Abstract
Background:
To report the effectiveness and safety of intravenous (IV) levetiracetam (LEV) in the treatment of critically ill children with acute repetitive seizures and status epilepticus (SE) in a children's hospital.
Materials and Methods:
We retrospectively analyzed data from children treated with IV LEV.
Results:
The mean age of the 108 children was 69.39 ± 46.14 months (1-192 months). There were 58 (53.1%) males and 50 (46.8%) females. LEV load dose was 28.33 ± 4.60 mg/kg/dose (10-40 mg/kg). Out of these 108 patients, LEV terminated seizures in 79 (73.1%). No serious adverse effects were observed but agitation and aggression were developed in two patients, and mild erythematous rash and urticaria developed in one patient.
Conclusion:
Antiepileptic treatment of critically ill children with IV LEV seems to be effective and safe. Further study is needed to elucidate the role of IV LEV in critically ill children.
Keywords: Critically ill children, effective, safe, levetiracetam (LEV)
Introduction
Levetiracetam (LEV) was approved in 2006 by the United States Food and Drug Administration for use in individuals less than 16 years of age. LEV is effective in both partial and generalized seizures.[1]
The treatment of critically ill patients in intensive care unit (ICU) and pediatric clinics with acute repetitive seizures (ARSs) or status epilepticus (SE) with classical antiepileptic dugs (AEDs) may have some limitations. Phenytoin may cause idiosyncratic drug rash, cardiovascular depression, hypotension, bradyarrhythmias, and thrombocytopenia.[2] Valproic acid may cause thrombocytopenia, pancreatitis, and hepatotoxicity.[3] LEV is one of the newer AEDs, which is now widely used in the treatment of childhood epilepsy. It has linear pharmacokinetics and low extent of metabolism. It is almost completely absorbed via oral route and it is unlikely to interact with other AEDs.[1] In comparison with other IV anticonvulsants, LEV has a few known adverse side effects, and thus it is potentially useful in treating critically ill pediatric patients. In the literature, there are a few data about its safety and efficacy in children.[4,5,6,7]
Here, we report our experience of using IV LEV in critically ill children.
Materials and Methods
This study is a retrospective case series of critically ill children who received IV LEV for the treatment of ARS or SE at our hospital between January 2011 and August 2014. Electroencephalography (EEG) was performed on critically ill patients who were kept in the ICU to exclude nonconvulsive seizures or nonconvulsive SE. We reviewed the medical records of those patients, retrospectively. Clinical and laboratory data such as age, gender, seizure type, etiology, mental retardation, neurologic deficits, previous history of epilepsy, the dosage of LEV, duration of therapy, side effects, length of ICU stay, number of oral AEDs, concomitant AEDs, and drug interactions obtained from neuroimaging findings, EEG findings, treatment response, renal and liver function test results, and hemogram were recorded.
ARS was defined as repeated myoclonic, clonic, tonic, or tonic-clonic seizures (each seizure lasting less than 5 min with recovery of consciousness between each seizure) that had persisted for at least 30 min irrespective of whether they had been treated with any emergency medication (benzodiazepine alone or benzodiazepine with either phenytoin or phenobarbital) or not. Convulsive SE was defined as a single and prolonged tonic-clonic seizure that had persisted for over 5 min irrespective of whether the child had received any emergency medication or not. Response to IV LEV was defined as complete termination of the seizure. Successful termination of the seizure was defined as occurring when all convulsive activities stopped within 10 min of completion of the infusions and did not recur within the following 24 h.
In our pediatric ICU, IV midazolam and diazepam are administered to individuals with ARS as the first step of treatment. If the seizures are not stopped even after this treatment, one or more of the second-line drugs, such as phenobarbital, phenytoin, valproate, and LEV, are used. LEV is preferred as the first treatment option in patients with coexisting comorbidities such as respiratory depression, cardiac arrhythmia, hepatic failure, and thrombocytopenia. Dosage of LEV was adapted to renal function, that is, it was reduced to 50% and 30% in the cases of clearances <30 mL/min and 10 mL/min, respectively. Maintenance dosage reduction is recommended in patients with renal impairment. IV LEV was infused with a loading dose of 10 mg/kg or 20 mg/kg over a period of 15 min depending on the child neurologist's instruction. The children underwent cardiopulmonary monitoring during the treatment. The serum LEV concentration could not be performed in our hospital.
