Abstract
Context:
Retinal migraine (RM) is considered as one of the rare causes of transient monocular visual loss (TMVL) and has not been studied in Indian population.
Objectives:
The study aims to analyze the clinical and investigational profile of patients with RM.
Materials and Methods:
This is an observational prospective analysis of 12 cases of TMVL fulfilling the International Classification of Headache Disorders-2nd edition (ICHD-II) criteria of RM examined in Neurology and Ophthalmology Outpatient Department (OPD) of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh from July 2011 to October 2012.
Results:
Most patients presented in 3rd and 4th decade with equal sex distribution. Seventy-five percent had antecedent migraine without aura (MoA) and 25% had migraine with Aura (MA). Headache was ipsilateral to visual symptoms in 67% and bilateral in 33%. TMVL preceded headache onset in 58% and occurred during headache episode in 42%. Visual symptoms were predominantly negative except in one patient who had positive followed by negative symptoms. Duration of visual symptoms was variable ranging from 30 s to 45 min. None of the patient had permanent monocular vision loss. Three patients had episodes of TMVL without headache in addition to the symptom constellation defining RM. Most of the tests done to rule out alternative causes were normal. Magnetic resonance imaging (MRI) brain showed nonspecific white matter changes in one patient. Visual-evoked potential (VEP) showed prolonged P100 latencies in two cases. Patent foramen ovale was detected in one patient.
Conclusions:
RM is a definite subtype of migraine and should remain in the ICHD classification. It should be kept as one of the differential diagnosis of transient monocular vision loss. We propose existence of “acephalgic RM” which may respond to migraine prophylaxis.
Keywords: Acephalgic retinal migraine, headache, ocular migraine, retinal migraine, transient monocular visual loss
Introduction
Galezowski first introduced the concept of “ophthalmic megrim” in four patients with permanent monocular visual loss temporally related to migrainous headache in 1882.[1] The term “retinal migraine” (RM) was coined by Carroll in 1970.[2] It is attributed to vasospasm within the retinal or ciliary circulation. It is also known by different names such as “anterior visual pathway migraine,” “ophthalmic migraine,” “monocular migraine”, or “ocular migraine” as unilateral visual loss may not be restricted exclusively to the retina and may involve choroid and optic nerve.[3,4]
RM is a rare entity whose prevalence is unknown. RM is included in International Classification of Headache Disorders-2nd edition (ICHD-II) as a subtype of migraine and is a diagnosis of exclusion.[5] The existence of this entity is controversial and some advocate removal of this from ICHD. However, recently ICHD-3 criteria beta version has been introduced and RM is included in it.[6]
With this background in mind, the present study was aimed to study the causes of transient monocular visual loss (TMVL) in Outpatient Department (OPD) and to study the clinical and investigational profile of patients with RM.
Materials and Methods
This is an observational study which prospectively analyzed 12 patients of RM who presented in Neurology and Ophthalmology OPD of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh from July 2011 to October 2012. Patients presenting with TMVL with migrainous headache were screened and patient fulfilling the ICHD-II criteria[5] for RM (1.4) [Figure 1] were included in the study. Institutional ethical committee permission was obtained.
Figure 1.
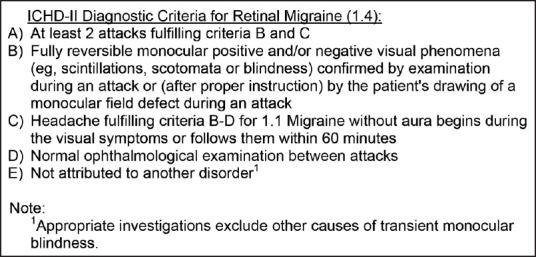
ICHD-II diagnostic criteria for retinal migraine (1.4). ICHD-II = International Classification of Headache Disorders-2nd edition
Patients in whom the transient monocular visual symptoms can be better explained by some other cause as per abnormalities in the investigations were excluded from the study. A questionnaire-based direct interview was used to collect information on disease profile, demographic variables, risk factors, and medications used. Clinical parameters noted were age, sex of the patient, age of onset, characteristics of the visual symptoms, blood pressure, and duration and frequency of the attacks of migraine headache and RM. Investigations performed were complete blood count, erythrocyte sedimentation rate (ESR), prothrombin time (PT), activated partial thromboplastin time (aPTT), fasting blood glucose (FBS), lipid profile, antinuclear antibody (ANA), antineutrophilic cytoplasmic antibodies (ANCA), rheumatoid factor (RF), antiphospholipid antibodies (APLA), renal function test, liver function test, magnetic resonance imaging (MRI) brain with MR angiography (MRA) of intracranial and neck vessels, digital subtraction angiography (DSA) if required, carotid Doppler study, transcranial Doppler (TCD) for right-to-left shunt (RLS), transthoracic echocardiography (TTE), transesophageal echocardiography (TEE) in case of detection of RLS in TCD, visual field charting, visual acuity, direct ophthalmoscopic examination, and tonometry. Visual testing was performed as soon as possible after the onset of TMVL and not at the time of attack.
