Abstract
Transcranial Doppler (TCD) is the only diagnostic tool that can provide continuous information about cerebral hemodynamics in real time and over extended periods. In the previous paper (Part 1), we have already presented the basic ultrasound physics pertaining to TCD, insonation methods, and various flow patterns. This article describes various advanced applications of TCD such as detection of right-to-left shunt, emboli monitoring, vasomotor reactivity (VMR), monitoring of vasospasm in subarachnoid hemorrhage (SAH), monitoring of intracranial pressure, its role in stoke prevention in sickle cell disease, and as a supplementary test for confirmation of brain death.
Keywords: Cerebral circulatory arrest, emboli, intracranial pressure (ICP), ischemic stroke, sickle-cell disease, transcranial Doppler (TCD), vasomotor reactivity (VMR), vasospasm
Introduction
Most of the advanced applications of transcranial Doppler (TCD) are possible due to its ability of continuous monitoring of blood flow in an intracranial artery over extended periods.[1] Thus, the dynamic changes in cerebral hemodynamics as a result of the natural history of an intracranial disease and complications of a disease or secondary to an external provocative stimulus can be observed in real time and aid in the diagnostic, therapeutic as well as prognostic processes.[2] Some of these routinely used applications are described in the manuscript.
TCD monitoring for spontaneous emboli
A flow-limiting stenosis in a cervical or intracranial artery can produce cerebral ischemia by regional hypoperfusion, artery-to-artery embolization, or a combination of both. Extended monitoring of an intracranial artery distal to the steno-occlusive site can detect spontaneous embolic signals [microembolic signals (MES)] and quantify the embologenic potential of the atherosclerotic plaque.
The best method for monitoring of MES is to use a TCD probe fixation headframe that enables continuous recording of spectral flow from the desired intracranial artery for extended periods. In routine practice, detection of even a single MES during 40 min of monitoring is considered as clinically significant. One example is shown in Figure 1.
Figure 1.
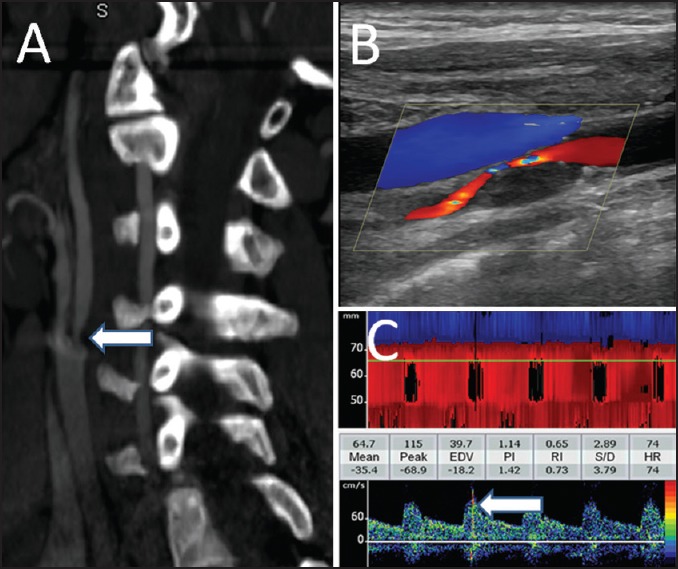
Neuroimaging findings in a patient with severe and symptomatic carotid artery stenosis. Computerized tomographic angiography of the neck shows a focal stenosis of proximal internal carotid artery (a). The plaque characteristics and flow through the residual lumen are shown on the cervical duplex ultrasonography (b). Continuous monitoring of the ipsilateral middle cerebral artery showed multiple microembolic signals (c)
The International Cerebral Hemodynamics Society describes MES as:
Random occurrence during the cardiac cycle.
Brief duration (usually <0.1 s).
High intensity (>3 dB over background).
Primarily unidirectional signal (if fast Fourier transform is used).
Audible component (chirp, whistle, or pop).
