americana) Biosci Biotechnol Biochem. 1965;59:683–8. doi: 10.1271/bbb.59.683. [DOI] [PubMed] [Google Scholar]
- 72.Janossy G, Greaves MF. Lymphocyte activation. II. Discriminating stimulation of lymphocyte subpopulation by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972;10:525–36. [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell PA, Hartman AL, Abel CA. Stimulation of B cells, but not T cells or thymocytes, by a sialic acid-specific lectin. Immunology. 1982;45:155–62. [PMC free article] [PubMed] [Google Scholar]
- 74.Miller K. The stimulation of human B and T lymphocytes by various lectins. Immunobiology. 1983;165:132–46. doi: 10.1016/S0171-2985(83)80055-2. [DOI] [PubMed] [Google Scholar]
- 75.de Miranda-Santos IK, Delgado M, Bonini PV, Bunn-Moreno MM, Campos-Neto A. A crude extract of Artocarpus integrifolia contains two lectins with distinct biological activities. Immunol Lett. 1992;31:65–71. doi: 10.1016/0165-2478(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 76.Dwyer JM, Johnson C. The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol. 1981;46:237–49. [PMC free article] [PubMed] [Google Scholar]
- 77.Hesketh TR, Bavetta S, Smith GA, Metcalfe JC. Duration of the calcium signal in the mitogenic stimulation of thymocytes. Biochem J. 1983;214:575–9. doi: 10.1042/bj2140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schechter B, Lis H, Lotan R, Novogrodsky A, Sharon N. The requirement for tetravalency of soyabean agglutinin for induction of mitogenic stimulation of lymphocytes. Eur J Immunol. 1976;6:145–9. doi: 10.1002/eji.1830060302. [DOI] [PubMed] [Google Scholar]
- 79.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex type Asn-linked oligosaccharides containing terminal Sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–85. [PubMed] [Google Scholar]
- 80.Leavitt RD, Felsted RL, Bachur NR. Biological and biochemical properties of Phaseolus vulgaris isolectins. J Biol Chem. 1977;252:2961–6. [PubMed] [Google Scholar]
- 81.Trowbridge IS. Isolation and chemical characterization of a mitogenic lectin from Pisum sativum. J Biol Chem. 1974;249:6004–12. [PubMed] [Google Scholar]
- 82.Macewicz LL, Suchorada OM, Lukash LL. Influence of Sambucus nigra bark lectin on cell DNA under different in vitro conditions. Cell Biol Int. 2005;29:29–32. doi: 10.1016/j.cellbi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Falasca A, Franceschi C, Rossi CA, Stirpe F. Mitogenic and hemagglutinating properties of a lectinpurified from Hura crepitans seeds. Biochim Biophys Acta. 1980;632:95–105. doi: 10.1016/0304-4165(80)90252-4. [DOI] [PubMed] [Google Scholar]
- 84.Kilpatrick DC. Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol. 1999;11:55–65. doi: 10.1007/BF02789176. [DOI] [PubMed] [Google Scholar]
- 85.Gilboa-Garber N, Mizrahi L. A new mitogenic D-Galactosephilic lectin isolated from seeds of the coral-tree Erythrina corallodendron. Comparison with Glycine max (Soyabean) and Psendomonas aerunginosa lectins. Can J Biochem. 1981;59:315–20. doi: 10.1139/o81-044. [DOI] [PubMed] [Google Scholar]
- 86.Lam SK, Ng TB. Lectins: Production and practical applications. Appl Microbiol Biotechnol. 2011;89:45–55. doi: 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adar R, Streicher H, Rozenblatt S, Sharon N. Synthesis of soyabean agglutinin in bacterial and mammalian cells. Eur J Biochem. 1997;249:684–9. doi: 10.1111/j.1432-1033.1997.t01-3-00684.x. [DOI] [PubMed] [Google Scholar]
- 88.Stubbs ME, Carver JP, Dunn RJ. Production of pea lectin in Escherichia coli. J Biol Chem. 1986;261:6141–4. [PubMed] [Google Scholar]
- 89.Peroutka RJ, Iii, Orcutt SJ, Strickler JE, Butt TR. SUMO fussion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol Biol. 2011;705:15–30. doi: 10.1007/978-1-61737-967-3_2. [DOI] [PubMed] [Google Scholar]
- 90.Upadhyay SK, Saurabh S, Rai P, Singh R, Chandrashekar K, Verma PC, et al. SUMO fusion facilitates expression and purification of garlic leaf lectin but modifies some of its properties. J Biotechnol. 2010;146:1–8. doi: 10.1016/j.jbiotec.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto K, Maruyama IN, Osawa T. Cyborg lectins: Novel leguminous lectins with unique specificities. J Biochem. 2000;127:137–42. doi: 10.1093/oxfordjournals.jbchem.a022575. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg RB, Hoschek G, Vodkin LO. An insertion sequence blocks the expression of a soyabean lectin gene. Cell. 1983;33:465–75. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- 93.Liener IE. Phytohemagglutinins: Their nutritional significance. J Agric Food Chem. 1974;22:17–22. doi: 10.1021/jf60191a031. [DOI] [PubMed] [Google Scholar]
