Abstract
Male Lepidoptera often possess specialized scales, called hair pencils that emit volatiles that are critical to mating success. Spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), males will display hair pencils to females before attempting copulation. The importance of volatiles on these hair pencils is, however, not clear. We compared the proportion of successful copulations in unmanipulated mating pairs to pairs where males had their hair pencils either removed or chemically washed, and to pairs where females were antennectomized. Mean proportions of successful matings were significantly lower in pairs where hair pencils had been manipulated or where females had been antennectomized compared with unmanipulated mating pairs. There was no significant difference in mating success between treatments where hair pencils had been manipulated; however, mating success was significantly lower in hair pencil treatments than in antennectomized treatments. Mean copulation proportions in hair pencil/antennectomized treatments were also significantly less than in respective sham-operated treatments. Our results suggest that volatiles are associated with hair pencils, and they may be required for mating success in C. fumiferana.
Keywords: Choristoneura fumiferana, hair pencil, pheromone
Mating in Lepidoptera typically begins with the attraction of males to females with long-range female sex pheromones (Cardé and Haynes 2004). Upon locating the female, males undertake a repeatable courtship sequence that precedes attempted copulation. Before entering into copulation, males from many species will typically expose to the female specialized scent-releasing organs such as coremata, androconial cells, or hair pencils from which a pheromone or blend of pheromone components may be emitted (Birch et al. 1990). Release of male pheromones during courtship has been shown to perform several roles, including attraction of females at various ranges and arrestment of female movement, which increases mating success (Birch et al. 1989, 1990). The importance of hair pencils to mating success has been demonstrated in a number of species in several studies (Birch et al. 1990, Lassance and Löfstedt 2009). For example, Fitzpatrick and McNeil (1988) and Royer and McNeil (1992) both observed significantly lower mating success in noctuid and pyralid mating pairs where males had hair pencils removed versus mating pairs containing unmanipulated males. In both studies, the authors concluded that the presence of hair pencils and their associated volatiles were critical for mating success.
In addition to Noctuidae and Pyralidae, these structures and associated compounds have been observed in several other families of Lepidoptera, including Arctiidae, Pyralidae, and other families (Birch et al. 1990, Hillier and Vickers 2004). Hair pencils have also been observed in several tortricid species (Grant 1978), but only in the tortricid Oriental fruit moth (Grapholita molesta Busck) have the specific role and composition of hair pencil volatiles been determined (George 1965, Baker and Cardé 1979a,b,c). In G. molesta, males spread their hair pencils in front of the female and release a blend of several volatiles that attract the female to the male. Upon reaching the male’s abdomen, the female arrests her movement, and the male immediately copulates with the female (Baker and Cardé 1979b). Similar mating behaviors have been observed in the spruce budworm (Choristoneura fumiferana [Clemens]) whereby the male will also spread his abdominal hair pencils in the direction of the female before attempting copulation (Palaniswamy et al. 1979). These authors also described the abdominal hair pencils in male C. fumiferana as modified scales arising between the intersegmental membrane between the eighth and ninth abdominal segments, on which reside a number of dentiform papillae that resemble similar papillae found on female sex glands. Whereas females have been shown to respond to volatiles derived from male hair pencil extracts in electroantennogram (EAG) analyses (Palaniswamy et al. 1979), the exact roles of the hair pencils, their volatiles, and the importance to mating success in C. fumiferana mating pairs are not clear.
The sex pheromone chemistry of female C. fumiferana is well known (Silk and Kuenen 1986, 1988), with females emitting a primary component pheromone of 95:5 E/Z11-tetradecenal (95/5 E/Z11-14:Ald) that is derived from a hydrolysis of an acetate (acetate esterase) within the female sex pheromone gland followed by an oxidation of the alcohol by an oxygen-dependent alcohol oxidase (Silk and Kuenen 1988). The identification, synthesis, and application of female pheromones have been useful in detection and management of C. fumiferana (Silk and Eveleigh 2016), and similar research on male-derived compounds will be complementary to existing mating disruption and detection protocols involving female sex pheromones.