Patients with cerebrovascular ischemia or high-grade internal carotid artery (ICA) stenosis can also undergo TCD monitoring for emboli to detect and quantify cerebral embolization.[3] The presence of MES on TCD distal to a high-grade asymptomatic stenosis of internal carotid artery identifies patients at a higher risk of stroke.[4] This information may help in optimizing the management strategy. Recent studies have shown that the MES count may be reduced with 1-week treatment with dual antiplatelet agents (aspirin plus clopidogrel) versus aspirin alone, both in patients with extracranial carotid artery stenosis[5] as well as intracranial stenosis.[6]
Right-to-left shunt (RLS) detection
Patent foramen ovale (PFO) is the commonest source of RLS circulatory shunt, occurring in approximately 25% of the general population. Patients with cerebrovascular ischemia due to paradoxical embolism can undergo TCD “bubble” test to detect RLS.
TCD is considered to be more sensitive and specific than transesophageal echocardiography for RLS detection[7] as well as for quantifying its “functional-potential.”[8] Power motion mode in the currently available TCD machines further enhances the sensitivity due to the overlapping and contiguous multiple gates.
Technique
Intravenous access should be obtained with an 18-gauge needle inserted into the antecubital vein. At least one middle cerebral artery (MCA) should be insonated. The contrast mixture is prepared by mixing 9 mL normal saline, 1 mL of air, and a few drops of the patient's own blood via a three-way stopcock. At least 10-16 strong mixes are required to produce sufficient number of microbubbles. The contrast mixture is injected and continuous TCD monitoring of MCA is performed for MES. Valsalva maneuver (VM) is performed 4-6 s after the contrast injection to ensure the arrival of microbubbles into the right atrium. VM increases the right atrial pressure and facilitates the travel of microbubbles from the right atrium to the left atrium. Considerable (about 20%) reduction in MCA flow velocities indicates an adequate VM. TCD monitoring is performed for another 16-20 s. Since the RLS grade is affected by the body position during the test, we recommend performing the procedure in supine and sitting positions.[9]
RLS can be quantified by employing the following grading systems.[10]
International Consensus Criteria (ICC)
Grade 0: No MES.
Grade 1: MES count 1-10.
Grade 2: MES count 11-30, and
Grade 3: MES count more than 30 with “shower” or “curtain” appearance.
Spencer's Logarithmic Scale
Grade 0: No MES.
Grade 1: MES count 1-10.
Grade 2: MES count 11-30.
Grade 3: MES count 31-100.
Grade 4: MES count 101-300.
Grade 5: MES count more than 300.
Vasomotor reactivity (VMR)
VMR represents the response of cerebral circulation to various vasomotor stimuli for maintaining a near-constant blood flow. Vasomotor changes in response to various stimuli can be studied in real-time by TCD. Carbon dioxide (CO2) is the strongest vasodilatory stimulus to cerebral circulation. Increased levels of CO2 cause vasodilatation and the response is reflected by increased flow velocities on TCD. Formal testing of evaluating VMR requires a proper setup with controlled conditions, especially the arterial and/or end-tidal CO2 levels. A simple method for clinical use was described by Markus et al.[11] where MCA velocities are monitored during 30 s of voluntary breath-holding and calculating the breath-holding index (BHI). The subject is asked to hold his/her breath for a minimum of 30 s while MCAs were being monitored with TCD. A deep breath or gasp/sigh is not allowed before starting breath holding. Similarly, breathing is started as gentle inspiration. MCA flow velocities are noted 4 s after breathing is restarted. The following formula is used to calculate BHI:

where MFV is the mean flow velocity. BHI of <0.69 is considered to represent an impaired cerebral vasodilatory reserve (CVR) regulated by the parasympathetic nervous system. A decreased BHI represents failure of the collateral flow to maintain adequate cerebral perfusion in response to the hypercapnic challenge. It is always advisable to monitor both MCAs simultaneously. A normal flow acceleration in the contralateral (normal) MCA establishes the adequacy of hypercapnic challenge and the expected response in the ipsilateral MCA. BHI can be used to identify patients at a higher risk of stroke among the cohort of asymptomatic carotid stenosis or previously symptomatic carotid occlusion.[12] It can be used to monitor the progression of stenosis as well as to select patients for further neuroimaging and therapeutic decision-making.[13] An example of the clinical use of CVR is shown in Figure 2.
