Abstract
Background:
Thalassemia is one of the most common monogenic disorders characterized by reduced production of globin chains. Although regular red blood cell (RBC) transfusion support is the main treatment for these patients, it may be associated with complications such as RBC alloimmunization.
Aim:
The study aimed to determine the incidence of alloimmunization and autoimmunization to RBC antigens in β-thalassemia major patients from Zanjan, Zanjan Province, Iran.
Materials and Methods:
A total of 49 β-thalassemia major patients comprising 24 females and 25 males (mean age: 18.59 ± 8.16 years; range: 2-40 years) from Northwest Iran were included in a cross-sectional study. Alloantibody screening and identification were done using 3-cell and 10-cell reagent red blood cells, respectively. Autoantibody detection was performed using direct Coomb's test.
Results:
The incidence of alloimmunization was 16.32% with 10 alloantibodies identified in 8 patients. The most common clinically significant alloantibody identified in alloimmunized patients was anti-Kell (K-antigen) (60%) followed by anti-Rhesus (Rh) (E, c-antigens). The rate of alloimmunization was significantly lower in patients transfused with leukoreduced RBCs compared with those transfused with nonleukoreduced RBCs (9.53% vs 57.14%, P = 0.001). There was no significant correlation between alloantibody formation and the age, gender, hemoglobin levels, number of transfused units, and splenectomy.
Conclusion:
Transfusion of leukoreduced and phenotypically matched red blood cells for Kell (K) and Rh (E, c) antigens may help reduce the alloimmunization rate in Iranian β-thalassemia major patients. Moreover, autoimmunization to RBC antigens was rare in our patients.
Keywords: Alloantibody, autoantibody, leukoreduced, leukoreduced packed red blood cells (RBCs), b-thalassemia major
Introduction
Thalassemia is an autosomal recessive disorder characterized by decreased synthesis of one or more hemoglobin polypeptide chains.[1] The lack of polypeptide chain interferes with hemoglobin synthesis, erythroid precursor maturation, and function.[1] β-thalassemia major patients have severe anemia due to partial or total suppression of β-globin chain synthesis.[1] Regular red blood cell (RBC) transfusion support is the main treatment for these patients, which alleviates anemia by significantly suppressing the hyperactive erythropoiesis as well as inhibiting the excessive iron absorption from the gastrointestinal tract.[2] One of the adverse complications of RBC transfusion is alloimmunization to erythrocyte antigens, especially in chronically transfused patients.[3] Alloimmunization further complicates the transfusion therapy by inducing hemolytic transfusion reactions in some patients as well as limiting the availability of compatible blood.[3]
Different rates of alloimmunization to RBC antigens have been reported in β-thalassemia major patients ranging from 2.87% to 37% in various studies.[4,5] Alloantibodies against Rhesus (Rh) and Kell blood group systems are the most common alloantibodies identified in a number of the studies.[6,7,8] Transfusion of leukoreduced and extended phenotype matched RBCs has been recommended to prevent alloimmunization.[3,9] Moreover, RBC autoantibodies may develop in transfusion-dependent thalassemia patients.[10] Although autoantibodies appear less frequently than alloantibodies, they can result in clinical hemolysis and cause difficulties in finding compatible blood for transfusion therapy.[10] The aim of this study was to evaluate the frequency of alloimmunization and autoimmunization to erythrocyte antigens in β-thalassemia major patients and to identify the common alloantibodies.
Materials and Methods
Patients and blood samples
Our study population consisted of 49 β-thalassemia major patients (females, n = 24; males, n = 25) from the northwest of Iran. The patients were regular recipients of blood transfusion at intervals of 2-5 weeks. The mean age of the patients was 18.59 ± 8.16 years (range: 2-40 years). The clinical and transfusion records of all the patients were examined for age and sex at first transfusion, splenectomy, frequency of transfusion, exposure to leukoreduced blood, and the presence of alloimmunization or autoimmunization. After informed consent, 5 mL of blood was collected from each patient in vacutainer tubes containing ethylene diamine tetraacetic acid disodium (Na2 -EDTA), and the plasma was separated and stored at -20˚C until the tests were performed in batches. This study was approved by the Ethics Committee of Zanjan University of Medical Sciences, Zanjan, Zanjan Province, Iran.