Results
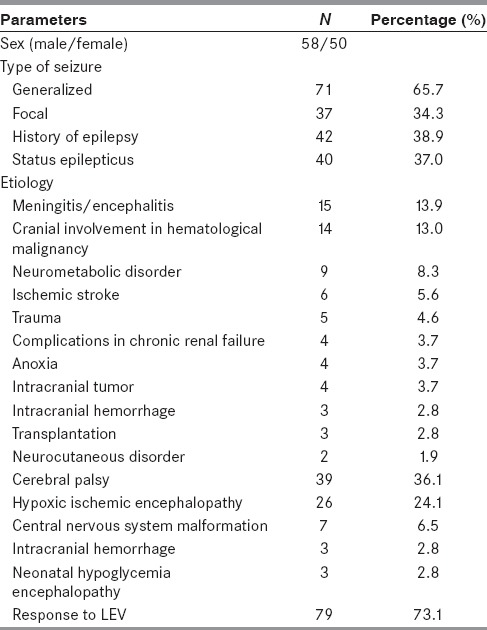
One hundred eight children were identified who received IV LEV for the treatment of SE or ARS in the pediatric ICU. The mean age of the patients was 69.39 ± 46.14 months (1-192 months). Of these 108 patients, 58 (53.7%) were boys and 50 (46.3%) were girls. Generalized and focal seizures were identified in 71 patients (65.7%) and 37 patients (34.3%), respectively.
The mean IV LEV loading dose was 28.33 ± 4.60 mg/kg/dose (10-40 mg/kg), the mean IV maintenance dose was 33.7 mg/kg/dose (20-40 mg/kg), and the median IV treatment period was 3-5 days (2-5 days) for LEV.
Forty two patients (38.9%) who presented with SE or ARS were also diagnosed with epilepsy before LEV was administered. Sixty three patients (58.3%) were given LEV as the first treatment option, and 45 patients were administered IV phenytoin. We reviewed side effects in only three patients (2.8%). Agitation and aggression were developed in two patients, and mild erythematous rash and urticaria developed in one patient after IV LEV.
Good response to LEV was detected in 79 of 108 patients (73.1%). The demographic details of these patients are summarized in Table 1.
Table 1.
Demographic data of the patients
Discussion
LEV is a second-generation antiepileptic drug considered effective in a broad spectrum of seizure types. It is partly metabolized by only plasma hydrolases; 60-70% of the unchanged drug and the inactive metabolite is eliminated by renal excretion, and the remaining 30% was elimination through the fecal route. There is no report on the interactions between LEV and any other drug, except for a few case reports.[8,9]
IV LEV has been reported to be effective and well tolerated and, therefore, clinically useful in a range of clinical situations. It is especially effective in the management of acute seizures in neonates, a severe epilepsy syndrome, myoclonic SE, following neurosurgery, in patients with brain tumors or other malignancies who are undergoing chemotherapy, and in the treatment of critically ill patients with multiorgan failure or those who are receiving a number of medications.[10,11,12,13,14,15]
We found LEV-terminated seizures in 73.1% (79/108) of the patients. In a study by İşgüder et al., it is found in 78.2% of the whole study population (133 patients).[16] In other studies, such as McTague et al. and Reiter et al., IV LEV treatment was reported to have totally controlled seizures in 62% and 67% of children, respectively.[6,11]
SE is one of the most common neurological emergencies. The neurological morbidity rate of pediatric patients with SE has been shown to be as high as 57%, and the mortality rate has been reported to be as high as 32%.[17,18] Therefore, phenytoin has been commonly used in patients with SE or ARS, which has numerous side effects that reduce its usefulness in critically ill patients. The treatment agents such as midazolam, pentobarbital, high dose of phenobarbital, propofol, inhaled anesthesia, and/or ketamine, that can induce coma and help in achieving anesthetic effects, are commonly used for refractory SE.[18] However, these medications necessitate long-term mechanical ventilation and increase the risk of complications, such as hemodynamic instability and metabolic disorders, that arise from the treatment. Studies are underway to investigate the effects of newer IV AEDs such as valproic acid and LEV.[3,5] Several studies suggest that IV LEV can be used successfully in patients with SE unresponsive to benzodiazepines or other initial therapy.[6,7] Kim et al. reported cases of seizure termination in 6 (43%) out of the 14 patients with SE.[19] Two other previous studies, Kirmani et al. and Gallentine, have reported that IV LEV treatment controlled seizures in 46.8% and 66.7% of children with SE, respectively.-[20,21] We detected seizure termination in 46.3% of the patients with SE in our study.