Figure 2.
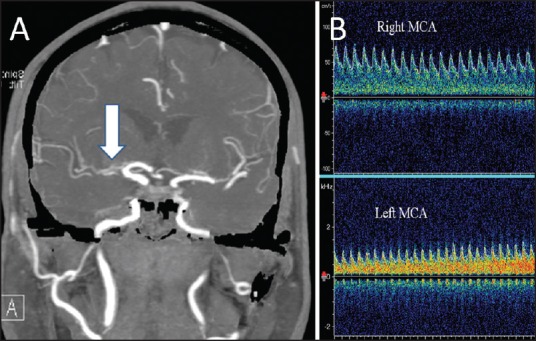
Assessment of cerebral vasomotor reactivity in a patient with large artery stenosis. Computerized tomographic angiography of the brain (a) Shows a severe stenosis of the right middle cerebral artery (MCA). Panel B shows the flow response in both MCAs. A normal flow acceleration during 30 s of voluntary breath-holding is noted in the left MCA. However, the right MCA shows a paradoxical reduction in the flow velocities, suggestive of an exhausted vasodilatory reserve with intracranial steal phenomenon (the reversed Robin Hood syndrome)
Some centers test the sympathetic nervous system control of intracranial flow too, largely for research purposes. The patient is asked to hyperventilate and MCA flow is continuously monitored. Hyperventilation induces hypocapnea that results in cerebral vasoconstriction, leading to a reduction of flow velocities. When combined with hypercapnic challenge (breath-holding), testing during hypocapnea (hyperventilation) is used to calculate cerebral VMR and calculated as:
VMR= MFVhypercapnea-MFVhypocapnea/MFVat rest × 100
A value more than 65% indicates normal VMR while the value of less than 33% reflects an exhausted VMR. VMR between 33% and 65% represents borderline impaired autonomic control.
TCD in sickle cell disease
Children with sickle-cell disease (SCD) carry a significant stroke risk, with 11% of all homozygous sickle cell (HbSS) patients developing ischemic stroke before the age of 20 years. These strokes primarily result from stenosis or occlusion of the distal intracranial internal carotid arteries and/or proximal MCA.
TCD can identify children with the highest risk of the first-ever stroke[14] and those in need of blood transfusion.[15] In a pivotal trial,[15] TCD detection of time averaged maximum mean flow velocity of 200 cm/s on two separate examinations was used to determine the need for blood transfusion that resulted in about 90% relative risk reduction of first-ever stroke.
The skill of TCD testing in children with sickle cell anemia is taught through standard tutorials with sonographers receiving specialized certificates, and diagnostic criteria for interpreting physicians are well defined. TCD is performed according to stroke prevention in sickle-cell disease (STOP) trial protocol, which is different from routine clinical TCD examination. STOP protocol uses time-averaged mean of the maximum (TAMM) velocities of the MCA, and/or TICA recorded on two separate occasions separated by at least 2 weeks.
Stroke prevention in sickle-cell disease-transcranial Doppler scanning protocol:
Transtemporal head diameter is measured and recorded. The nomogram for expected depths for the patient's head diameter is reviewed.
Sample volume is set to 6 mm.
Velocity scale is set to 200-250 cm/s.