Alloantibody screening and identification
Commercially available triplet antibody screen reagent red blood cells (Alpha Laboratories-Z451, 40 Parham Dr, Eastleigh SO50 4NU, United Kingdom) were used for screening of irregular alloantibodies by the standard tube method. New antibodies to RBC antigens were detected using standard blood bank methods (saline, 37°C with albumin, and Coomb's phases). Antibody screen-positive samples were subjected to antibody identification procedure using a 10-cell panel of reagent red blood cells (Alpha Laboratories-Z471, 40 Parham Dr, Eastleigh SO50 4NU, United Kingdom) with known antigens.
Autoantibody detection
A polyspecific antiglobulin reagent was used to perform direct antiglobulin test (DAT) using a 3-5% suspension of patients’ RBCs and appropriate controls. In cases with positive DAT result, the test was repeated using a monospecific antiglobulin reagent. The results were read macroscopically and microscopically, and all negative results were confirmed by adding Coomb's control cells.
It is important to remember that a DAT test may be positive in many conditions other than the presence of autoantibody including intravenous administration of immunoglobin G (IgG), patients with neonatal hemolytic disease, bone marrow transplantation, administration of some drugs, infections, acute and chronic leukemia and is rarely positive in healthy individuals.[11] We assumed the presence of autoantibody in patients with positive DAT result since the prevalence of the abovementioned conditions was quite rare in our study population. However, definitive identification of an autoantibody requires that all DAT positive samples are subjected to the elution process, and the eluted antibody is subsequently identified using a panel of reagent RBCs. The specificity of antibody should be compared with a predefined phenotype of patient red blood cells to determine if it is directed against a self-antigen (autoantibody) or a foreign antigen (alloantibody). Because of limitations in our laboratory and considering that only one of the studied patients had positive DAT, we did not perform elution and adsorption studies.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 16 (SPSS Inc, Chicago, IL, USA). All data were statistically analyzed using Student's t-test and chi-square test. P < 0.05 was considered statistically significant.
Results
A total of 49 β-thalassemia major patients including 25 (51%) males and 24 (49%) females were enrolled in the study. The mean age of the alloimmunized and nonalloimmunized patients was 21.12 ± 6.19 years and 18.12 ± 8.46 years, respectively. The difference in mean age was not statistically significant by Student's t-test (P = 0.85). Additionally, the correlation between the age at the start of transfusion and alloimmunization status was not statistically significant (P = 0.78). The age distribution of β-thalassemia major patients is represented in Table 1.
Table 1.
Age distribution of thalassemia patients
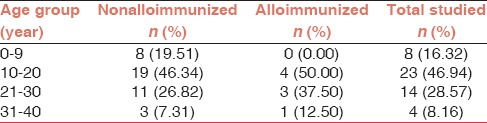
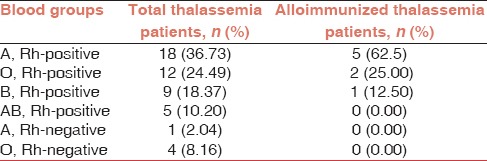
In the current study, eight subjects were found to be positive for RBC alloantibodies. Thus, the prevalence of alloimmunization in our study population was 16.32% with 95% confidence interval (CI) of 8.5-29.0%. Collectively, 10 alloantibodies were detected in eight alloimmunized patients, and the most commonly identified alloantibody was anti-K (Kell) followed by anti-Rh (E, c antigens) [Table 2]. The profiles of alloimmunized thalassemia patients are given in Table 3. As shown in the Table, six alloimmunized patients (75%) developed single alloantibody while two alloimmunized patients (25%) developed dual alloantibody. Moreover, only one (2.04 %) β-thalassemia major patient developed autoantibody as well as alloantibody. Table 4 represents the incidence of ABO blood groups among alloimmunized and nonalloimmunized patients. As shown in this table, the prevalence of antibody was higher in patients with blood group A (62.5%). Chi-square test showed significant differences in the distribution of blood group A between the alloimmunized and nonalloimmunized patients (P = 0.01).