Patients with cancer have an additional high risk for drug-drug interactions, nearly 6% when two drugs are prescribed together.[22] Drug-drug interactions between chemotherapeutic agents and older AEDs have been documented.[22] Newer AEDs are preferably used in children who are treated for cancer. We used IV LEV in 14 patients with hematological malignancy. We found no significant pharmacokinetic interactions and side effects in those patients. But Parentelli et al. reported a case of leukemia with potential risk of drug-drug interaction between LEV and methotrexate.[9]
It should not be a surprise to note that LEV is used in the setting of seizure prevention or seizure treatment in patients with central nervous system (CNS) malignancies. Depending on the type and location of tumor and the age of the patients who are diagnosed with CNS malignancies, chance of developing seizures can vary from 20% to 45%.[14] Some authorities suggest the use of LEV or gabapentin can be used as a first-line therapy for the treatment of seizures in patients with brain tumors.[14,23] We used IV LEV in four patients with brain tumor and their seizures were well controlled.
If seizures occur after stroke, treatment should follow the specific guidelines for the management of seizures as in other neurological conditions. Kutlu et al. reported on the treatment of early and late seizures in the setting of ischemic or hemorrhagic stroke and found that in 82.4% of patients seizures were successfully controlled with IV LEV.[24]
Etiology is a strong determinant of prognosis, and drug response to AEDs treatment. In convulsive SE, the response rate of LEV was statistically higher in the unknown etiology than the efficacy in the symptomatic etiology. But, Khongkhatithum et al., determined no significant difference between the unknown or symptomatic etiologies.[25] Also, İşgüder et al. described that there was no significant difference of the response rate between the different etiologies of seizures (symptomatic, cryptogenic, and idiopathic).[16]
A large case report in critically ill adults suggested LEV monotherapy was associated with fewer complications compared with other AEDs, especially phenytoin.[26] Recently, there are reports that LEV may be safe and effective for treating SE and ARS in children.[6,15,16,17] Nau et al. reported no adverse hemodynamic events or cardiac arrhythmias in their study.[27] Goraya et al. observed no serious adverse effect in 10 children treated with IV LEV.[5] İşgüder et al. described side effects such as agitation and aggression in 3/133 patients.[16] We found agitation and aggression in two patients, and mild erythematous rash and urticaria in one patient. LEV was discontinued in those three patients, and their symptoms were resolved. But, we detected no interaction with other AEDs and medications.
In conclusion, LEV appears to be safe for critically ill patients in children. Future prospective studies should compare LEV with conventional AEDs, such as fosphenytoin, in terms of safety and efficacy in treating critically ill patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ramael S, Daoust A, Otoul C, Toublanc N, Troenaru M, Lu ZS, et al. Levetiracetam intravenous infusion: A randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia. 2006;47:1128–35. doi: 10.1111/j.1528-1167.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 2.Varelas PN, Spanaki M. Management of seizures in the critically ill. Neurologist. 2006;12:127–39. doi: 10.1097/01.nrl.0000195827.34370.63. [DOI] [PubMed] [Google Scholar]
- 3.Yu KT, Mills S, Thompson N, Cunanan C. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia. 2003;44:724–6. doi: 10.1046/j.1528-1157.2003.41302.x. [DOI] [PubMed] [Google Scholar]
