Abstract
Glioblastoma is the most common primary brain tumour in adults. Prognosis is poor: even with the current gold-standard first-line treatment—maximal safe resection and combination of radiotherapy with temozolomide chemotherapy—the median overall survival time is only approximately 15–17 months, because the tumour recurs in virtually all patients, and no commonly accepted standard treatment for recurrent disease exists. Several targeted agents have failed to improve patient outcomes in glioblastoma. Immunotherapy with immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab has provided relevant clinical improvements in other advanced tumours for which conventional therapies have had limited success, making immunotherapy an appealing strategy in glioblastoma. This Review summarizes current knowledge on immune checkpoint modulators and evaluates their potential role in glioblastoma on the basis of preclinical studies and emerging clinical data. Furthermore, we discuss challenges that need to be considered in the clinical development of drugs that target immune checkpoint pathways in glioblastoma, such as specific properties of the immune system in the CNS, issues with radiological response assessment, and potential interactions with established and emerging treatment strategies.
Introduction
Glioblastoma is the most common primary tumour of the CNS in adults, representing approximately 50% of all gliomas and 15% of primary brain tumours.1 The median age at diagnosis of glioblastoma is 64 years and the prognosis of patients with glioblastoma is poor, with median overall survival time of approximately 15–17 months.2
Despite advances in therapy, such as the widespread adoption of temozolomide for chemotherapy in newly diagnosed glioblastoma in 2005,3,4 improvements in survival for patients with glioblastoma have been modest.5,6 The current standard of care for newly diagnosed glioblastoma is maximal resection of the tumour, followed by radiotherapy and temozolomide.7 Unfortunately, glioblastoma ultimately relapses in almost all patients, and none of the current treatments can effectively prolong survival after relapse.7 Consequently, given the poor prognosis and limited treatment options for patients with glioblastoma, considerable interest has been directed in the development of new therapeutic approaches for this disease.
In the past 5 years, immunotherapy with immune checkpoint inhibitors has provided clinical advances in the treatment of other tumours for which conventional therapies have had limited success.8-14 These drugs facilitate effective antineoplastic immune response by suppressing co-inhibitory receptors and pathways that are activated by tumours to suppress T-cell response against tumour cells. Of particular rele vance is the finding that immune checkpoint inhibitors can induce deep and durable remissions that sometimes last for several years, and that even though treatment-related toxicities and adverse events can be considerable, they are manageable in most cases.8-14
The FDA approved the first two checkpoint inhibitors that target programmed cell death protein 1 (PD1) in late 2014 (pembrolizumab and nivolumab for unresectable or metastatic melanoma), and approved nivolumab for non-small-cell lung cancer (NSCLC) in March 2015.15,16 The first large phase III trial of nivolumab in patients with glioblastoma (NCT02017717) was initiated in 2014. In this Review, we summarise the involvement of immune checkpoint pathways in cancer, and evaluate the potential of immune checkpoint modulators in glioblastoma. We discuss preclinical data and emerging clinical studies on immune checkpoint inhibitors in glioblastoma. We also consider challenges that could occur in the clinical development of these agents in brain tumours, which might arise from specific characteristics of the CNS immune system, issues with radiological response assessment, and potential interactions with established and emerging treatment strategies. The aim of this Review is to promote rational and focused investigations into the clinical utility of immune checkpoint inhibitors in this devastating disease.
Immune checkpoint modulators
Immune checkpoint system
The interaction of tumour cells with the immune system (Figure 1) is a major determinant of cancer pathogenesis. The immune system attempts to eliminate tumour cells via a response cycle that comprises several steps, beginning with the release of antigens from tumour cells at cell death, followed by the presentation of these antigens by antigen-presenting cells (APCs) to T cells that are then primed and activated against cancer-specific antigens in the lymph nodes.17 These cytotoxic T cells, referred to as CD8+ cells, migrate to tumour sites where they infiltrate the tumours, specifically recognize the cancer cells, and elicit tumour-cell death, which then causes the release of more tumour-associated antigens, thereby continuing the cycle.17 Throughout this process, various ligand–receptor interactions, or checkpoint pathways, between APCs and T cells and between tumor cells and T cells provide signals to stimulate or inhibit T-cell activation, and to regulate the duration and extent of the immune response.17
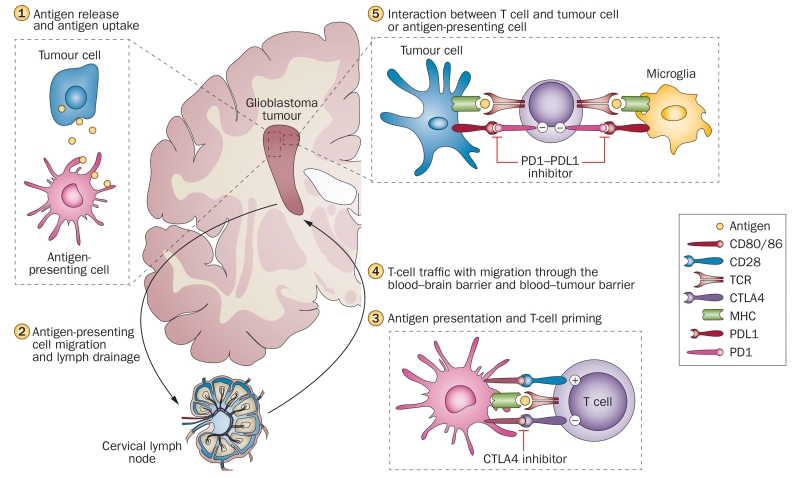
Figure 1.