Here, we demonstrate the importance of hair pencils and associated volatiles to C. fumiferana mating success by comparing the mating success of unmanipulated mating pairs to treatments containing (1) males with hair pencils removed; (2) hair pencils washed with a solvent; and (3) antennectomized females. Based on the importance of hair pencil volatiles to mating success observed in previous studies, we predicted that the removal of hair pencil volatiles from the mating sequence—through ablation or solvent rinsing of hair pencils or through female antennectomization—would disrupt mating success.
Methods
Overwintering second-instar C. fumiferana larvae were obtained from the Canadian Forest Service’s Insect Production Services (IPS) in Sault Ste. Marie, ON, Canada (Dedes 2014), and were raised on artificial diet (McMorran 1965), also produced at IPS. Adults were kept on a 16:8 (L:D) h cycle, and were 1–3-d-old for the study. Mating trials were conducted in a wind tunnel (23–25°C, 60–70% relative humidity) flowing at 0.2 km/h (0.056 m/s) that was housed in a darkened room illuminated by several red incandescent lights. Treatments were compared using cages (20 by 17 by 14 cm) consisting of a cardboard box with plastic window-screen coverings on all four sides and the roof. Fifteen replicates of each treatment were completed (n = 15). In addition to a control group containing unmanipulated males and females, treatments consisted of (1) males with hair pencils removed plus normal females; (2) males with hair pencils washed with 5 μl of 99% hexane plus normal females; and (3) normal males plus antennectomized females. One cage was used consistently for each treatment with five males and five females in the cage at one time. As males in treatments 1 and 2 and females in treatment 3 required anesthetization in order to remove or wash body parts, all insects were anesthetized with CO2 prior to placement into cages to control for any potential artifacts of CO2 exposure on insect behavior. Hair pencils of males in treatment 1 were removed using fine forceps. In treatment 2, male hair pencils were washed with hexane (99%) applied with a 5-μl micropipette (Drummond Wiretrol, Broomall, PA). In treatment 3, female antennae were removed close to the head using microscissors. To determine if CO2 anesthetization affected mating success, we also conducted under identical conditions a separate evaluation of mating success of normal mating pair groups (n = 15) that were not anesthetized with CO2.
For the experiment, cages were placed in a wind-tunnel environment during the second hour of scotophase when pheromone calling peaks (Silk and Kuenen 1986). Females were introduced to the cages first, followed by males an hour later. As normal duration of copulation has been reported to be 3 h (Outram 1971, Delisle and Hardy 1997, Delisle and Simard 2002), cages were checked ∼3 h after the introduction of males, and the number of pairs in copula were counted so that any possible copulation events within the cages would be observed. To confirm that any possible reductions in proportions of mating pairs were attributable to the presence or absence of hair pencils or female antenna and not handling, sham-operated treatments containing mating pairs that simulated the effects of handling on the insects were also observed. These included (1) males with scales removed from the dorsum of the abdomen + normal females; (2) males with 3 μl of hexane (99%) applied to the dorsum of the abdomen + normal females; and (3) normal males + females with their left hind leg amputated between the femur and tarsus. These were compared with hair pencil/female antennectomization treatments 1–3 to ensure that any possible effects on copulation success were not attributable to trauma sustained during handling. As with hair pencil/female antennectomization treatments 1–3, 15 replicates of each treatment were completed (n = 15) with five males and five females being used for each replicate.
To assess differences in mating success among hair pencil/female antennectomized treatments, we used a generalized linear model (proc GENMOD, dist = binomial, link = logit; SAS Institute 1999), followed by CONTRAST statements (Wald tests; SAS Institute 1999) to compare all possible pairs of hair pencil/female antennectomization treatments. To determine if trauma associated with handling influenced copulation success, Student’s t-tests comparing the mean proportion of moths in copula of hair pencil/female antennectomized treatments versus their respective sham-operated treatments were also completed. A one-way analysis of variance (ANOVA) that compared the mean proportions of moths in copula for sham-operated treatments and the control was also completed. The level of statistical significance was set at α = 0.05 for all analyses.
