Abstract
Settings:
This study was done in a tertiary care hospital having bed strength of more than 700 beds at SDM Medical College of Medical Sciences and Hospital, Dharwad, located in Northern Karnataka.
Aim:
The study was done to ascertain prevalence of Gilbert's syndrome in healthy blood donors and review the literature about feasibility of utilizing blood components from Gilbert's syndrome donors.
Materials and Methods:
The study was done for 18 months and 7030 whole blood units were collected and all the units were subjected to mandatory transfusion-transmitted screening and all the plasma bags which were icteric on visual inspection were subjected to hematological and biochemical investigations to rule out other causes of hyperbilirubinemia.
Results:
Seven thousand and thirty units were collected and 445 (6.3%) were discarded due to various reasons. Of them, 50 units (0.71%) had Gilbert's syndrome. All had unconjugated hyperbilirubinemia and other hematological and liver function tests were within normal range. Statistical analysis was done to find mean, median, and standard deviation from mean and standard error of mean with lower and upper confidence limits.
Conclusion:
Majority of blood donors whose plasma is icteric are suffering from Gilbert's syndrome (GS). This disease does not cause any harm to donor or patient but raises a lot of concern as many severe disorders also manifest in similar way. The available literature shows that all blood components can be used from donors suffering from GS. There should be introspection. Proper guidelines are to be framed about the use and discarding of blood components in donors with GS.
Keywords: Blood safety, fresh frozen plasma, Gilbert's syndrome, hyperbilirubinemia, jaundice
Introduction
Gilbert's syndrome (GS) is a benign, inherited condition manifesting as unconjugated hyperbilirubinemia without any other comorbidity. The genetic basis of GS and associated gene has been identified in 1995.[1] It requires neither treatment nor long-term medical attention. Its clinical importance lies in the fact that the mild hyperbilirubinemia may be mistaken for a sign of occult, chronic, or progressive liver disease or hemolytic process. It should be diagnosed by exclusion.[2] The prevalence is about 8%. Males predominate over females.[3]
Gilbert first described this syndrome, at the beginning of 20th century (Gilbert and Lereboullet, 1901), but he included a number of diseases pertaining to liver, spleen, and hematemesis.[4,5]
Most cases of GS become evident after exercise, stress, drug administration, prolonged fasting, or intercurrent diseases, and drugs such as paracetamol, nilotinib, and pazopanib can induce hyperbilirubinemia in GS patients.[6]
Bilirubin is the end product of heme breakdown. Majority (80%) originates from the degradation of erythrocyte hemoglobin in the reticuloendothelial system; the rest 20% is derived from inefficient erythropoiesis in bone marrow and degradation of other heme proteins.[7]
The normal serum bilirubin levels in adults ranges from 0.3 to 1.2 mg/dl. Jaundice is manifested when equilibrium between bilirubin production and excretion is disturbed. It is clinically evident when the serum bilirubin levels rise above 2.5 mg/dl.
The free bilirubin is toxic, and hence it should be detoxified. Bilirubin is detoxified in the liver by enzyme bilirubin uridine diphosphate- glucuronyltransferase 10 (UGT1A1). This enzyme conjugates one or two molecules of glucuronic acid with free bilirubin in converting it from water insoluble (toxic) to soluble form (non toxic). This helps in excretion. Multiple genetic mutations of this enzyme UGT1A1, gene cause hereditary unconjugated hyperbilirubinemias such as —Crigler-Najjar syndrome types I, II, and Gilbert's syndrome.[8]
Materials and Methods
The study was carried out in voluntary blood donors who donated whole blood to blood bank at tertiary care hospital in Northern Karnataka from 1st January 2012 to 30th June 2013. The whole blood was collected only from those voluntary donors who were found fit as per standard questionnaire, including medication history, physical examination, and investigations as per Directorate General of Health Services and National AIDS Control Organisation guidelines. Blood (450 mL) collected was separated into various components such as packed red blood cells, fresh frozen plasma, platelet concentrate, cryoprecipitate, as per standard protocol in refrigerated centrifuge (HAREUS CRYOFUGE 5500i).