Overview of the immune response and major immune checkpoint molecules in the immune cycle of glioblastoma. Antigens released from degenerating tumour cells are taken up by antigen-presenting cells, microglia and macrophages (1). Antigens are trafficked to lymph nodes via migration of antigen-presenting cells, and via drainage through lymphatic vessels in the meningeal sinuses (2). In the lymphatic tissues, antigen presentation and T-cell priming takes place. This interaction is tightly regulated by a multitude of co-inhibitory (CTLA4) and co-stimulatory (CD80, CD86, CD28) immune checkpoint molecules, and could be modulated by specific therapeutic antibodies, such as the CTLA4 inhibitor ipilimumab (3). Activated T cells reach the tumour via the blood stream and migration through the blood–brain or blood–tumour barrier (4). Tumour-associated immunosuppressive factors, including immune checkpoint molecules, inhibit tumour cell destruction by T cells. PDL1 is expressed on tumour cells and microglia and inhibits T cells via binding to PD1. PD1–PDL1 inhibitors (for example, nivolumab, pembrolizumab) block this immunosuppressive mechanism and thereby increase tumour cell lysis by lymphocytes (5). Abbreviations: CTLA4, cytotoxic T-lymphocyte-associated antigen 4; MHC, major histocompatibility complex; PDL1, programmed cell death 1 ligand 1; PD1, programmed cell death protein 1; TCR, T-cell receptor.
Two signals are involved in the activation or inhibition of the T-cell response: the primary signal occurs when antigens are presented through the MHC to the T-cell receptor, and the secondary signal that is either co-stimulatory or co-inhibitory and determines the T-cell response.18-20 Checkpoint molecules reflect these signals, and can be either co-stimulatory or co-inhibitory: CD28, CD80, CD86, CD40L, CD137, TNFRSF4 (also known as OX40), CD58, and CD28 promote immune activation, whereas cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), PD1, lymphocyte activation gene 3 (LAG-3), TIGIT, and T-cell immunoglobulin and mucin domain-3 (TIM3; also known as hepatitis A virus cellular receptor 2) suppress immune activation.18,20-23 These immune checkpoint pathways are exploited by tumour cells to evade immune detection, and can be targets for therapies.17,20 It is important to note, however, that much of the data on the function of these molecules has been derived from models of melanomas and other tumour types; at present, the exact involvement of checkpoint pathways in brain tumour pathogenesis is unknown.
Clinical experience with checkpoint inhibitors
Clinical data and ongoing clinical studies
Immunotherapies that specifically target co-inhibitory checkpoints have proven highly successful in several types of advanced tumour. Inhibition of CTLA4 is one approach that has shown clinical benefit.24 Ipilimumab, a fully humanized monoclonal antibody against CTLA4, was approved in 2011 by the FDA and the European Medicines Agency for the treatment of unresectable or metastatic melanoma, and has also shown benefit in patients with brain metastases of advanced melanoma, especially in patients who did not require corticosteroids for oedema at enrolment.8,9,25-28
Another effective approach is the inhibition of the pathway involving PD1, PDL1, and PDL2.29 Nivolumab and pembrolizumab are monoclonal antibodies that inhibit the PD1 receptor and its interaction with its ligands PDL1 and PDL2, thereby overcoming PD1 pathway-mediated inhibition of the antitumour immune response. Both agents were approved in 2014 by the FDA for the treatment of unresec table or metastatic melanoma and disease progression following ipilimumab treatment.11,15,16,30-32 In addition, nivolumab was approved by the FDA for NSCLC in March 2015. These agents have also shown benefit in other advanced tumours, including those associated with Hodgkin lymphoma and renal cell cancer.12,33-37
Several other PD1–PDL1 checkpoint inhibitors—pidilizumab and AMP-224 that target PD1, and MEDI4736, MPDL3280A, and MSB0010718C that target PDL1—are under investigation for both solid tumours and haematological malignancies. Modulation of other co-inhibitory checkpoints, such as TIM3 and lymphocyte activation gene 3 protein (LAG3), is also being explored (Table 1). TIM3 regulates T-cell exhaustion,38 and high expression of TIM3 has been found in tumours from patients with melanoma, NSCLC, lymphoma and other cancers.20,39 Similarly, LAG3 has been shown to be highly expressed in tumour-infiltrating lymphocytes (TILs) from patients with melanoma, colorectal cancer or fibrosarcoma, and seems to act synergistically with PD1 to control the expansion of activated T cells.40,41 Moreover, agents that activate co-stimulatory molecules are being investigated; for example MEDI6469, which targets TNFRSF4, is undergoing phase I clinical testing in a variety of advanced solid tumours.