Results
Over the 1.5 year period of the study, 20 patients of TMVL with headache were encountered, of these 12 patients were diagnosed as RM and included in the study. The remaining eight were excluded from the study as TMVL and headache was found to be due to alternative causes on the basis of investigations and detailed ophthalmological evaluation [Figure 2].
Figure 2.
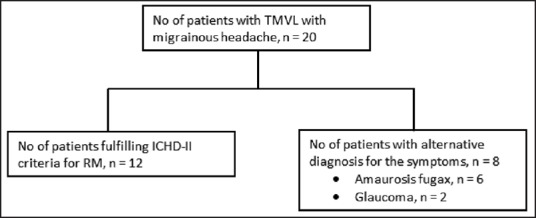
Causes of transient monocular visual symptoms with migrainous headache. TMVL = Transient monocular visual loss, RM = Retinal migraine
Baseline characteristics, demographic profile, and clinical features are depicted in Table 1. Of 12 cases diagnosed as RM, sex distribution was equal (male (M) = female (F)) with mean age of 33 years (range 15-55 years). Majority of patients in 4th decade were males with female patients showing more even distribution with peak in 2nd and 5th decade. Thirty-three percent (4/12) patients had family history of migraine, but no family history of RM was noted in study population. No history of any precipitating cause for visual symptoms was identified. All patients had episodic headaches except one patient who had associated chronic migraine.
Table 1.
Baseline demographic profile, clinical features, and selected investigations of 12 cases with RM
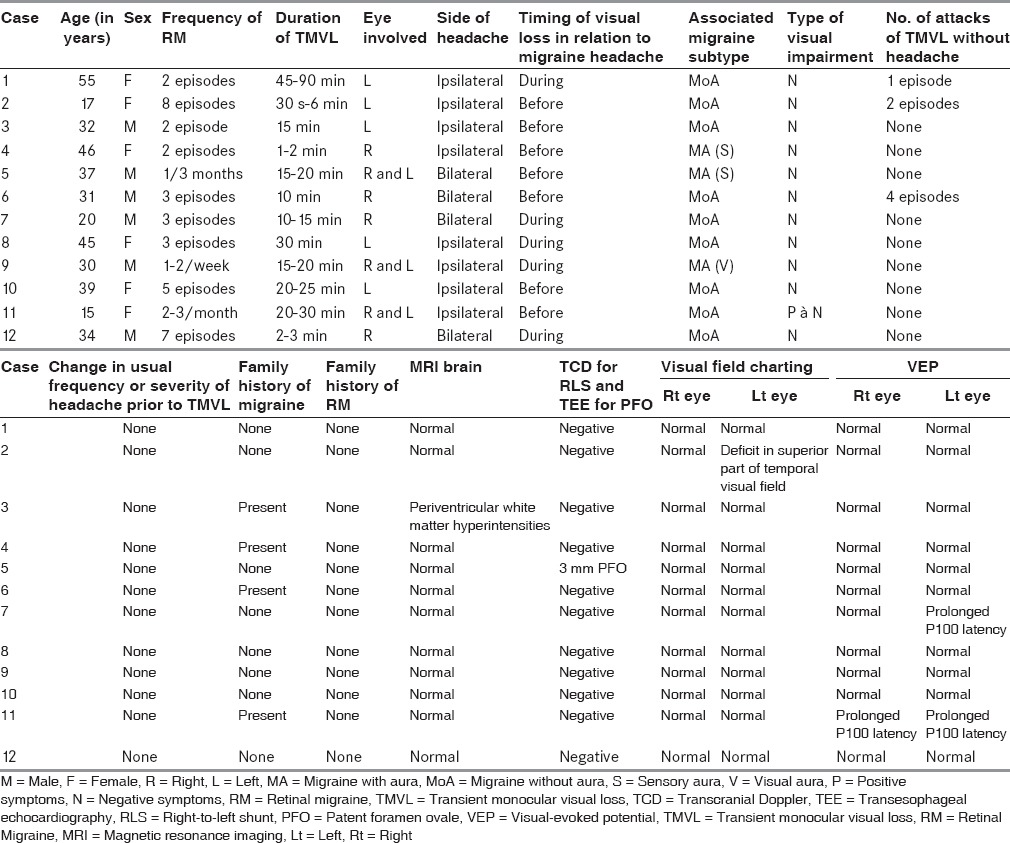
Clinical features
Eleven patients out of the study population of 12 had a history of migraine headache, migraine without aura (MoA), or migraine with aura (MA). One patient (case 2) had developed two episodes of unexplained TMVL without headache when she presented; but from third attack of TMVL, she started having headaches fulfilling criteria for both MoA and RM. Including case 2, 75% had MoA and 25% had MA. Aura was sensory in two patients, whereas one patient had visual aura with fortification spectra in both eyes followed by headache and TMVL occurring simultaneously. Headache was ipsilateral to visual symptoms in 67% and bilateral in 33%. Seventy-five percent (9/12) patients had TMVL limited consistently to one side, while rest had involvement of either side during separate attacks. One patient (case 11) had positive phenomenon in the form of monocular flashes of light followed by loss of vision and headache. Rest 11 patients had negative phenomenon in the form of scotoma, blurring of vision, and partial or complete blackouts. Visual field defects as described by patients were altitudinal or quadrantic. There was complete resolution of visual symptoms in all patients.