Plasma bags before storing them for freezing were observed visually for any abnormalities such as suspended particles and yellow or greenish-yellow discoloration (icteric) plasma.
Inclusion criteria: All healthy voluntary whole blood donor units whose plasma after component separation showed icteric discoloration.
Exclusion criteria: All whole blood donor units whose plasma after component separation showed icteric plasma and on further investigations showed transfusion-transmitted infections (TTI) reactivity or abnormal hematological or biochemical values.
All donor units which were included in the study were subjected to investigations to rule out any liver diseases or hemolytic anemia. The following samples were taken for investigations-2 mL of whole blood samples from segments of the primary bag were subjected to hematological complete blood count in (Sysmex KX-21-3 Part) or (XT-1800 i Sysmex-5 Part) with peripheral smear and reticulocyte count. Direct and indirect Coombs tests were done.
Three milliliters of plasma samples were collected from plasma bag segments in bulbs, and transported (in dark closed box) to biochemistry laboratory for liver function tests, namely estimation of total and direct serum bilirubin, aspartate and alanine aminotransferases, alkaline phosphatase, serum proteins, albumin, and lactic dehydrogenase. Biochemical tests were done on (Hitachi-902) automated analyzer and (Bio System-350) semi-automated analyzer.
All bags were mandatorily screened for TTI, namely HIV, hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) by enzyme-linked immunosorbent assay (ELISA), and rapid card kits.
The ELISA kits used were from following manufactures—(bioMerieux) Vironostika—HIV Ag/Ab, hepanostika “ultra”—HBsAg, and hepanostika “ultra”—HCV; (SD Bio Standard diagnostic) SD HIV-1/2 ELISA 3.0, HbsAg-1/2 ELISA 3.0, HCV-1/2 ELISA 3.0, and syphilis by (Tulip Diagnostic) VDRL-Rapid Plasma Reagin (RPR) CARBOGEN; (Span Diagnostics) VDRL—RPR kit.
Rapid test kits used for malaria were—(SD Bio Standard diagnostic) Bioline M P—Ag-PF/Pan for HIV and HBsAg and HCV were screened by (Diagnostic Enterprises Mitra & Co)—Tridot and Hepacard. Syphilis by (SD Bio Standard diagnostic) VDRL Syphilis-3.0 anti TP card.
All units reactive to transfusion-transmitted diseases, abnormality with liver enzymes, and positive for hemolytic screening, namely complete blood count and peripheral smear showing features of hemolytic anemia, positive direct coombs test, increased reticulocyte count, and increased lactate dehydrogenase (LDH) were excluded the from study.
Results
A total of 7030 donors donated blood in the period. From them, 445 (6.3%) were discarded. Of these, 377 (5.2%) were TTI reactive in these, eight donors had icteric plasma. Total, 61 units showed icteric plasma. Out of these, 50 had Gilbert syndrome, eight showed TTI reactivity, and three had abnormal liver enzyme levels. In the rest 15 units, reasons for discarding were quantity not sufficient-10, leakage-2, and atypical antibody present-3. All the donors having Gilbert's syndrome had serum bilirubin greater than 1.2 mg/dl and increased unconjugated fraction. All these cases were investigated for various biochemical and hematological parameters. All the results [Table 1], show that except for increased unconjugated bilirubin levels all other parameters were in normal limits.*
Table 1.