Results
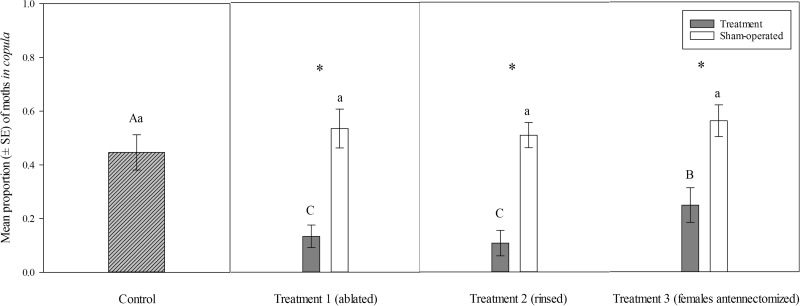
There was a significant difference in the mean proportion of moths in copula across hair pencil/female antennectomized treatments (χ2 = 30.85, df = 3, P < 0.0001). The proportion of moths in copula (mean ± SE) in the control group (0.447 ± 0.065) was significantly higher than that of treatment 1 (males with hair pencils washed with 5 μl of 99% hexane plus normal females) (0.107 ± 0.047) and treatment 2 (males with hair pencils ablated plus normal females) (0.133 ± 0.042), and treatment 3 (normal males plus antennectomized females) (0.247 ± 0.064) (Wald test, P < 0.05; Fig. 1). There was no significant difference in mating success between treatments 1 and 2, however, mating success was significantly lower in treatments 1 and 2 than in treatment 3 (Wald test, P < 0.05; Fig. 1). Mean proportions of moths in copula for all three hair pencil/antennectomized treatments were also significantly less than their respective sham-operated treatments (Student’s t-tests, P < 0.01, Fig. 1). There were no significant differences in the mean proportion of moths in copula between the control and sham-operated treatments (one-way ANOVA, F3, 56 = 0.618, P = 0.606). Mean proportion of moths in copula in mating pairs not anesthetized with CO2 was 0.747 ± 0.041 (n = 15).
Fig. 1.
Mean (± SE) proportions of C. fumiferana mating pairs in copula in cages (n = 15 cages per treatment group). Control group contained five unmanipulated males and five unmanipulated females per cage. Treatments include (1) five males with hair pencils ablated + five unmanipulated females per cage; (2) five males with hair pencils washed with 5 µl of 99% hexane + five unmanipulated females per cage; and (3) five unmanipulated males + five antennectomized females per cage. Sham-operated treatments include (1) mating pairs containing five males with scales removed from the dorsum of the abdomen + five normal females; (2) mating pairs containing five males with 3 µl of hexane (99%) applied to the dorsum of the abdomen + five normal females; and (3) mating pairs with five normal males + five females with their left hind leg amputated between the femur and tarsus. ‘*’ represents significant differences between mean proportions of mating pairs in copula of treatment and sham-operated mating pairs (Student’s t-test, P < 0.01). Different uppercase letters represent significant differences between mean proportions of mating pairs in copula for control and hair pencil/female antennectomization treatments (Wald test, P < 0.05). Different lowercase letters represent significant differences between mean proportions of mating pairs in copula for control and sham-operated treatments (one-way ANOVA, P < 0.05).
Discussion
Our data point strongly to the conclusion that mating success is related to the presence of hair pencil volatiles in males. In treatments where hair pencils had either been ablated or chemically washed, the mean proportion of moths in copula was much lower than in the control. Mating success was also significantly lower in pairs where females had been antennectomized. Significantly higher proportions of moths in copula in sham-operated treatments confirm that decreases in mating success are not related to insect handling or trauma. These results suggest that male hair pencils and their associated volatiles serve an important role in the mating success of C. fumiferana.
Decreases in mating success caused by the ablation of hair pencils have been observed in other species, including Pseudaletia unipuncta [Haworth] (Lepidoptera: Noctuidae) (Fitzpatrick and McNeil 1988) and Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) (Royer and McNeil 1992, Lassance and Löfstedt 2009). Although mating was significantly lower in our hair pencil treatments, some mating instances were observed. These may be because hair pencil ablation may not have been 100% complete, with volatiles remaining after ablation or washing, a conclusion also discussed by Royer and McNeil (1992). Hair pencils are also found on the wing folds of males in several tortricids (Grant 1978), including C. fumiferana (Palaniswamy et al. 1979). Although it is possible that pheromones may also be emitted from these regions as well, detectable amounts are likely obscured based on the significant reduction in mating success in pairs where hair pencils had been manipulated.