Table 2.
Frequency of various anti-red blood cell alloantibodies in alloimmunized β-thalassemia major patients

Table 3.
Profile of alloimmunized β-thalassemia major patients
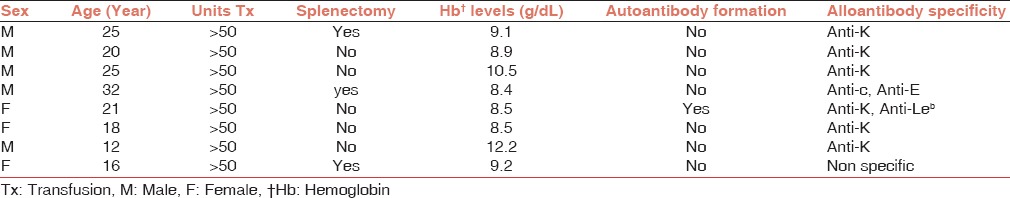
Table 4.
Alloimmunization incidence by blood groups
Moreover, our results revealed that 5 out of 25 males (20%) and 3 out of 24 females (12.5%) showed evidence of alloimmunization. Chi-square test showed no significant correlation between gender and alloimmunization status (P = 0.47).
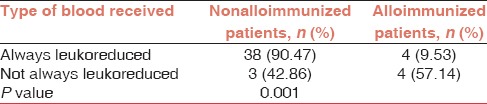
The majority of patients (85.71%) received ABO and Rh (D) matched, homologous leukoreduced packed RBC while a minority of them (14.29%) received ABO and Rh (D) matched, homologous nonleukoreduced packed RBC. As shown in Table 5, the rate of alloimmunization was significantly higher in patients receiving nonleukoreduced packed RBC compared with those receiving leukoreduced packed RBC (57.14% vs 9.53%) (P = 0.001).
Table 5.
Alloimmunization incidence in patients receiving leukoreduced and nonleukoreduced packed red blood cell
Moreover, 17 out of 49 patients (34.7%) underwent splenectomy, out of which only 3 patients were alloimmunized and the remaining 14 patients were not alloimmunized. The comparison of splenectomy as a risk factor between patients with and without alloimmunization (37.5% vs 34.1%) was not statistically significant (P = 0.85). Finally, the correlation between hemoglobin levels (P = 0.93), number of transfused units (P = 0.7), and age at the start of transfusion (P = 0.45) and alloimmunization was not significant. Table 6 represents the antigen matching stringency levels based on different antigen sets.
Table 6.
Antigen matching stringency levels[9]

Discussion
In the present study, we examined the prevalence of common alloantibodies in multitransfused β-thalassemia major patients from the northwest of Iran. We reported an alloimmunization rate of 16.32%, with 95% CI of 8.5-29.0%. The rates of alloimmunization in other parts of Iran have been reported as follows: 7.4% (Tehran), 5.34% (Fars Province), 18.7% (Southwest of Iran), 2.87% (northeast of Iran), and 17.9% (southeast of Iran).[5,12,13,14,15]
The most common clinically significant alloantibodies detected in our study were directed against K antigen in the Kell system and E, c antigens in the Rh system. Similarly, Ameen et al. reported anti-Kell and anti-Rh as the most commonly identified alloantibodies in their study.[16] In a recent multicenter study from Iran, Azarkeivan et al. screened 835 thalassemia patients for unexpected RBC alloantibody and reported an alloimmunization rate of 12.1% with anti-K as the most commonly identified alloantibody.[17] Furthermore, Saied et al. investigated 95 Egyptian β-thalassemic patients and reported anti-Kell and anti-E as the most commonly identified alloantibodies.[7]
The frequency of alloimmunization in our thalassemia patients (16.32%) was lower than that reported in thalassemia patients from Taiwan (37%), Egypt (28.4%), and Kuwait (30%)[4,7,8] but was higher than the frequency reported in patients from Pakistan (3.75%), Italy (5.2%), Greece (3.7%), India (5.64%), and Malaysia (8.6%).[18,19,20,21,22] One of the most important reasons for the relatively high alloimmunization rate in our thalassemia patients may be transfusion of nonleukoreduced RBC units in a number of patients. As indicated in Table 5, over 50% of our alloimmunized thalassemia patients received nonleukoreduced RBC units.