The transient monocular visual symptoms preceded the onset of headache in 58% (7/12) and occurred during headache in 42% (5/12). Onset of TMVL after resolution of migraine headache was not noted in any patient. Three patients had episodes of TMVL without associated headache in addition to the attacks fulfilling criteria for RM. In case 2, as noted, initial two episodes of TMVL were not associated with headache, but patient was later diagnosed as RM based on subsequent attacks and she responded to usual migraine therapy.
The duration of visual symptoms showed marked variability ranging from 30 s to 45 min. The duration of visual symptoms was less than 30 min, except in one patient who had only one episode lasting 90 min (case 1). Frequency of RM was variable with most patients not showing any fixed pattern of recurrence. Only three patients (case 5, 9, and 11) showed a consistent pattern of recurrence as depicted in Table 1. No change in frequency or severity of preexisting migraine headache was noted prior to attack of RM.
Investigations
Complete blood count was normal in all patients, except two patients who showed mild anemia (hemoglobin (Hb)-11g/dl). Renal function test, liver function test, FBS, lipid profile, serum electrolytes coagulation profile, and ESR were within normal limits. ANA, ANCA, RA factor, and APLA were negative in all patients. MRI brain was normal in all but one patient (case 3) showing nonspecific periventricular white matter hyperintensities. MRA intracranial and neck vessels was normal. Ophthalmological evaluation was done between attacks. Direct ophthalmoscopy, visual acuity, and tonometry were normal in all patients. Perimetry in case 2 showed deficit in superior part of temporal visual field. Visual-evoked potential (VEP) showed prolongation of P100 latency in left (symptomatic) side in case 7 and in both sides (both eyes symptomatic) in case 11. One patient had positive findings for RLS in TCD study that was further evaluated by TEE which showed a 3 mm sized patent foramen ovale (PFO).
Discussion
RM is an extremely rare cause of transient monocular vision loss and is a diagnosis of exclusion; as elaborated by Hill et al.,[7] Table 2 lists the other important differential diagnosis of TMVL.[8]
Table 2.