We observed that mating success in pairs containing antennectomized females was significantly lower than in the control group. This result may be due to the female not being able to detect hair pencil volatiles, causing disruptions to the mating sequence. Antennectomization of females has been shown to reduce mating success in O. nubilalis (Royer and McNeil 1992) and Heliothis virescens F. (Lepidoptera: Noctuidae) (Hillier and Vickers 2004). Palaniswamy et al. (1979) also observed EAG responses in female C. fumiferana antenna that were exposed to male C. fumiferana hair pencil extracts. Although these studies support our conclusion, it is possible that antennectomization may also prevent the female from detecting her own sex pheromones and thus potentially disrupt her own pheromone production. Palaniswamy and Seabrook (1978, 1985) observed that C. fumiferana female pheromone production can be induced by the detection of female sex pheromone. If pheromone emittance does not occur, then male attraction and subsequent mating may be reduced due to an inability of the male to locate the female through pheromone detection. The significantly higher amount of successful matings in the antennectomized treatment compared with mating pairs where hair pencils had been manipulated is also interesting as it suggests that other olfactory organs could exist. Kent et al. (1986) suggested that sensilla on the labial palps of several hawkmoth (Manduca spp. [Hübner] (Lepidoptera: Sphingidae)) species could be secondary olfactory receptors. Ablation of labial palps in male C. fumiferana did not, however, affect mating success (Sanders and Lucuik 1992). Future experiments analyzing the structure of female labial palps may be useful in determining if such structures exist.
Observed mating success in the control group (∼42%) was much lower than that observed by Sanders and Lucuik (1992) (82%). We attribute this to CO2 anesthetization of all insects prior to observations. Mating trials conducted with insects not exposed to CO2 anesthetization yielded mating proportions (74.1 ± 4.10%) more similar to those of Sanders and Lucuik (1992), thereby indicating that CO2 anesthetization was a likely factor in the relatively low mating successes observed in the control group. Insect handling also did not significantly influence mating success, a result also observed by Sanders and Lucuik (1992) and Royer and McNeil (1992) in C. fumiferana and O. nubilalis, respectively.
At present, the chemical composition of this potential volatile or blend of male volatiles is unknown. Studies have shown hair pencil blends to vary across species. In G. molesta, male hair pencil volatile blends include mellein, methyl jasmonate, methyl 2-epi-jasmonate, and trans-ethyl cinnamate (Nishida et al. 1982), whereas (E)-2-methyl-2-butenoic acid (tiglic acid) is the primary component in hair pencil extracts from male Conogethes punctiferalis [Guenée] (Lepidoptera: Pyralidae) (Kimura and Honda 1999). Analyses of C. fumiferana hair pencil extracts through GC-MS analyses and synthesis of compounds and bioassays are currently underway. Although the specific role of these compounds in the mating sequence of C. fumiferana is also unknown, other studies have demonstrated that hair pencil volatiles could fulfil a variety of roles, including acting as female attractants (Baker et al. 1981, Sinnwell et al. 1985, Krasnoff and Dussourd 1989, Krasnoff and Roelofs 1989, Landolt and Heath 1989) and as compounds that arrest female behaviors, including sex pheromone production (Hendricks and Shaver 1975). Other studies have suggested that females may be able to assess mate quality based on hair pencil volatile composition (Baker and Cardé 1979b), and others suggest that hair pencil volatiles may inhibit competing males from approaching females both pre- (Lecomte et al. 1998, Hillier et al. 2007) and post- (Andersson et al. 2003) copulation. This latter possibility may be useful in interfering with the mating behavior of C. fumiferana, possibly in conjunction with female 95:5 E/Z11-14:Ald compounds. Further studies on the composition and identity of male-derived hair pencil compounds in conjunction with behavioral assays are required.
Acknowledgments
We thank Katie Burgess for her help with laboratory work. This research was funded by the Atlantic Canada Opportunities Agency project “Early Intervention Strategies to Suppress a Spruce Budworm Outbreak” and by Natural Resources Canada – Canadian Forest Service. All experiments reported here comply with the laws of Canada.