Different parameters in donors with GS
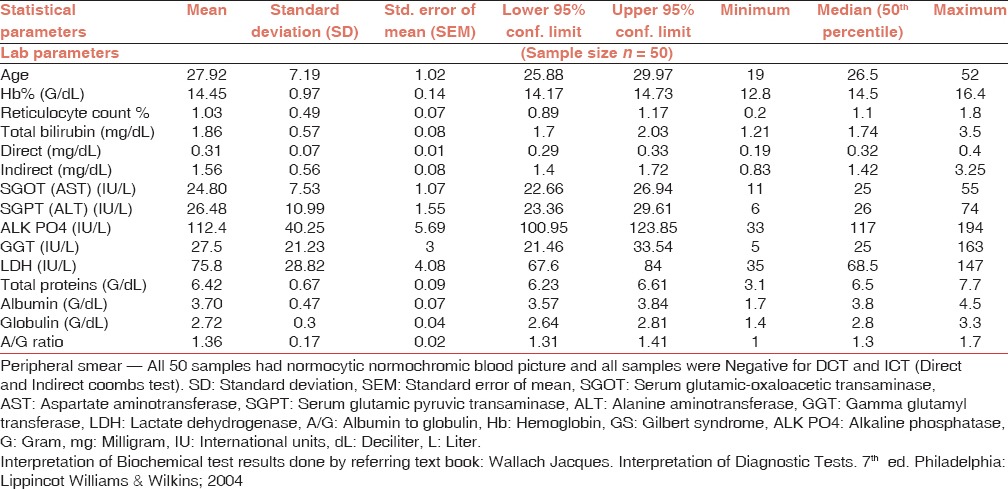
Hematological investigations: In this study the age of donors with GS ranged from 19 to 52 years with mean age being 27.92 ± 7.19 years. Mean hemoglobin level was 14.45 ± 0.97 G% (range: 12.8-16.4). Mean reticulocyte count was 1.03 ± 0.49% (range: 0.2-1.80).
Biochemistry values: Mean levels were serum bilirubin of 1.86 ± 0.57 mg% (range: 1.21-3.5). The hyperbilirubinemia was predominantly unconjugated (about 83.87% fraction being unconjugated) with mean of 1.56 ± 0.56 mg% (range: 0.83-3.25) in all the donors. No donor had clinical jaundice or history of jaundice. None of the donors gave history of transfusion, hepatitis like illness, or exposure to hepatotoxic agents.
Liver enzymes study showed — mean plasma levels of aspartate aminotransferase amongst cases were 24.8 ± 7.53 IU/L (range 11-55) whereas those of alanine aminotransferase were 26.48 ± 10.99 (range 6-74 IU/L). LDH levels were 75.8 ± 28.8 IU/L (range 35-147 IU/L). All other liver function tests, namely alkaline phosphatase, gamma glutamyl transferase enzyme levels, total plasma proteins, albumin, globulins, and albumin to globulin ratio were within normal limits.
There was no clinical or biochemical evidence of hemolysis with normal levels of LDH; a normal complete blood count, peripheral blood smear, and a normal reticulocyte count. All 50 cases were negative for TTI screening - HIV, hepatitis B and C, malaria, and syphilis.
Discussion
The donors analyzed for icteric plasma and hyperbilirubinemia did not have clinical jaundice, any history of jaundice since 1-year, any deranged liver function tests, any pathology for increased indirect hyperbilirubinemia, and history of recent medication. Hence, they were diagnosed as suffering from Gilbert's syndrome. As per drug controlling authorities’ guidelines, all such units (packed red blood cell, platelet concentrate, and fresh frozen plasma) were discarded.
In India, maintaining the inventory of platelet concentrates and packed red blood cell concentrates are proving cumbersome due to poor voluntary donation and high TTI positivity. The additional problem of icteric plasma confounds to present problems.
For any blood banker or transfusion medicine consultant, the main products to target for discarding will be fresh frozen plasma and platelet concentrates as these products contain most of increased unconjugated bilirubin. The immediate thought to avoid transfusion of such products will be in neonates and infants due to immature liver and adult patients having liver diseases.
Hence, after going through the literature and studies the solutions that emerge are.
J. L. Naimane et al. has argued that normal donor with Gilbert's syndrome can donate blood and components which can be utilized to even neonate without any harm by giving following justification—“One might question whether neonates with preexisting hyperbilirubinemia represent a group at special risk from the extra bilirubin load in the plasma.” The following hypothetical example suggests that the bilirubin load in these infants produces negligible changes in serum bilirubin concentration.