Table 1.
Examples of immune checkpoint inhibitors in development
| Agent | Target | Tumour type(s) | Highest phase trial |
Indication in the highest-phase trial |
|---|---|---|---|---|
| Ipilimumab | CTLA4 | Glioblastoma, NSCLC, SCLC, gastric cancer, melanoma, ovarian, pancreatic cancer, renal cancer, multiple myeloma, lymphomas |
Marketed | Advanced melanoma |
| Tremelimumab | CTLA4 | NSCLC, mesothelioma, squamous cell cancer of the head and neck, other solid tumours |
III | Mesothelioma |
| Nivolumab | PD1 | Colorectal cancer, glioblastoma, hepatocellular carcinoma, NSCLC, SCLC, squamous cell cancer of the head and neck, breast cancer, bladder cancer, gastric cancer,melanoma, ovarian cancer, pancreatic cancer, renal cancer, multiple myeloma, lymphomas |
Marketed | Metastatic melanoma, non-small cell lung cancer |
| Pembrolizumab | PD1 | NSCLC, glioblastoma, squamous cell cancer of the head and neck, pancreatic cancer, renal cell cancer, other advanced solid tumours, lymphoma |
Marketed | Metastatic melanoma |
| Pidilizumab | PD1 | Multiple myeloma, glioblastoma, lymphoma | III | Lymphoma |
| AMP224 | PD1 | Advanced solid tumours, colorectal cancer | I | Advanced solid tumours, colorectal cancer |
| AMP514/ MEDI0680 |
PD1 | Advanced malignancies, aggressive B-cell lymphomas |
II | Aggressive B-cell lymphomas |
| BMS936559 | PDL1 | Advanced solid tumours | I | Several |
| MEDI4736 | PDL1 | NSCLC, squamous cell cancer of the head and neck, glioblastoma, and other advanced solid tumours |
III | Non-small cell lung cancer |
| MPDL3280A | PDL1 | Bladder cancer, NSCLC, renal cell carcinoma, and other advanced solid tumours |
III | Non-small cell lung cancer |
| MSB0010718C | PDL1 | Advanced solid tumours | II | Merkel cell carcinoma |
| BMS986016 | LAG-3 | Advanced solid tumours, chronic lymphocytic leukaemia, Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma |
I | Advanced solid tumours, chronic lymphocytic leukaemia, Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma |
| IMP321 | LAG-3 | Advanced pancreatic cancer, metastatic breast cancer, metastatic kidney cancer, metastatic melanoma |
I/II | Melanoma |
| Lirilumab | KIR | Advanced solid tumours, multiple myeloma, Hodgkin lymphoma, non-Hodgkin lymphoma, acute myeloid leukaemia |
II | Acute myeloid leukaemia |
| IPH2101 | KIR | Squamous cell cancer of the head and neck, multiple myeloma |
II | Multiple myeloma |
| 1-7F9 | KIR | Multiple myeloma, acute myeloid leukemia | II | Multiple myeloma |
| KW-6002 | A2aR | Preclinical | Preclinical | Not applicable |
Abbreviations: A2aR, adenosine A2a receptor; CTLA4, cytotoxic T-lymphocyte associated protein 4; KIR, killer-cell immunoglobulin-like receptors; LAG-3, lymphocyte activation gene 3; NSCLC, non-SCLC; PD1, programmed cell death protein 1; PDL1, programmed cell death 1 ligand 1; SCLC, small-cell lung cancer.