References Cited
- Andersson J., Borg-Karlson A.-K., Wiklund C. 2003. Antiaphrodisiacs in pierid butterflies: a theme with variation! J. Chem. Ecol. 29: 1489–1499. [DOI] [PubMed] [Google Scholar]
- Baker T. C., Cardé R. T. 1979a. Analysis of pheromone-mediated behaviours in male Grapholita molesta, the oriental fruit moth (Lepidoptera: Totricidae). Environ. Enomol. 8: 956–968. [Google Scholar]
- Baker T. C., Cardé R. T. 1979b. Courtship behavior of the oriental fruit moth (Grapholitha molesta): experimental analysis and consideration of the role of sexual selection in the evolution of courtship pheromones in the Lepidoptera. Ann. Entomol. Soc. Am. 72: 173–188. [Google Scholar]
- Baker T. C., Cardé R. T. 1979c. Endogenous and exogenous factors affecting periodicities of female calling and male sex pheromone response in Grapholita molesta (Busck). J. Insect Physiol. 25: 943–950. [Google Scholar]
- Baker T. C., Nishida R., Roelofs W. L. 1981. Close-range attraction of female oriental fruit moths to herbal scent of male hairpencils. Science 214: 1359–1361. [DOI] [PubMed] [Google Scholar]
- Birch M. C., Lucas D., White P. R. 1989. The courtship behavior of the cabbage moth, Mamestra brassicae (Lepidoptera: Noctuidae) and the role of male hair-pencils. J. Insect Behav. 2: 227–239. [Google Scholar]
- Birch M. C., Poppy G. M., Baker T. C. 1990. Scents and eversible scent structures of male moths. Annu. Rev. Entomol. 35: 25–58. [Google Scholar]
- Cardé R. T., Haynes K. F. 2004. Structure of the pheromone communication channel in moths, pp. 282–332. In Cardé R. T., Millar J. G. (eds.), Advance in insect chemical ecology. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Dedes J. 2014 Spruce budworm (Choristoneura fumiferana). Natural Resources Canada, Ottawa, Canada. Available at: http://publications.gc.ca/collections/collection_2014/rncan-nrcan/Fo124‐11‐2014-eng.pdf. [Accessed 21 January 2014].
- Delisle J., Hardy M. 1997. Male larval nutrition influences the reproductive success of both sexes of the spruce budworm, Choristoneura fumiferana (Lepidoptera: Totricidae). Func. Ecol. 11: 451–463. [Google Scholar]
- Delisle J., Simard J. 2002. Factors involved in the post-copulatory neural inhibition of pheromone production in Choristoneura fumiferana and C. rosaceana females. J. Insect. Physiol. 48: 181–188. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S. M., McNeil J. N. 1988. Male scent in lepidopteran communication: the role of male pheromone in mating behaviour of Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae). Mem. Entomol. Soc. Can. 120: 131–151. [Google Scholar]
- George J. A. 1965. Sex pheromone of the oriental fruit moth Grapholita molesta (Busck) (Lepidoptera: Totricidae). Can. Entomol. 97: 1002–1007. [Google Scholar]
- Grant G. 1978. Morphology of the presumed male pheromone glands on the forewings of tortricid and phycitid moths. Ann. Entomol. Soc. Am. 71: 423–431. [Google Scholar]
- Hendricks D., Shaver T. 1975. Tobacco budworm: male pheromone suppressed emission of sex pheromone by the female. Environ. Entomol. 4: 555–558. [Google Scholar]
- Hillier N. K., Vickers N. J. 2004. The role of heliothine hairpencil compounds in female Heliothis virescens (Lepidoptera: Noctuidae) behavior and mate acceptance. Chem. Senses. 29: 499–511. [DOI] [PubMed] [Google Scholar]
- Hillier N. K., Kelly D., Vickers N. J. 2007. A specific male olfactory sensillum detects behaviorally antagonistic hairpencil odorants. J. Insect. Sci. 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. S., Harrow I. D., Quartararo P., Hildebrand J. C. 1986. An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projections in Manduca sexta and certain other sphinx moths and silk moths. Cell Tiss. Res. 245: 237–245. [DOI] [PubMed] [Google Scholar]