- 4.Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42:1574–9. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 5.Goraya JS, Khurana DS, Valencia I, Melvin JJ, Cruz M, Legido A, et al. Intravenous levetiracetam in children with epilepsy. Pediatr Neurol. 2008;38:177–80. doi: 10.1016/j.pediatrneurol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.McTague A, Kneen R, Kumar R, Spinty S, Appleton R. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: Experience from a children's hospital. Seizure. 2012;21:529–34. doi: 10.1016/j.seizure.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Incecik F, Hergüner MO, Altunbasak S. The efficacy and side effects of levetiracetam on refractory epilepsy in children. J Pediatr Neurosci. 2012;7:19–22. doi: 10.4103/1817-1745.97614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisodiya SM, Sander JW, Patsalos PN. Carbamazepine toxicity during combination therapy with levetiracetam: A pharmacodynamic interaction. Epilepsy Res. 2002;48:217–9. doi: 10.1016/s0920-1211(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 9.Parentelli AS, Phulpin-Weibel A, Mansuy L, Contet A, Trechot P, Chastagner P. Drug-drug interaction between methotrexate and levetiracetam in a child treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:340–1. doi: 10.1002/pbc.24371. [DOI] [PubMed] [Google Scholar]
- 10.Khan O, Chang E, Cipriani C, Wright C, Crisp E, Kirmani B. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44:265–9. doi: 10.1016/j.pediatrneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Reiter PD, Huff AD, Knupp KG, Valuck RJ. Intravenous levetiracetam in the management of acute seizures in children. Pediatr Neurol. 2010;43:117–21. doi: 10.1016/j.pediatrneurol.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Haberlandt E, Sigl SB, Scholl-Buergl S, Karall D, Rauchenzauner M, Rostasy K. Levetiracetam in the treatment of two children with myoclonic status epilepticus. Eur J Paediatr Neurol. 2009;13:546–9. doi: 10.1016/j.ejpn.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Milligan TA, Hurwitz S, Bronmield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71:665–9. doi: 10.1212/01.wnl.0000324624.52935.46. [DOI] [PubMed] [Google Scholar]
- 14.Usery JB, Michael LM, 2nd, Sills AK, Finch CK. A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J Neurooncol. 2010;99:251–60. doi: 10.1007/s11060-010-0126-8. [DOI] [PubMed] [Google Scholar]
- 15.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med. 2009;10:505–10. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.İşgüder R, Güzel O, Ağın H, Yılmaz Ü, Akarcan SE, Celik T, et al. Efficacy and safety of IV levetiracetam in children with acute repetitive seizures. Pediatr Neurol. 2014;51:688–95. doi: 10.1016/j.pediatrneurol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: A meta-analysis. J Child Neurol. 1999;14:602–9. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 18.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461–7. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Lee JH, Ryu HW, Lim BC, Hwang H, Chae JH, et al. Effectiveness of intravenous levetiracetam as an adjunctive treatment in pediatric refractory status epilepticus. Pediatr Emerg Care. 2014;30:525–8. doi: 10.1097/PEC.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 20.Kirmani BF, Crisp ED, Kayani S, Rajab H. Role of intravenous levetiracetam in acute seizure management of children. Pediatr Neurol. 2009;41:37–9. doi: 10.1016/j.pediatrneurol.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 21.GallentineWB, Hunnicutt AS, Husain AM. Levetiracetamin children with refractory status epilepticus. Epilepsy Behav. 2009;14:215–8. doi: 10.1016/j.yebeh.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Yap KY, Chui WK, Chan A. Drug interactions between chemotherapeutics regimens and antiepileptics. Clin Ther. 2008;30:1385–407. doi: 10.1016/j.clinthera.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 23.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–30. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 24.Kutlu G, Gomceli YB, Unal Y, Inan LE. Levetiracetam monotherapy for late post stroke seizures in the elderly. Epilepsy Behav. 2008;13:542–4. doi: 10.1016/j.yebeh.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Khongkhatithum C, Thampratankul L, Wiwattanadittakul N, Visudtibhan A. Intravenous levetiracetam in Thai children and adolescents with status epilepticus and acute repetitive seizures. Eur J Paediatr Neurol. 2015;19:429–34. doi: 10.1016/j.ejpn.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Rüegg S, Naegelin Y, Hardmeier M, Winkler DT, Marsch S, Fuhr P. Intravenous levetiracetam: Treatment experience with the first 50 critically ill patients. Epilepsy Behav. 2008;12:477–80. doi: 10.1016/j.yebeh.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Nau KM, Divertie GD, Valentino AK, Freeman WD. Safety and efficacy of levetiracetam for critically ill patients with seizures. Neurocrit Care. 2009;11:34–7. doi: 10.1007/s12028-009-9185-0. [DOI] [PubMed] [Google Scholar]