Adverse effects
The most important treatment-related adverse effects associated with immune checkpoint inhibition are inflammatory and autoimmune events. Of note, some data suggest a correlation between treatment response and immune-related adverse events, although this association requires further investigation.42 Adverse effects are particularly common in patients treated with the CTLA4 inhibitor ipilimumab: severe cases of colitis, pneumonitis and hypophysitis have been reported, among other serious immune-related toxicities.8,9,43-45 Patients receiving ipilimumab monotherapy for melanoma were much more likely to discontinue treatment because of adverse events—such as diarrhoea, colitis, rash, or fatigue—than were patients treated with nivolumab (13.2% vs 5.1%, respectively). Combination therapy with nivolumab plus ipilimumab was associated with particularly high rate of discontinuation because of severe adverse effects (29.4%).45
Adverse events have also been reported with the PD1 inhibitors nivolumab and pembrolizumab, although these agents seem to have a more favourable safety profile, perhaps suggesting a more restricted role of PD1 in inhibiting the immune response.8,10,11,30-32,46 Because of these experiences, detailed algorithms have been developed to manage specific immune-related adverse events—such as skin-related, gastrointestinal, hepatic and endocrine events—and these algorithms are now well established in clinical practice.47
A key challenge in the development of immunotherapy for CNS tumours will be to balance the intensity of the immune response with the potential for inflammatory and autoimmune events, including autoimmunity directed at the brain (allergic encephalomyelitis).46,48,49 Moreover, any increase in intracranial pressure and cerebral oedema that is associated with enhanced inflammatory response against tumour manifestations, owing to effective immune checkpoint inhibition, could reduce tolerability.49 Of note, autoimmune events in the CNS have not been reported in the approximately 250 patients with brain metastases from melanoma who were treated with ipilimumab, though long-term follow-up data from such patients have not yet been published.25,26,28
Biomarkers for response to checkpoint inhibition
In several cancer types, including melanoma and lung cancer, expression of PDL1 in the tumour positively correlates with response to inhibitors of the PD1–PDL1 axis, although responses have also been observed in patients with PDL1-negative tumours and the true predictive role of this marker is under intense investigation.10,50 Importantly, several immunohistochemical assays for the assessment of PDL1 expression exist and evaluation of PD1 as potential predictive biomarker will require detailed comparison of analytical test performance using various antibodies and cut-offs. Interestingly, studies in patients with melanoma and lung cancer have demonstrated that response to immuno therapy could be related to genetic signatures: patients with distinct neoantigen signatures responded better to immune checkpoint inhibition.51,52
The immune system and glioblastoma
CNS immune privilege revisited
The traditional assumption has been that immune responses in the CNS were limited, because the blood–brain barrier, an absence of a conventional lymphatic drainage system, and low levels of APCs, MHC, and T cells, provided immune privilege or immune isolation for the brain.19,48,53 This view has recently been challenged, as it has become clear the CNS actively communicates with the immune system. In 2015, a lymphatic system within the CNS was discovered; this system drains CNS antigens from the cerebrospinal fluid into the cervical lymph nodes, thus facilitating immune surveillance of the CNS.54 It is also clear that some immune cells readily migrate into the CNS and have a crucial role in the pathobiology of various neurological diseases such as multiple sclerosis, CNS infections and neuro degenerative disorders; these cells are also present in brain tumours including gliomas.48,53,55 The CNS contains high numbers of microglia, which are the main effector cells of the innate immune system in the CNS and exert a number of critical functions including cytotoxicity via nitric oxide release, phagocytosis, and T-cell activation through antigen presentation.
In glioblastoma, increased permeability of the of the blood–brain barrier, associated with pathologically structured microvessels and vascular endothelial growth factor (VEGF) expression, could also contribute to the interaction between tumour cells and the immune system. Interestingly, tumour cells have been detected in the peripheral circulation of patients with glioblastoma; further studies should determine whether and how these cells are involved in immune stimulation.56,57
Tumour-associated immunosuppression
Several mechanisms within the glioblastoma microenvironment facilitate the tumour’s evasion of the immune response, making glioblastoma a particularly immunosuppressive tumour. Glioblastoma tumours express various potent immunosuppressive factors, such as prostaglandin E2, TGF-β, indoleamine 2,3-dioxygenase (IDO), IL-10 and STAT3.58-60 Moreover, ineffective presentation of tumour antigens by APCs or recruitment of immunosuppressive cells such as regulatory T cells (TREG cells), or myeloid-derived suppressor cells to the tumour microenvironment seem to contribute to immune evasion of tumour cells in glioblastoma.20,61-63
Increased PDL1 expression in glioblastoma
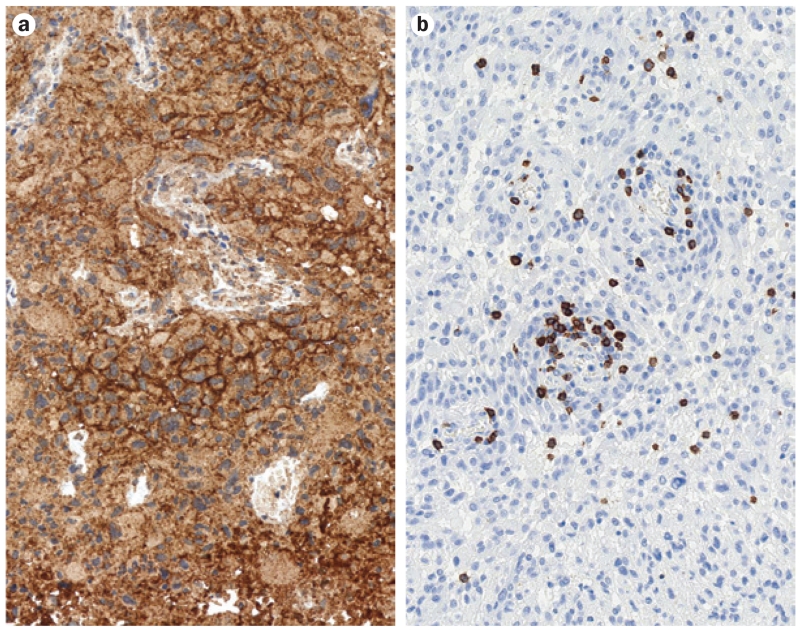
Expression and activity of immune checkpoint molecules—in particular PDL1—that inhibit T cells, has emerged as an important immunosuppressive mechanism in glioblastoma.64-66 In an analysis of 135 glioblastoma specimens, diffuse or fibrillary PDL1 expression was present in 88% of samples from patients with newly diagnosed glioblastoma and in 72% of samples from patients with recurrent glioblastoma (Figure 2), although no correlation between PDL1 expression and survival was found.65
Figure 2.