Factors affecting alloimmunization are complex, and at least three contributing factors may explain the differences in alloimmunization rate: RBC antigenic difference between the donor and recipient, the recipient's immune status, and the immunomodulatory effect of the allogenic blood transfusion on recipient's immune system.[10] In addition, differences in immunogenicity of various RBC antigens may affect the rate of alloimmunization. The Kell and Rh blood group system antigens are strong immunogens; therefore, the majority of studies including ours have reported alloantibody specificity to Kell and Rh blood group systems.[14,15,17,18]
Moreover, the sensitivity of the laboratory method used for antibody screening and identification (gel centrifugation techniques vs classical tube techniques) significantly affects the efficiency of antibody identification procedures and alloimmunization incidence. A study by Delaflor-Weiss et al. demonstrated increased overall incidence of clinically significant antibodies identified by the gel method as compared to the tube method without significant differences in the nonspecific reactions.[23]
In our study, patients transfused with nonleukoreduced RBCs had a higher rate of alloimmunization compared with patients transfused with leukoreduced RBCs. The immunomodulatory role of white blood cells in transfused blood may explain this observation. Leukocytes in blood components may downregulate T-helper cell type 1 (Th1) immune response and direct the recipient toward T-helper cell type 2 (Th2) responses. Such skewing toward type 2 immunity may enhance alloantibody formation.[24] Our results were consistent with the results of Singer et al. and Hussein et al. who showed a lower rate of alloimmunization in patients transfused with leukoreduced RBCs.[3,10] The standard of leukoreduction in Iran is fewer than 5 × 106 donor white blood cells per component.
Unlike alloimmunization, the rate of autoimmunization was very low (2.14%) in our study. A low rate of autoimmunization, together with a high rate of alloimmunization, has been reported in other studies.[3,22]
Currently, providing compatible blood for alloimmunized thalassemia patients presents a major challenge. Transfusion of extended phenotype matched blood can be useful in the prevention of alloimmunization.[9,24] Klapper et al.[9] used an extended phenotype matched blood transfusion based on the levels of antigen matching stringency [Table 6]. Retrospectively, if our thalassemia patients had been transfused with extended phenotype matched blood for Kell- K, Rh-E, and Rh-c antigens or level 2 antigen matching stringency [Table 6], the alloimmunization rate would have been reduced by 75% (in six out of eight alloimmunized patients).
It has been reported that an early age at the start of transfusion therapy may offer some level of immune tolerance and protect against alloimmunization.[10,20,25] However, in our study, the incidence of alloimmunization was not influenced by the age at which transfusion was started. One possible explanation for this finding may be the small sample size of alloimmunized patients in our study. Moreover, the number of transfused blood units, genetic and ethnic background of the patients and blood donors, and the immune status of the patients may affect the alloimmunization rate. Our result was consistent with other studies reporting no association between the age at the start of transfusion therapy and alloimmunization rate.[3,12,14,22]
Regarding the frequency of blood transfusion in patients, the average number of transfused units per year was 18.25 U/year in alloimmunized patients compared with 17.50 U/year in nonalloimmunized patients. The correlation between alloimmunization and the frequency of blood transfusion was not significant in our study, which was inconsistent with the results of other studies.[25] The possible reasons for these controversial results may be explained by the fact that in addition to the number of transfused units, many concomitant factors such as ethnic similarity between patients and blood donors, the time interval between blood transfusions, and type of transfused blood (leukoreduced vs nonleukoreduced) may affect the alloimmunization incidence in different studies.[3,7,10,26]
Splenectomy is also considered as a risk factor for alloimmunization.[10] However, in the current study, there was no statistically significant difference between splenectomized and nonsplenectomized patients regarding the alloimmunization rate (37.5% vs 34.1%; P = 0.85). This is a contrast to the study of Hussein et al. (2014) who observed a higher rate of alloimmunization in splenectomized patients.[3] However, our results were in accordance with other studies that reported an insignificant association between splenectomy and alloimmunization rate in thalassemia patients.[25,27] These conflicting results may be related to various factors including RBC transfusion burden, timing of initial RBC antigen exposure (presplenectomy vs postsplenectomy), and the life span of transfused RBC.[28]
Conclusion
The rate of RBC alloimmunization is relatively high in our patients. Determination of RBCs phenotype before beginning chronic blood transfusion as well as selective transfusion of leukoreduced and phenotypically matched RBC units for Kell (K) and Rh (E, c) antigens may help reduce the alloimmunization rate in chronic transfusion patients.