Anterior TCD examination begins with the identification of anterior temporal window. MCA signal is acquired and tracked to as shallow a depth as possible; for most children, this should be to a depth less than 40 mm (in STOP, this depth was referred to as M1MCA, indicating the shallowest depth of the MCA that could be recorded). Optimized TCD signal is recorded. MCA stem is then “tracked” by advancing the sample volume by 2-mm increments, optimizing and recording the signal.
The MCA is “tracked” (recording in 2-mm increments of depth) to the internal carotid bifurcation (lCA). The anterior cerebral artery (ACA) is tracked to 4-6 mm deeper than the ICA bifurcation and recorded as ACA. The sample volume depth is then returned to the ICA bifurcation, the probe is angled slightly inferiorly, the depth is increased by 4-6 mm, and the Doppler flow velocity waveforms in the distal ICA are recorded, labeled as dICA.
The sample depth is then returned to the ICA bifurcation, the probe is angled slightly posteriorly until the posterior cerebral artery (PCA) is detected. The sample volume depth is then decreased to the shallowest depth at which signal can be detected. The PCA is tracked in 2 mm increments to the midline, where bidirectional flow from top of the basilar (TOB) is recorded.
The opposite side is then examined (through the transtemporal window) and recorded using this same technique.
Posterior examination was performed as per the standard TCD scanning protocol. As stenoses only very rarely affect the vertebrobasilar system in children with sickle-cell disease, high velocities here were generally secondary to increased volume flow (collateralization of flow).
Ophthalmic TCD was not part of the STOP protocol.
STOP classification for risk stratification and treatment strategy:
Normal: TAMM velocity <170 cm/s — a repeat assessment is indicated.
Conditional: TAMM 170-200 cm/s in the MCA and/or terminal ICA — in children with no previous records, a repeat TCD is planned in about 2 weeks
Abnormal: TAMM >200 cm/s in the MCA and/or terminal ICA — urgent blood transfusion is arranged.
TCD as supplementary test for confirmation of brain death
Clinical evaluation of brain-stem functions with various provocative measures is the gold standard for the diagnosis of brain death.[16] However, the diagnosis of brain death may be delayed considerably if only clinical assessments are considered. This phenomenon could have serious implications, especially in centers involved in aggressive organ transplantation programs.
On its own, TCD cannot be used to diagnose brain death since this is a clinical diagnosis. TCD can be used to help in diagnosing cerebral circulatory arrest in adults and children (older than 6 months). TCD can be used to monitor progressive flow changes toward cerebral circulatory arrest. Once a reverberating signal is found, it should be monitored for at least 30 min in the both MCAs and the basilar artery (BA) to avoid false-positive findings. A prolonged absence of brain perfusion can be detected using an oscillating (reverberating) flow pattern, systolic spike, or absent flow signals, and this process will lead to brain death.
Scanning protocol and algorithm if cerebral circulatory arrest is suspected:
Document arterial blood pressure at the time of TCD examination.
Assess both MCAs (starting depth 50 mm) and BA (80 mm).
Positive MCA or BA end-diastolic flow is found = No cerebral circulatory arrest.
Absent end-diastolic flow = Uncertain cerebral circulatory arrest (too early or too late).
Reversed minimal end-diastolic flow = Possible cerebral circulatory arrest [continue monitoring, document diastolic blood pressure (BP) ≥50 mmHg].
Reverberating flow = Probable cerebral circulatory arrest (confirm in both MCAs at 50-60 mm and BA at 80-90 mm).
An example of cerebral circulatory arrest on TCD is shown in Figure 3.
Figure 3.