PDL1 expression and tumour-infiltrating lymphocytes in glioblastoma. Expression of the immunosuppressive molecule PDL1 and sparse infiltration with cytotoxic lymphocytes are found in the majority of glioblastoma cases. a | Most samples from glioblastoma show prominent expression of PDL1 on tumour cells. Brown indicates areas immunolabelled with monoclonal anti-PDL1 antibody 5H1. b | Glioblastoma typically harbours sparse infiltration with tumour-infiltrating lymphocytes, accentuated around microvessels. Brown indicates immunolabelled CD8+ T cells. Both light microscopy images taken with an original magnification of ×200. Abbreviation: PD1, programmed cell death protein 1; PDL1, programmed cell death 1 ligand 1.
It is important to note that the level of PDL1 expression in the healthy CNS tissue that surrounds glioblastomas is very low. The glioblastoma tumours themselves seem to be more likely to express PDL165 than are other tumour types (~30% of melanomas30 and 25–36% of NSCLC tumours67). Interestingly, examination of 446 glioblastoma samples from The Cancer Genome Atlas showed that PDL1 gene expression differed according to molecular subtype of glioblastoma: the mesenchymal subtype showed much higher PDL1 expression than did other subtypes.65 This finding is in line with other studies showing that the mesenchymal glioblastoma subtype is particularly immunogenic, with overexpression of genes involved in antitumour pro-inflammatory responses, including both adaptive and innate immunity, and immunosuppression.68,69
On a functional level, PDL1 produced by glioma cell lines inhibits T-cell activation and reduces the production of cytokines—such as IFN-γ, IL-2 and IL-10—by lymphocytes.70,71 Moreover, glioma cells were also shown to upregulate PDL1 expression in circulating monocytes and tumour-infiltrative macrophages via modulation of autocrine–paracrine IL-10 signalling.66 Microglia strongly inhibit T-cell function via PD1–PDL1 signalling in in vitro models of inflammatory and autoimmune CNS disorders,72 and PDL1 expression has also been reported in microglial cells in human glioblastoma specimens.65 Notably, PD1-expressing TILs and PDL1 expression has also been observed in other CNS neoplasms such as brain metastases and primary CNS lymphoma; suggesting thera peutic relevance of targeted inhibition of the PD1–PDL1 axis in these tumour types.73-75
Overall, the recent success of immune checkpoint inhibitors in other tumour types and the accumulating data showing a prominent involvement of checkpoint molecules in immune evasion of glioblastoma provide a sound rationale for clinical trials with such agents in this tumour.
Tumour-associated immune cells in glioblastoma
The inflammatory infiltrates in glioblastomas are usually relatively sparse and are comprised of various cell types including CD8+ cells, CD4+ (T-helper) cells, CD20+ cells (also known as B cells), TREG cells, natural killer cells, microglia and macrophages.65,76-78 PD1-expressing T cells are found in approximately one-third of glioblastoma samples.65 Usually, TILs are found predominantly in the perivascular area, but also in the tumour tissue. These lymphocytes are particularly numerous in mesenchymal glioblastomas with NF1 and RB1 mutations.78 TILs have been reported to correlate with survival times in glioblastoma; however, the studies that reported this correlation were small and retrospective in nature, meaning that the finding should be validated in further investigations.76,77 Future studies should also investigate whether density or composition of TIL infiltrates could be used as predictive biomarkers for response to immunomodulatory therapy in glioblastoma.
Checkpoint inhibitors in glioblastoma
Preclinical evidence
Modulation of the checkpoint pathways has been investigated with in vitro and in vivo models.59,79-82 Activation of specific co-stimulatory receptors, such as OX40, and blockade of specific co-inhibitory receptors, such as PD1 and CTLA4, induced tumour regression and promoted long-term survival in animal models of glioma.79,80,82 The immunosuppressive potential of various combinations of co-inhibitory and co-stimulatory checkpoint inhibitors (for example, PDL1, CTLA4 and CD137) and/or radiation have also been explored in murine models of glioblastoma. Both the combination of several immunosuppressive drugs—such as combined treatment with IDO, CTLA4 and PDL159—and the combination of immune checkpoint inhibition with radiotherapy82-83 prolonged survival. In addition, infiltration of TILs and the ratio of effector T cells to TREG cells were frequently altered, indicating enhanced immune function.59,79-83
Directions for future clinical trials
Given the prominent role of the PD1–PDL1 axis in glioblastoma pathophysiology, several clinical trials have been initiated to determine the potential of PD1–PDL1 checkpoint inhibitors for glioblastoma, both as monotherapy and in combination with other agents (Table 2). In addition, a number of clinical trials are evaluating immune checkpoint molecules in brain metastases, and could yield important information on adverse events, response patterns, and effects of combination with other therapies such as corticosteroids or radiation. In the foreseeable future, the results of these trials should provide further direction regarding the utility of targeting immune checkpoints in CNS tumours.