- 94.Reisner Y, Itzicovitch L, Meshorer A, Sharon N. Hematopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Natl Acad Sci U S A. 1978;75:2933–6. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Dupont B, Good RA, et al. Transplantation for acute leukemia with HLA-A and B nonidentical parental marrow cells fractionated with soyabean agglutinin and sheep red blood cells. Lancet. 1981;2:327–31. doi: 10.1016/s0140-6736(81)90647-4. [DOI] [PubMed] [Google Scholar]
- 96.Reisner Y. Differential agglutination by soyabean agglutinin of human leukemia and neuroblastoma cell lines: Potential application to autologous bone marrow transplantation. Proc Natl Acad Sci U S A. 1983;80:6657–61. doi: 10.1073/pnas.80.21.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slocombe GW, Newland AC, Yeatman NW, Macey M, Jones HM, Knott L. Allogenic bone marrow transplantation for adult leukemia with soybean lectin fractionated marrow. Bone Marrow Transplant. 1986;1:31–9. [PubMed] [Google Scholar]
- 98.Schiff SE, Kurtzberg J, Buckley RH. Studies of human bone marrow treated with soyabean lectin and sheep erythrocytes: Stepwise analysis of cell morphology, phenotype and function. Clin Exp Immunol. 1987;68:685–93. [PMC free article] [PubMed] [Google Scholar]
- 99.Ben-Yosef R, Or R, Naparstek E, Kapelushnik J, Samuels S, Slavin S, et al. Should soybean agglutinin purging be performed in breast cancer patients undergoing autologous stem cell transplantation. A retrospective analysis of 48 patients? Am J Clin Oncol. 1997;20:419–23. doi: 10.1097/00000421-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 100.Meijer E, Slaper-Cortenbach IC, Thijsen SF, Dekker AW, Verdonck LF. Increased incidence of EBV - Associated lymphoproliferative disorders after allogenic stem cell transplantation from matched unrelated donors due to a change of T cell depletion technique. Bone Marrow Transplant. 2002;29:335–9. doi: 10.1038/sj.bmt.1703362. [DOI] [PubMed] [Google Scholar]
- 101.Smogorzewska EM, Brooks J, Annett G, Kapoor N, Crooks GM, Kohn DB, et al. T cell depleted haploidentical bone Marrow transplantation for the treatment of children with severe combined immunodeficiency. Arch Immunol Ther Exp (Warsz) 2000;48:111–8. [PubMed] [Google Scholar]
- 102.Bayraktar UD, de Lima M, Saliba RM, Maloy M, Castro-Malaspina HR, Chen J, et al. Ex-vivo T cell depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mandai M, Ikeda H, Jin ZB, Iseki K, Ishigami C, Takahashi M. Use of lectins to enrich mouse ES-derived retinal progenitor cells for the purpose of transplantation therapy. Cell Transplant. 2010;19:9–19. doi: 10.3727/096368909X476599. [DOI] [PubMed] [Google Scholar]
- 104.Toyoda M, Yamazaki-Inoue M, Itakura Y, Kuno A, Ogawa T, Yamada M, et al. Lectin microarray analysis of pleuripotent and multipotent stem cells. Genes Cells. 2011;16:1–11. doi: 10.1111/j.1365-2443.2010.01459.x. [DOI] [PubMed] [Google Scholar]
- 105.Dodla MC, Young A, Venable A, Hasneen K, Rao RR, Machacek DW, et al. Differing lectin binding profiles among human embryonic stem cells and derivatives aid in the isolation of neural progenitor cells. PLoS One. 2011;6:e23266. doi: 10.1371/journal.pone.0023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang YC, Nakagawa M, Garitaonandia I, Slavin I, Altun G, Lacharite RM, et al. Specific lectin biomarkers for isolation of human pleuripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011;21:1551–63. doi: 10.1038/cr.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Z, Wang M, Xiang Q, Sun Z, Zhang R. The lectin of Dolichos biflorus agglutinin recognizes glycan epitopes on the surface of a subset ofcardiac progenitor cells. Cell Biol Int. 2013;37:1238–45. doi: 10.1002/cbin.10162. [DOI] [PubMed] [Google Scholar]
- 108.Mikkola M, Toivonen S, Tamminen K, Alfthan K, Tuuri T, Satomaa T, et al. Lectin from Erythrina cristagalli supports undifferentiated growth and differentiation of human pluripotent stem cells. Stem Cells Dev. 2013;22:707–16. doi: 10.1089/scd.2012.0365. [DOI] [PubMed] [Google Scholar]
- 109.Colucci G, Moore JG, Feldman M, Chrispeels MJ. cDNA cloning of FRIL, a lectin from Dolichos lablab, that preserves hematopoietic progenitors in suspension culture. Proc Natl Acad Sci U S A. 1999;96:646–50. doi: 10.1073/pnas.96.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yao H, Xie X, Li Y, Wang D, Han S, Shi S, et al. Legume lectin FRIL preserves neural progenitor cells in suspension culture in vitro. Clin Dev Immunol. 2008;2008:531317. doi: 10.1155/2008/531317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tateno H, Matsushima A, Hiemori K, Onuma Y, Ito Y, Hasehira K, et al. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell specific probe rBc2LCN. Stem Cells Transl Med. 2013;2:265–73. doi: 10.5966/sctm.2012-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]