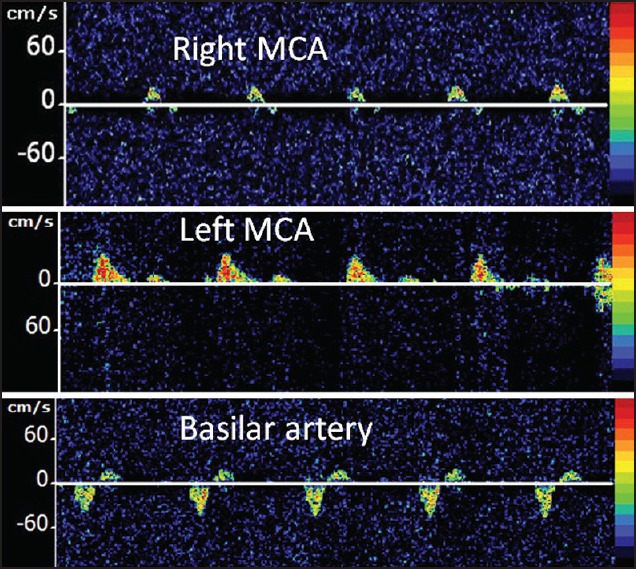
TCD findings from a 6-year-old male who underwent abdominal surgery for perforated bowel, which was complicated by recurrent episodes of ventricular fibrillation and cardiac arrest, requiring cardiopulmonary resuscitation. This TCD was performed on day 4 due to absent pupilary reflex and poor gag reflex. Alternating flow spectra were obtained from the right middle cerebral artery (MCA) and basilar artery while left MCA demonstrated only systolic spikes, suggestive of cerebral circulatory arrest
TCD monitoring of vasospasm in subarachnoid hemorrhage
Arterial vasospasm is a complication of subarachnoid hemorrhage (SAH), which becomes symptomatic in more than 25% of patients leading to a delayed ischemic deficit (DID).
TCD can be used to detect asymptomatic vasospasm onset, follow spasm progression and facilitate triple-H-therapy, identify patients with severe vasospasm, monitor the effect of therapies and interventions, and detect spasm resolution.
Various studies have shown the usefulness of TCD in diagnosing vasospam in anterior and posterior circulations following SAH.[17,18] More specifically, TCD can detect the development of vasospasm days before it can become clinically apparent (days 2-5 following SAH onset), and this information can be used by the treating physician. After vasospasm is diagnosed, TCD can help in monitoring its progression and response to various therapeutic measures as well as selecting patients for additional endovascular interventions.
Sloan et al. showed that TCD was highly specific (100%) for vertebral and basilar artery vasospasm when mean flow velocities were ≥80 cm/s and ≥95 cm/s, respectively.[19] Based on the available evidence, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology has recently stated that TCD is useful for the detection of vasospasm following spontaneous SAH.
Suggested protocol
First, TCD should be obtained as soon as possible after the diagnosis of SAH. This would include a complete standard TCD with additional flow velocities obtained from the cervical internal carotid arteries (ICA) using the TCD probe via submandibular approach. Flow velocities obtained from the extracranial ICA are used to calculate the Lindegaard's ration (defined as the ration of MFV of MCA and submandibular ICA). The flow velocities obtained from the cervical ICA via a duplex machine are not acceptable since these are obtained at about 60° insonation angle.
TCD evaluations using a standardized protocol should be used on a daily (or even more frequently) basis to monitor the progress of vasospasm.
In patients with vasospasm, especially if a change in the treatment approach was made due to TCD findings, TCD should be repeated within 6-8 h to evaluate the effect of treatment.
Information about intracranial pressure, if available, should be taken into account during the interpretation of TCD findings.
In case of doubtful findings, other imaging modalities should be employed.
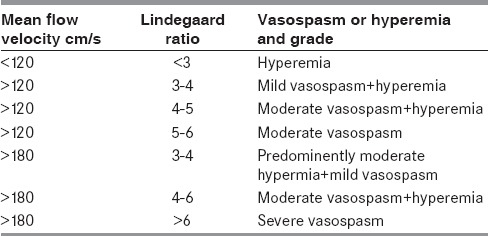
The established diagnostic criteria (Table 1 shows the diagnostic criteria for MCA vasospasm used at National University Hospital, Singapore) should be used for the diagnosis and monitoring of vasospasm.
Table 1.