Suppose that a 1 kg premature infant receives 10 mL of frozen plasma with serum bilirubin concentration of 3 mg/dl. The bilirubin load will be 3 × 10/100 = 0.3 mg. When diluted in the infant's estimated plasma volume of approximately 50 mL, this amount of bilirubin could raise the infant's serum bilirubin concentration by approximately 0.6 mg/dl. Extravascular diffusion would further attenuate this rise.[9]
Existing regulations prohibiting the issue of components in which the plasma is abnormal in color (particularly if icteric) present a paradox and a dilemma when the donor has GS. When the donors with icteric plasma are subjected to investigations, they show only mild unconjugated hyperbilirubinemia, normal complete blood counts, no hemolytic features, and normal liver enzymes, particularly alanine aminotransferase levels in which case their blood should be safe for transfusion.
Arora et al. in their study found a prevalence of 0.91% for asymptomatic hyperbilirubinemia in healthy donors. They have also argued to have a firm policy about such cases.[10]
A similar study by Koul et al. was conducted in Kashmir in 1000 randomly selected blood donors. All were previously unaware of a hyperbilirubinemic state. Mean serum bilirubin levels were 2.64 mg/dl. The mean serum bilirubin level in our study was 2.19 mg/dl. They concluded that GS is seen in 3% of healthy voluntary blood donors in Kashmir.[2] This prevalence will cause huge inventory loss.
Lee C Claridge et al. in a study has elaborated on specific guidelines to diagnose Gilbert syndrome which are.
Unconjugated hyperbilirubinemia (conjugated bilirubin is within the normal range ≤20% of total bilirubin), with other liver and biliary tree function tests being normal, a negative hemolysis screen. He concluded that over-investigation is the greatest danger to patients with this benign condition. Patient advice and education have to be given accordingly.[11]
As differential diagnosis of unconjugated hyperbilirubinemia apart from GS are—drug-induced hemolysis, glucose-6—phosphate dehydrogenase deficiency, other hemolytic anemias such as thalassemia trait, sickle cell trait, and other causes of immune and nonimmune hemolytic anemia which would manifest earlier itself with anemia and donors will be on treatment and follow-up. In spite of this, all such asymptomatic cases can be investigated for complete blood count, peripheral smear and serum LDH levels, and other biochemical parameters which will be abnormal in such scenarios.
Jashnani et al. in their study found it in approximately 1.5% of the blood units collected annually. They were in dilemma and felt that guidelines regarding cut off, issuing such units, and discarding of such units need urgent attention from central authorities.[12]
Many studies have shown that there is no significant relationship between Gilbert's syndrome (promoter polymorphism) and hyperbilirubinemia in G6PD deficient neonates.[13]
Conclusion
Blood components are either discarded or returned to blood centers because of a finding of icteric plasma. This is in accordance with existing regulations of the Food and Drug Administration and standards of the American Association of Blood Banks.[9]
Patients with a record of GS have lower mortality from any cause, which may represent the beneficial properties of moderately raised bilirubin. Whether the relationship between GS and lower mortality is causal, remains to be proven. Mortality rates observed for people with GS in the general population are almost half those of people without evidence of GS.[14]
The population incidence of GS could be as high as 6% and that the sex incidence is approximately equal.[15] By definition, bilirubin levels in Gilbert syndrome are lower than 6 mg/dl, though most patients exhibit levels lower than 3 mg/dl.[16]
UGT1A1* 28 genotyping identifies patients at risk for drug toxicity and can increase drug safety by dose individualization. Rapid and facile UGT1A1*28 genotyping is, therefore, of great clinical importance.[17]
As existing rules and regulations prohibit the issue of blood and components if plasma is abnormal in color. Now with the present literature, it is up to the regulatory agencies of the blood bank to decide a reassessment of existing policies and regulations and frame guidelines accordingly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Col U. S. Dinesh, professor and HOD, Department of Pathology and Dr. Ravikala Rao, professor, Department of Pathology, for their guidance and support.
We thank Mr. Mahadev Walishetti, technical supervisor, Mr. Gururaj Joshi and Mr. Rudragouda Pattanshetti, technicians, blood bank SDM College of Medical Sciences and Hospital, Dharwad, for their help in doing in this study.
Footnotes
*(Wallach Jacques. Interpretation of Diagnostic Tests. 7th ed. Philadelphia: Lippincot Williams & Wilkins; 2004).