Table 2.
Representative clinical trials of immune checkpoint inhibitors in glioblastoma and brain metastases
| National Clinical Trial registration number |
Mechanism of tested agent |
Therapy and/or treatment groups | Tumour type | No. of patients |
Phase |
|---|---|---|---|---|---|
| NCT02017717 | Anti-PD1, anti-CTLA4 |
Nivolumab (anti-PD1) Nivolumab + ipilimumab (anti-CTLA4) Bevacizumab (control group) | Recurrent glioblastoma | n = 372 | III |
| NCT01952769 | Anti-PD1 | Pidilizumab (two cohorts) | Relapsed glioblastoma, diffuse intrinsic pontine glioma |
n = 30 | I/II |
| NCT02311920 | Anti-PD1, anti-CTLA4 |
TMZ + nivolumab TMZ + ipilimumab TMZ + nivolumab + ipilimumab |
Newly-diagnosed glioblastoma or gliosarcoma |
n = 42 | I |
| NCT02336165 | Anti-PDL1 | MEDI4736 MEDI4736 + radiotherapy MEDI4736 + bevacizumab |
Newly-diagnosed or recurrent glioblastoma |
n = 84 | II |
| NCT02115139 | Anti-CTLA4 | Ipilimumab + whole-brain radiotherapy | Melanoma BM | n = 66 | II |
| NCT02097732 | Anti-CTLA4 | Ipilimumab followed by stereotactic radiosurgery, followed by ipilimumab Stereotactic surgery followed by ipilimumab |
Melanoma BM | n = 40 | II |
| NCT02107755 | Anti-CTLA4 | Ipilimumab followed by stereotactic radiosurgery |
Oligometastatic melanoma | n = 32 | II |
| NCT01703507 | Anti-CTLA4 | Ipilimumab + whole-brain radiotherapy Ipilimumab + stereotactic radiosurgery |
Melanoma BM | n = 24 | I |
| NCT01950195 | Anti-CTLA4 | Ipilimumab + stereotactic radiosurgery | Melanoma BM | n = 30 | I |
| NCT02337491 | Anti-PD1 | Pembrolizumab monotherapy Pembrolizumab + bevacizumab |
Recurrent glioblastoma | n = 79 | II |
| NCT02085070 | Anti-PD1 | Pembrolizumab | Non-small cell lung cancer BM or melanoma BM |
n = 64 | II |
Abbreviations: BM, brain metastases; CTLA4, cytotoxic T-lymphocyte antigen-4; PD1, programmed cell death protein 1; PDL1, programmed cell death 1 ligand 1; TMZ, temozolomide.
Combination immunotherapy
The multitude of immunosuppressive mechanisms observed in glioblastoma might necessitate combination of several immunomodulatory agents to achieve an optimal therapeutic activity. Indeed, emerging data from patients with melanoma indicate that combination of inhibitors that target different immune checkpoint molecules can increase efficacy in comparison with monotherapy, at least in some subgroups of patients: the combination of ipilimumab and nivolumab is more effective than ipilimumab alone in previously untreated patients with melanoma, especially in patients with PDL1-negative tumours.45 The combination treatment had higher adverse event rates than did monotherapy, but adverse events were manageable and were not associ ated with treatment-related deaths.45 Other emerging checkpoint molecules that might be targeted effectively include OX40 and LAG3; future studies will show whether combined targeting of these molecules increases therapeutic activity.
Overall, combination of various immune checkpoint modulators in patients with glioblastoma shows promise; however, deeper insights into the interplay of co-stimulatory and co-inhibitory molecules is needed for rationally designing clinical studies to explore such strategies. In addition, recent studies indicate a role for vaccination against tumour-associated antigens in patients with glioma who have epithelial growth factor receptor variant III (EGFRvIII) or isocitrate dehydrogenase (IDH) mutations.84,85
Gliomas, including glioblastomas, have been shown to have relatively low mutation rates86 and are, therefore, presumed to have relatively low basal immune stimulation compared with tumour types that have high response rates to immunotherapies, such as those associated with melanoma and NSCLC. Combination of vaccination and immune checkpoint inhibition could offer synergistic antitumour activity and should be explored in future studies.