Financial support and sponsorship
Zanjan University of Medical Sciences (grant number: A-11-836-2).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MJ. Update on thalassemia: Clinical care and complications. Hematol Oncol Clin North Am. 2010;24:215–27. doi: 10.1016/j.hoc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Hussein E, Desooky N, Rihan A, Kamal A. Predictors of red cell alloimmunization in multitransfused Egyptian patients with beta-thalassemia. Arch Pathol Lab Med. 2014;138:684–8. doi: 10.5858/arpa.2013-0016-OA. [DOI] [PubMed] [Google Scholar]
- 4.Wang LY, Liang DC, Liu HC, Chang FC, Wang CL, Chan YS, et al. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfusion Med. 2006;16:200–3. doi: 10.1111/j.1365-3148.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghian MH, Keramati MR, Badiei Z, Ravarian M, Ayatollahi H, Rafatpanah H, et al. Alloimmunization among transfusion-dependent thalassemia patients. Asian J Transfus Sci. 2009;3:95–8. doi: 10.4103/0973-6247.53884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal I, Ahmed N. Frequency of red cell alloimmunization and autoimmunization in thalassemia major children. Biomedica. 2014;30:25–8. [Google Scholar]
- 7.Saied DA, Kaddah AM, Eldin RMB, Mohaseb SS. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent Egyptian thalassemic patients. J Pediatr Hematol Oncol. 2011;33:409–14. doi: 10.1097/MPH.0b013e3182208154. [DOI] [PubMed] [Google Scholar]
- 8.el-Danasoury AS, Eissa DG, Abdo RM, Elalfy MS. Red blood cell alloimmunization in transfusion-dependent Egyptian patients with thalassemia in a limited donor exposure program. Transfusion. 2012;52:43–7. doi: 10.1111/j.1537-2995.2011.03234.x. [DOI] [PubMed] [Google Scholar]
- 9.Klapper E, Zhang Y, Figueroa P, Ness P, Stubbs J, Abumuhor I, et al. Toward extended phenotype matching: A new operational paradigm for the transfusion service. Transfusion. 2010;50:536–46. doi: 10.1111/j.1537-2995.2009.02462.x. [DOI] [PubMed] [Google Scholar]
- 10.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 11.Bıçakçı Z, Öztürkmen S, Akyay A, Olcay L. False positive result of the direct antiglobulin test (DAT): The role of the elevated level of immunoglobulin G. Pediatr Hematol Oncol. 2012;29:611–9. doi: 10.3109/08880018.2012.695440. [DOI] [PubMed] [Google Scholar]
- 12.Shamsian BS, Arzanian MT, Shamshiri AR, Alavi S, Khojasteh O. Frequency of red cell alloimmunization in patients with-major thalassemia in an Iranian Referral Hospital. Iran J Pediatr. 2008;18:149–53. [Google Scholar]
- 13.Karimi M, Nikrooz P, Kashef S, Jamalian N, Davatolhagh Z. RBC alloimmunization in blood transfusion-dependent beta-thalassemia patients in southern Iran. Int J Lab Hematol. 2007;29:321–6. doi: 10.1111/j.1365-2257.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 14.Keikhaei B, Hirad Far A, Abolghasemi H, Mousakhani H, Ghanavat M, Moghadam M, et al. Red blood cell alloimmunization in patients with thalassemia major and intermediate in southwest Iran. IJBC. 2013;6:41–6. [Google Scholar]
- 15.Amin M, Gholamhossein T, Majid N, Marziyeh H, Narges S, Akbar D, et al. Prevalence of alloimmunization against RBC antigens in thalassemia major patients in south east of Iran. J Blood Disorders Transf. 2013;4:1–4. [Google Scholar]