Differential diagnosis of transient monocular visual loss[8]
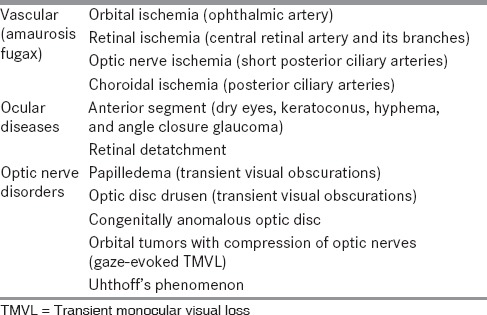
We included only those patients who met the criteria for ICHD-II classification of RM and after excluding all other possible causes of TMVL.
The paper by Grosberg et al.,[9] is one of the most comprehensive reviews of RM in which he described six new cases and reviewed cases fulfilling the criteria for RM which were reported in literature. For comparison we included patients with transient visual symptoms, while excluding the patients with PMVL. The comparison is depicted in Table 3.
Table 3.
Comparison between observations in present study and subgroup of patients with TMVL in the review by Grosberg et al.[9]
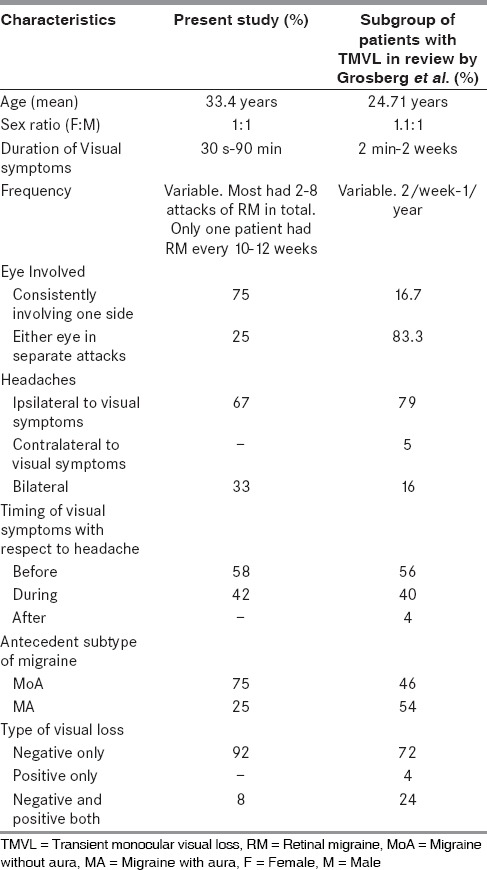
The demographic characteristics of the RM seen in our patients differed from the common types of migraine, MoA, and MA. The average age of onset is later with most patients in their 3rd and 4th decade when compared with adolescent age of onset in the common migraine types. Sex distribution was equal with F:M being 1:1, which is in contrast to 3:1 in the common migraine types.[10] These findings are comparable with previous reports [Table 3]. Family history of RM was not noted in the study population. Two families with familial RM have been described by Lewinshtein et al.[11]
The duration of visual symptoms in present study ranged from 30 s to 45 min which was within limit set by ICHD- II criteria. One patient had only one episode lasting more than 60 min reversible symptoms lasting 2 weeks have been reported by Grosberg et al.[9] Attacks of RM were comparatively infrequent in Indian population as noted in Table 3.
No change in frequency or severity of preexisting migraine headache was noted prior to attack of RM. Such phenomenon has been observed in ophthalmoplegic migraine.[12]
Majority of our patients (75%) had MoA as the antecedent migraine type, whereas MA (54%) was more common in the review by Grosberg et al.[9] The side of headache was predominantly ipsilateral in both studies [Table 3]. Visual symptoms occurred either just before (58%) or during (42%) headache. Onset of visual symptoms after the migraine headache was not seen in present study, but was reported in one patient in the review by Grosberg et al. [Table 3]. Visual symptoms were predominantly negative with one patient showing positive symptom followed by negative symptoms. Similar predominance of negative symptoms was demonstrated in previous reports [Table 3].
MA is the most common cause of transient visual disturbance, especially in patients less than 45 years.[13] Migrainous aura occurs in a hemianopic distribution and involves both the eyes compared to vision loss of RM which is monocular and develops rapidly within 5 min.[14] Out of the three patients with MA seen in our study, only one patient had visual aura in the form of fortification spectra in both the eyes. We included only those patients who had monocular visual defect either on the basis of history or by making a drawing wherever possible. All our patients had almost acute onset vision loss which developed rapidly and disappeared within 45 min.