Suggested diagnostic criteria for diagnosis and quantification of vasospasm in the middle cerebral artery by transcranial Doppler
TCD monitoring of intracranial pressure (ICP)
Depending upon the clinical requirement, ICP measurement or monitoring is performed via intraventricular drain, hollow skull bolt, or lumbar puncture. However, the invasive nature of these methods limits their routine use. TCD is noninvasive that can evaluate cerebral blood flow hemodynamics in major intracranial arteries at the bedside. TCD-derived pulsatility index (PI), calculated as the difference between flow velocities measured during systole (Vs) and diastole (Vd), divided by the mean flow velocity (Vm) [(Vd-Vs)/Vm], provides useful information about ICP. However, this relationship is complex and dependent on a number of physiological factors and disease states. In a recent study, we performed TCD simultaneously with lumbar puncture and looked into the relationship between TCD-PI and opening pressure.[20] We found that TCD-PI has acceptable accuracy parameters in differentiating patients with ICP of more than 20 cm of cerebrospinal fluid. Interestingly, serial estimations in a single patient over short period of time are reliable for an indirect assessment of longitudinal changes in ICP. An example for the use of TCD in the assessment and monitoring of ICP is shown in Figure 4.
Figure 4.
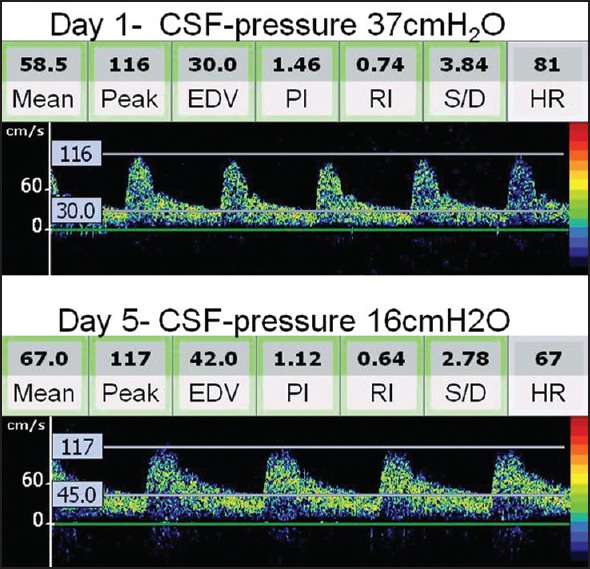
Serial transcranial Doppler (TCD) spectra and intracranial pressure (ICP) monitoring in a patient with encephalitis. TCD performed on day 2 shows a high resistance Doppler spectra [note the pulsatility index (PI) as 1.46]. Clinical improvement in this patient was associated with reduction of ICP (16 cm of water) and normalization of TCD-derived PI to 1.12
Conclusion
TCD provides real-time information about cerebral hemodynamics and permits extended monitoring with excellent temporal resolution. Advanced applications of TCD are integral parts of the armamentarium of neurologists for evaluating various mechanisms of cerebral ischemia. Furthermore, TCD helps in planning and monitoring the disease process, effectiveness of treatment as well as aids in establishing the prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sharma VK, Tsivgoulis G, Lao AY, Alexandrov AV. Role of transcranial Doppler ultrasonography in evaluation of patients with cerebrovascular disease. Curr Neurol Neurosci Rep. 2007;7:8–20. doi: 10.1007/s11910-007-0016-4. [DOI] [PubMed] [Google Scholar]
- 2.Yeo LL, Sharma VK. Role of transcranial Doppler ultrasonography in cerebrovascular disease. Recent Pat CNS Drug Discov. 2010;5:1–13. doi: 10.2174/157488910789753576. [DOI] [PubMed] [Google Scholar]
- 3.Spencer MP, Thomas GI, Nicholls SC, Sauvage LR. Detection of middle cerebral artery emboli during carotid endarterectomy using transcranial Doppler ultrasonography. Stroke. 1990;21:415–23. doi: 10.1161/01.str.21.3.415. [DOI] [PubMed] [Google Scholar]
- 4.King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: A systematic review and meta-analysis. Stroke. 2009;40:3711–7. doi: 10.1161/STROKEAHA.109.563056. [DOI] [PubMed] [Google Scholar]