- 16.Ameen R, Al-Shemmari S, Al-Humood S, Chowdhury RI, Al-Eyaadi O, Al-Bashir A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion. 2003;43:1604–10. doi: 10.1046/j.1537-2995.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 17.Azarkeivan A, Ansari S, Ahmadi MH, Hajibeigy B, Maghsudlu M, Nasizadeh S, et al. Blood transfusion and alloimmunization in patients with thalassemia: Multicenter study. Pediatr Hematol Oncol. 2011;28:479–85. doi: 10.3109/08880018.2011.568595. [DOI] [PubMed] [Google Scholar]
- 18.Usman M, Moin S, Moinuddun M, Ahmad S, Perveen R, Azmi MA, et al. Frequency of red cell alloimmunization among patients with transfusion dependent beta thalassemia in Pakistan. UHOD. 2011;9:12. [Google Scholar]
- 19.Sirchia G, Zanella A, Parravicini A, Morelati F, Rebulla P, Masera G. Red cell alloantibodies in thalassemia major. Results of an Italian cooperative study. Transfusion. 1985;25:110–2. doi: 10.1046/j.1537-2995.1985.25285169198.x. [DOI] [PubMed] [Google Scholar]
- 20.Spanos T, Karageorga M, Ladis V, Peristeri J, Hatziliami A, Kattamis C. Red cell alloantibodies in patients with thalassemia. Vox Sang. 1990;58:50–5. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 21.Dhawan HK, Kumawat V, Marwaha N, Sharma RR, Sachdev S, Bansal D, et al. Alloimmunization and autoimmunization in transfusion dependent thalassemia major patients: Study on 319 patients. Asian J Transfus Sci. 2014;8:84–8. doi: 10.4103/0973-6247.137438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslina MN, Ariffin N, Illuni Hayati I, Rosline H. Red cell immunization in multiply transfused Malay thalassemic patients. Southeast Asian J Trop Med Public Health. 2006;37:1015–20. [PubMed] [Google Scholar]
- 23.Delaflor-Weiss E, Chizhevsky V. Implementation of gel testing for antibody screening and identification in a community hospital, a 3-year experience. Lab Medicine. 2005;36:489–92. [Google Scholar]
- 24.Sood R, Makroo RN, Riana V, Rosamma NL. Detection of alloimmunization to ensure safer transfusion practice. Asian J Transfus Sci. 2013;7:135–9. doi: 10.4103/0973-6247.115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Sewefy D, Al Feky M, Abdel Fatah M, El Sakhawy Y, Ragab I, El Sayed H. Clinically significant red blood cell antibodies in multitransfused Egyptian thalassemic patients. Egypt J Haematol. 2014;39:171–6. [Google Scholar]
- 26.Sharma RR, Marwaha N. Leukoreduced blood components: Advantages and strategies for its implementation in developing countries. Asian J Transfus Sci. 2010;4:3–8. doi: 10.4103/0973-6247.59384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirzaeian A, Tamaddon G, Naderi M, Hosseinpour M, Sargolzaie N. Prevalence of alloimmunization against RBC antigens in thalassemia major patients. Zahedan J Res Med Sci. 2013;15:55–8. [Google Scholar]
- 28.Hendrickson JE, Stowell SR, Smith NH, Girard-Pierce KR, Hudson KE, Zimring JC. Transfused RBCs can be immunogenic in splenectomized mice: Of inflammation, adjuvants, and anamnestic responses. Transfusion. 2012;52:P1–030A. [Google Scholar]