Local delivery of immunotherapies
Local delivery of immune checkpoint inhibitors to the tumour tissue, with the goal of achieving maximum therapeutic effect while limiting systemic toxicity, is a theoretically appealing opportunity. At present, however, it is unclear to what extent the therapeutic effect of immune checkpoint inhibitors relies on local activity in the tumour tissue microenvironment, as opposed to activity in lymph nodes or other peripheral components of the immune system. Furthermore, the feasibility of local delivery methods, such as drug-releasing wafers and convection-enhanced delivery, is limited in patients with glioblastoma.87
Predictive immunotherapy response biomarkers
Well designed translational research projects that accompany clinical trials are of paramount importance to identify predictive biomarkers for response to immune checkpoint inhibition in glioblastoma. Such studies should investigate the predictive value of the expression of immune checkpoint molecules (such as PDL1) and the presence, density or composition of TILs in tumour tissues. In addition, high-dimensional profiling of tumour tissue samples for immune-related gene expression patterns and molecular tumour subtypes seems reasonable, and might help distinguish responsive or insensitive patient subpopulations.88 ‘Liquid biopsies’ to identify blood biomarkers might also be useful.
Guidelines for response evaluation
One of the difficulties with immunotherapy is that responses are not sufficiently explained by existing criteria for solid tumours, such as the Response Evaluation Criteria in Solid Tumours (RECIST), which updated earlier tumour response criteria from the World Health Organization.89 Experience with ipilimumab in metastatic melanoma showed that response to immunotherapy does not follow the same pattern as seen with conventional chemotherapeutic agents. Measureable response can take longer to achieve with immunotherapy than with conventional treatments, and durable stable disease could in fact indicate response. Additionally, response can occur even after disease progression (as measured by conventional criteria).90 An initial increase in tumour burden, typically representing failure for chemotherapeutic agents, can arise as a result of continued tumour growth prior to the stimulation of the immune response. However, it can also represent successful stimulation of the immune response, because movement of TILs into the tumour can lead to an apparent increase in the tumour size.90,91 As a result of these observations, specific immune response criteria were developed. In these criteria—unlike in RECIST—new lesions do not necessarily represent progressive disease, but are considered a part of the total tumour burden for comparison with baseline disease.90,92,93 In addition, responses are based on bidimensional measurements rather than the unidimensional measurements used in RECIST.
A multidisciplinary, multinational panel is currently drafting the Immunotherapy Response Assessment in Neuro-Oncology (iRANO) guidelines to standardize response assessment criteria in patients with neuro-oncological malignancies who are undergoing immunotherapy. The iRANO guidelines build on the response assessment criteria that were originally developed in 2010 to address response assessment challenges associated with imaging-based evaluation of patients with CNS tumours; these challenges include pseudoprogression linked to temozolomide chemoradiotherapy, and pseudoresponse linked to antiangiogenic agents, such as bevacizumab.94
A major focus of iRANO is to provide recommendations for management of patients with early progressive changes seen on imaging after initiation of an immunotherapeutic agent. These changes can be caused by actual tumour growth that precedes the development of a sufficient antitumour immune response, or by pseudoprogression associated with an inflammatory immune infiltrate. In such cases, early progressive imaging changes do not preclude ultimate clinical benefit; indeed, some patients with advanced solid tumours have shown late clinical benefit of immune checkpoint blockade.8 The iRANO criteria will permit continuation of therapy beyond initial progression in patients who are clinically stable, to obtain confirmation of true tumour progression on follow-up imaging. These criteria will also address important nuances specific to neuro-oncological malignancies, including management of cerebral oedema and corticosteroid dosage.
Concomitant therapy
As immunotherapy becomes more widely available, the potential increases for both synergies and adverse interactions between conventional glioblastoma therapies and immune checkpoint inhibitors. Thus, questions yet to be resolved include how to combine checkpoint inhibitors with current standards of care for glioblastoma—radiotherapy, temozolomide, bevacizumab and corticosteroids—and whether the use of these agents is associated with positive or negative interactions.
Radiotherapy
After surgical resection, radiotherapy is the backbone of standard treatment for newly diagnosed glioblastoma, and it is also commonly used in recurrent glioblastoma.7 Whole-brain radiation therapy or stereotactic radiation is also recommended for the treatment of brain metastases.95 Radiation elicits tumour necrosis, primarily by DNA damage and apoptosis, leading to changes in the tumour microenvironment, which can suppress the immune response.96-98 However, the unmasking of tumour antigens and the antigen release associated with radiotherapy might improve the efficacy of immune checkpoint inhibitors. Indeed, some patients show the so-called abscopal effect, a phenomenon where localized irradiation of a tumour shrinks both the irradiated tumour as well as a metastasis far from the irradiated site.97 The abscopal effect is now generally acknowledged to be immune-mediated: an upregulation and release of tumour antigens in the microenvironment caused by radiation-induced tumour cell death, which leads to stimulation of the immune response via activation of immune checkpoint pathways.96,97
The potential relationship between radiotherapy and the immune system suggests that the combination of radiation with an immune checkpoint inhibitor has a synergistic effect.95 In brain metastases from melanoma, for example, a retrospective analysis found evidence of the abscopal effect in over 50% of patients who received radiotherapy following treatment with ipilimumab, and median overall survival of patients who showed the abscopal effect was substantially longer (22 months) than that of patients who did not show the effect (8 months).99 The abscopal effect might benefit patients with glioma, for example, by eliciting an immune response against tumour cells outside of the radiation field.