Of 12 patients, three patients also had TMVL in absence of headaches. One patient, at onset of symptoms, presented just with TMVL and later evolved to have RM fulfilling the ICHD-II criteria. Hence, we propose the term “acephalgic retinal migraine” for such attacks and this entity should be considered in patients with unexplained TMVL, especially if patient is young, has a history of migraine headache, or does not have any risk factor for atherosclerotic vascular disease.
Neither did we include patients with permanent visual loss with migraine, nor did any patient of RM develop permanent visual loss in subsequent attacks. One patient showed a defect in perimetry examination, which corresponded to the field of vision involved during TMVL. VEP showed prolonged P100 latencies in two patients. In one patient the symptomatic eye (left) showed the abnormality and in another patient having TMVL in either eye in separate attacks, both eyes showed abnormalities. This reflects possible subclinical damage to retina during RM, which may accumulate after recurrent attacks. In comparison, Grosberg et al.,[9] described permanent monocular visual loss in 43% of patients. Only a long-term follow-up of patients can answer the question.
Ophthalmological examination was done in between attacks of RM and was found to be normal in all patients. Few cases of retinal artery spasm have been reported when examined during the episode of RM.
Only one patient having RM and MoA showed white matter changes in MRI brain, which were consistent with those described with migraine. PFO was detected in only one case, which is considerably less than general population, and hence does not point to any causative relationship.[15]
The pathogenesis of the transient visual symptoms is not clear. Transient compromise of blood supply to retina or possibly optic nerve head due to vasoconstriction is the most plausible explanation.[16,17] Retinal spreading depression akin to the well-described cortical spreading depression is also a hypothesis which was put forward based on experiments on frog and chick retina.[18,19,20] Occasionally, optic nerve and retinal infarction can occur due to retinal vascular changes secondary to retinal spreading depression.[21]
No drug trial has been reported in RM. Most of our patients responded to standard migraine prophylaxis. Aspirin has been reported anecdotally to be effective[22] and was prescribed to all study population. Considering vasospasm as the root cause of symptoms, some advocate use of verapamil, flunarizine, and nifedipine[23] for prophylaxis; and inhaled amyl nitrate therapy[24] for acute headache. Triptans and ergots are to be avoided because of their vasoconstrictive properties.
Our study has few limitations. Firstly, the sample size was very small. Secondly, ophthalmological examination was not conducted during the episodes of transient monocular vision loss. Thirdly, vasospasm of retinal or ciliary circulation was not documented in any of the patients.
In conclusion, though a rare entity, RM is one of the causes of TMVL with headache, especially in young patients without atherosclerotic risk factors. It should be considered in the differential diagnosis of transient monocular vision loss. It is a definite subtype of migraine and should remain in the ICHD classification as has been recently included in the ICHD-3 beta classification [Table 4].[6]
Table 4.