- 5.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 6.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. CLAIR Study Investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): A randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–97. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 7.Jauss M, Kaps M, Keberle M, Haberbosch W, Dorndorf W. A comparison of transesophageal echocardiography and transcranial Doppler sonography with contrast medium in the detection of patent foramen ovale. Stroke. 1994;25:1265–7. doi: 10.1161/01.str.25.6.1265. [DOI] [PubMed] [Google Scholar]
- 8.Belvís R, Leta RG, Martí-Fàbregas J, Cocho D, Carreras F, Pons-Lladó G, et al. Almost perfect concordance between simultaneous transcranial Doppler and transesophageal echocardiography in the quantification of right-to-left shunts. J Neuroimaging. 2006;16:133–8. doi: 10.1111/j.1552-6569.2006.00021.x. [DOI] [PubMed] [Google Scholar]
- 9.Lao AY, Sharma VK, Tsivgoulis G, Malkoff MD, Alexandrov AV, Frey JL. Effect of body positioning during transcranial Doppler detection of right-to-left shunts. Eur J Neurol. 2007;14:1035–9. doi: 10.1111/j.1468-1331.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 10.Lao AY, Sharma VK, Tsivgoulis G, Frey JL, Malkoff MD, Navarro JC, et al. Detection of right-to-left shunts: Comparison between the international consensus and spencer logarithmic scale criteria. J Neuroimaging. 2008;18:402–6. doi: 10.1111/j.1552-6569.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 11.Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23:668–73. doi: 10.1161/01.str.23.5.668. [DOI] [PubMed] [Google Scholar]
- 12.Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–7. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- 13.Low SW, Teo K, Lwin S, Yeo LL, Paliwal PR, Ahmad A, et al. Improvement in cerebral hemodynamic parameters and outcomes after superficial temporal artery-middle cerebral artery bypass in patients with severe stenoocclusive disease of the intracranial internal carotid or middle cerebral arteries. J Neurosurg. 2015;123:662–9. doi: 10.3171/2014.11.JNS141553. [DOI] [PubMed] [Google Scholar]
- 14.Adams R, McKie V, Nichols F, Carl E, Zhang DL, McKie K, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–10. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 15.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 16.Practice parameters for determining brain death in adults (summary statement). The Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1995;45:1012–4. doi: 10.1212/wnl.45.5.1012. [DOI] [PubMed] [Google Scholar]
- 17.Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, Mesiwala AH, et al. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59:360–6. doi: 10.1227/01.NEU.0000223502.93013.6E. [DOI] [PubMed] [Google Scholar]
- 18.Sloan MA, Haley EC, Jr, Kassell NF, Henry ML, Stewart SR, Beskin RR, et al. Sensitivity and specificity of transcranial Doppler Utrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39:1514–8. doi: 10.1212/wnl.39.11.1514. [DOI] [PubMed] [Google Scholar]
- 19.Sloan MA, Burch CM, Wozniak MA, Rothman MI, Rigamonti D, Permutt T, et al. Transcranial Doppler detection of vertebrobasilar vasospasm following subarachnoid hemorrhage. Stroke. 1994;25:2187–97. doi: 10.1161/01.str.25.11.2187. [DOI] [PubMed] [Google Scholar]
- 20.Wakerley BR, Kusuma Y, Yeo LL, Liang S, Kumar K, Sharma AK, et al. Usefulness of transcranial Doppler-derived cerebral hemodynamic parameters in the noninvasive assessment of intracranial pressure. J Neuroimaging. 2015;25:111–6. doi: 10.1111/jon.12100. [DOI] [PubMed] [Google Scholar]