- Kimura T., Honda H. 1999. Identification and possible functions of the hairpencil scent of the yellow peach moth, Conogethes punctiferalis (Guenée) (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 34: 147–153. [Google Scholar]
- Krasnoff S. B., Dussourd D. E. 1989. Dihydropyrrolizine attractants for arctiid moths that visit plants containing pyrrolizidine alkaloids. J. Chem. Ecol. 15: 47–60. [DOI] [PubMed] [Google Scholar]
- Krasnoff S. B., Roelofs W. L. 1989. Quantitative and qualitative effects of larval diet on male scent secretions of Estigmene acrea, Phragmatobia foliginosa, and Pyrrharctia isabella (Lepidoptera: Arctiidae). J. Chem. Ecol. 15: 1077–1093. [DOI] [PubMed] [Google Scholar]
- Landolt P. J., Heath R. R. 1989. Attraction of female cabbage looper moths (Lepidoptera: Noctuidae) to male-produced sex pheromone. Ann. Entomol. Soc. Am. 82: 520–525. [Google Scholar]
- Lassance J. M., Löfstedt C. 2009. Concerted evolution of male and female display traits in the European corn borer, Ostrinia nubilalis. BMC Biology 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte C., Thibout E., Pierre D., Auger J. 1998. Transfer, perception, and activity of male pheromone of Acrolepiopsis assectella with special reference to conspecific male sexual inhibition. J. Chem. Ecol. 24: 655–671. [Google Scholar]
- McMorran A. 1965. A synthetic diet for Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 97: 58–62. [Google Scholar]
- Nishida R., Baker T., Roelofs W. 1982. Hairpencil pheromone components of male oriental fruit moths, Grapholita molesta. J. Chem. Ecol. 8: 947–959. [DOI] [PubMed] [Google Scholar]
- Outram I. 1971. Aspects of mating in the spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 103: 1121–1128. [Google Scholar]
- Palaniswamy P., Seabrook W. D. 1978. Behavioural responses to the female eastern spruce budworm Choristoneura fumiferana (Lepidoptera: Tortricidae) to the sex pheromone of her own species. J. Chem. Ecol. 4: 544–551. [Google Scholar]
- Palaniswamy P., Seabrook W. D. 1985. The alteration of calling behaviour by female Choristoneura fumiferana when exposed to synthetic sex pheromone. Entomol. Exp. Appl. 37: 13–16. [Google Scholar]
- Palaniswamy P., Seabrook W. D., Ross R. 1979. Precopulatory behavior of males and perception of a potential male pheromone in spruce budworm, Choristoneura fumiferana. Ann. Entomol. Soc. Am. 72: 544–551. [Google Scholar]
- Royer L., McNeil J. N. 1992. Evidence of a male sex pheromone in the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae). Can. Entomol. 124: 113–116. [Google Scholar]
- Sanders C., Lucuik G. 1992. Mating behavior of spruce budworm moths, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 124: 273–286. [Google Scholar]
- SAS Institute . 1999. The SAS system version 8 for Windows. SAS Institute, Cary, NC. [Google Scholar]
- Silk P. J., Eveleigh E. S. 2016. Pheromone communication, behavior, and ecology in the North American Choristoneura genus. In Allison J. D., Cardé R. T. (eds.), Pheromone communication in moths: evolution, behavior and application. University of California Press, Oakland, CA, USA: (In press). [Google Scholar]
- Silk P. J., Kuenen L. 1986. Spruce budworm (Choristoneura fumiferana) pheromone chemistry and behavioral responses to pheromone components and analogs. J. Chem. Ecol. 12: 367–383. [DOI] [PubMed] [Google Scholar]
- Silk P. J., Kuenen L. 1988. Sex pheromones and behavioral biology of theconiferophagous Choristonerua. Annu. Rev. Entomol. 33: 83–101. [Google Scholar]
- Sinwell V., Schulz S., Francke W., Kittmann R., Schneider D. 1985. Identification of pheromones from the male swift moth Hepialus hecta L. Tetrahedron Letters. 26: 1707–1710. [Google Scholar]