Updated diagnostic criteria for retinal migraine: International Classification of Headache Disorders (ICHD)-3 beta

Unexplained TMVL may represent “acephalgic RM” and treatment trial with migraine prophylaxis should be offered. Long-term follow-up studies are required to answer the questions regarding PMVL in RM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Galezowski X. Ophthalmic megrim: An affection of the vasomotor nerves of the retina and retinal centre which may end in a thrombosis. Lancet. 1882;1:176–9. [Google Scholar]
- 2.Carroll D. Retinal migraine. Headache. 1970;10:9–13. doi: 10.1111/j.1526-4610.1970.hed1001009.x. [DOI] [PubMed] [Google Scholar]
- 3.Corbett JJ. Neuro-ophthalmic complications of migraine and cluster headaches. Neurol Clin. 1983;1:973–95. [PubMed] [Google Scholar]
- 4.Walsh Fb, WFja H. Clinical Neuro-ophthalmology. In: Walsh FB, Hoyt WF, editors. 3rd ed. Vol. 2. Baltimore: Williams and Wilkins; 1969. p. 1671. [Google Scholar]
- 5.Lance JW, Olesen J. 2nd ed. Vol. 24. Cephalalgia; 2004. The International Classification of headache disorders; pp. 9–160. [Google Scholar]
- 6.Olesen J. ICHD-3 beta is published. Use it immediately. Cephalalgia. 2013;33:627–8. doi: 10.1177/0333102413487610. [DOI] [PubMed] [Google Scholar]
- 7.Hill DL, Daroff RB, Ducros A, Newman NJ, Biousse V. Most cases labeled as “retinal migraine” are not migraine. J Neuroophthalmol. 2007;27:3–8. doi: 10.1097/WNO.0b013e3180335222. [DOI] [PubMed] [Google Scholar]
- 8.Biousse V, Newman NJ. Vision loss: Overview. Semin Neurol. 2007;27:199–210. doi: 10.1055/s-2007-979679. [DOI] [PubMed] [Google Scholar]
- 9.Grosberg BM, Solomon S, Friedman DI, Lipton RB. Retinal migraine reappraised. Cephalalgia. 2006;26:1275–86. doi: 10.1111/j.1468-2982.2006.01206.x. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in United States: Data from the American Migraine Study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewinshtein D, Shevell MI, Rothner AD. Familial retinal migraines. Pediatr Neurol. 2004;30:356–7. doi: 10.1016/j.pediatrneurol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Lal V, Sahota P, Singh P, Gupta A, Prabhakar S. Ophthalmoplegia with migraine in adults: Is it ophthalmoplegic migraine? Headache. 2009;49:838–50. doi: 10.1111/j.1526-4610.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 13.Tippin J, Corbett JJ, Kerber RE, Schroeder E, Thompson HS. Amaurosis fugax and ocular infarction in adolescents and young adults. Ann Neurol. 1989;26:69–77. doi: 10.1002/ana.410260111. [DOI] [PubMed] [Google Scholar]
- 14.Kowacs PA, Utiumi MA, Piovesan EJ. The visual system in migraine: From the bench side to the office. Headache. 2015;55:84–98. doi: 10.1111/head.12514. [DOI] [PubMed] [Google Scholar]
- 15.Diener HC, Kurth T, Dodick D. Patent foramen ovale and migraine. Curr Pain Headache Rep. 2007;11:236–40. doi: 10.1007/s11916-007-0196-2. [DOI] [PubMed] [Google Scholar]
- 16.Doyle E, Vote BJ, Casswell AG. Retinal migraine: Caught in the act. Br J Ophthalmol. 2004;88:301–2. doi: 10.1136/bjo.2003.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killer HE, Forrer A, Flammer J. Retinal vasospasm during an attack of migraine. Retina. 2003;23:253–4. doi: 10.1097/00006982-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Gouras P. Spreading depression of activity in amphibian retina. Am J Physiol. 1958;195:28–32. doi: 10.1152/ajplegacy.1958.195.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Martins-Ferreira H, de Castro GO. Light-scattering changes accompanying spreading depression in isolated retina. J Neurophysiol. 1966;29:715–26. doi: 10.1152/jn.1966.29.4.715. [DOI] [PubMed] [Google Scholar]
- 20.Martins-Ferreira H, Nedergaard M, Nicholson C. Perspectives on spreading depression. Brain Res Brain Res Rev. 2000;32:215–34. doi: 10.1016/s0165-0173(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 21.Codeluppi L, Bonifacio G, Chiari A, Ariatti A, Nichelli PF. Optic nerve involvement in retinal migraine. Headache. 2015;55:562–4. doi: 10.1111/head.12481. [DOI] [PubMed] [Google Scholar]
- 22.Gan KD, Mouradian MS, Weis E, Lewis JR. Transient monocular visual loss and retinal migraine. CMAJ. 2005;173:1441–2. doi: 10.1503/cmaj.050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterkorn JM, Kupersmith MJ, Wirtschafter JD, Forman S. Brief report: Treatment of vasospastic amaurosis fugax with calcium-channel blockers. N Engl J Med. 1993;329:396–8. doi: 10.1056/NEJM199308053290604. [DOI] [PubMed] [Google Scholar]
- 24.Walter JR, Birchfield WJ. Ocular migraine in a young man resulting in unilateral blindness and retinal oedema. J Pediatric Ophthalmol. 1971;8:173–6. [Google Scholar]