References
- 1.Rudenski AS, Halsall DJ. Genetic testing for Gilbert's syndrome: How useful is it in determining the cause of jaundice? Clin Chem. 1998;44(8 Pt 1):1604–9. [PubMed] [Google Scholar]
- 2.Koul PA, Geelani SA, Mudasir S. Benign jaundice in healthy blood donors in the Kashmir Valley of Indian Subcontinent. Internet J Intern Med. 2006;6:9. [Google Scholar]
- 3.Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson LL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. New York: The McGraw-Hill Companies Inc.; 2008. [Google Scholar]
- 4.Berthelot P, Dhumeaux D. New insights into the classification and mechanisms of hereditary, chronic, non-haemolytic hyperbilirubinaemias. Gut. 1978;19:474–80. doi: 10.1136/gut.19.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell LW, Hemingway E, Billing BH, Sherlock S. Idiopathic unconjugated hyperbilirubinemia (Gilbert's syndrome). A study of 42 families. N Engl J Med. 1967;277:1108–12. doi: 10.1056/NEJM196711232772102. [DOI] [PubMed] [Google Scholar]
- 6.Yu GP, Jiang QL, Fan ZP, Zhao J, Wei Q, Sun J, et al. Allogeneic hematopoietic stem cell transplantation for acute leukemia with Gilbert's syndrome. J Hematol Oncol. 2011;4:9. doi: 10.1186/1756-8722-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sticova E, Jirsa M. New insights in bilirubin metabolism and their clinical implications. World J Gastroenterol. 2013;19:6398–407. doi: 10.3748/wjg.v19.i38.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 9.Naiman JL, Sugasawara EJ, Benkosky SL, Mailhot EA. Icteric plasma suggests Gilbert's syndrome in the blood donor. Transfusion. 1996;36:974–8. doi: 10.1046/j.1537-2995.1996.36111297091741.x. [DOI] [PubMed] [Google Scholar]
- 10.Arora V, Kulkarni RK, Cherian S, Pillai R, Shivali M. Hyperbilirubinemia in normal healthy donors. Asian J Transfus Sci. 2009;3:70–2. doi: 10.4103/0973-6247.53875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claridge LC, Booth C, Gill PS. 10-minute consultation Gilbert's syndrome. Br Med J. 2011;342:1–2. doi: 10.1136/bmj.d2293. d2293. [DOI] [PubMed] [Google Scholar]
- 12.Jashnani KD, Karwande A, Puranik G. Icteric donor plasma: To transfuse or to discard? Indian J Pathol Microbiol. 2012;55:604–5. doi: 10.4103/0377-4929.107853. [DOI] [PubMed] [Google Scholar]
- 13.Zahedpasha Y, Ahmadpour M, Niaki HA, Alaee E. Relation between neonatal icter and Gilbert syndrome in gloucose-6-phosphate dehydrogenase deficient subjects. J Clin Diagn Res. 2014;8:63–5. doi: 10.7860/JCDR/2014/6674.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horsfall LJ, Nazareth I, Pereira SP, Petersen I. Gilbert's syndrome and the risk of death: A population-based cohort study. J Gastroenterol Hepatol. 2013;28:1643–7. doi: 10.1111/jgh.12279. [DOI] [PubMed] [Google Scholar]
- 15.Owens D, Evans J. Population studies on Gilbert's syndrome. J Med Genet. 1975;12:152–6. doi: 10.1136/jmg.12.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazer H, Katz J, editors. Unconjugated Hyperbilirubinemia. pp. 1–21. Available from: http://www.emedicine.medscape.com/article/178841-overview#aw2aab6b2b2 .
- 17.Ehmer U, Lankisch TO, Erichsen TJ, Kalthoff S, Freiberg N, Wehmeier M, et al. Rapid allelic discrimination by TaqMan PCR for the detection of the Gilbert's syndrome marker UGT1A1*28. J Mol Diagn. 2008;10:549–52. doi: 10.2353/jmoldx.2008.080036. [DOI] [PMC free article] [PubMed] [Google Scholar]