However, many questions regarding optimal treatment modalities remain to be answered: should these agents be used concomitantly or sequentially; should the checkpoint inhibitor be initiated before radiotherapy and continued throughout radiotherapy and beyond, or should a checkpoint inhibitor be administered after the completion of radiotherapy? In light of the potential synergisms, commencing checkpoint inhibitor treatment prior to radiotherapy would seem rational, however, clinical trials are needed to establish optimal combination strategies.100
Temozolomide
Temozolomide in combination with radiotherapy is the gold standard treatment for newly diagnosed glioblastoma, and temozolomide monotherapy can be used as maintenance therapy. However, the use of temozolomide is associated with myelosuppression, particularly leukopaenia and lymphopaenia.3,4,98,101 Although temozolomide-induced myelosuppression has been proposed to reduce the therapeutic effect of immunotherapies, some data demonstrate that lymphopaenia might in fact augment immunotherapy, because it could eliminate TREG cells or alter homeostatic mechanisms that limit the number of lymphocytes, thereby enabling rapid clonal expansion of tumour-specific effector T cells. However, these results should be interpreted with caution because the studies were small.102-105 However, despite these theoretical considerations, to date the interactions between temozolomide and checkpoint inhibitors are unclear and need to be elucidated in further studies.
Bevacizumab
Bevacizumab is a recombinant humanized mono clonal antibody that inhibits the activity of human VEGF. Bevacizumab is indicated in some countries for the treatment of glioblastoma with disease progression after a prior therapy, as well as for other tumour types, including those found in patients with metastatic colorectal cancer, metastatic renal cell carcinoma, NSCLC or cervical cancer.106 Some studies suggest that bevacizumab has the potential to elicit an immune response107,108 and could, therefore, have synergistic effects with immune checkpoint inhibitors. In patients with metastatic melanoma, bevacizumab increased the number of CD8+ lymphocytes, which might stimulate immune responses.107
Tumour-derived VEGF-A, a member of the VEGF family of pro-angiogenic factors, has been shown to have immunosuppressive functions via the prevention of dendritic cell maturation and decrease in T-cell number and function.108 Bevacizumab specifically targets VEGF-A, and increases dendritic cell numbers and function in solid tumours.108 In patients with colorectal cancer, bevacizumab has been shown to reduce the amount of TREG cells in peripheral blood.108 Thus, it is reasonable to assume that combination therapy with bevacizumab and an immune checkpoint inhibitor is a favourable approach; early, promising data from patients with metastatic melanoma treated with ipilimumab–bevacizumab combination therapy suggests this strategy to be worthy of further investigation.109,110
It must be noted that the data on the interaction between bevacizumab and the CNS immune function in gliomas are insufficient. Moreover, the interaction of immune checkpoint inhibitors with bevacizumab or other agents targeting the VEGF pathway cannot be predicted and needs to be evaluated carefully. The pronounced anti-oedematous and steroid-sparing effect of bevacizumab2,111 could be of particular interest for brain tumours; bevacizumab could help avoid the immunosuppressive effects of corticosteroids and manage immune-related brain oedema in patients treated with immunomodulatory agents.
Corticosteroids
Corticosteroids, especially dexamethasone, are commonly administered to patients with brain tumours to reduce brain oedema.112,113 Corticosteroids are also used according to established treatment algorithms to manage immune-related adverse events associated with checkpoint inhibitors.44,47 Consequently, it is possible that the use of steroids might inhibit any immune responses elicited by checkpoint inhibitors. However, clinical evidence indicates that, in cases of treatment-related adverse immune events, the judicious use of steroids has successfully reversed toxicity with no apparent compromise of antitumour activity.11,44,114 Limiting steroid use to the minimum necessary dose—as is currently done for all patients—is important not only to avoid steroid-associated adverse events, but also to control inflammatory adverse events in patients receiving immunotherapy without reducing the antitumour efficacy of these agents.
Conclusions
The approval of the first CTLA4 inhibitor, ipilimumab, and the recent approval of two PD1 checkpoint inhibitors for unresectable or metastatic melanoma (nivolumab and pembrolizumab) and NSCLC (nivolumab) have provided substantial improvements for these otherwise devastating diseases, and there is evidence of similar benefits in other advanced tumour types, such as metastatic renal cell carcinoma.13
The potential for immune checkpoint inhibitors to benefit patients with glioblastoma is of great interest, because these patients have a poor prognosis and few effective treatment options. However, many issues specific to CNS tumours—both primary tumours and brain metastases—must be addressed before the value of checkpoint inhibitors in CNS cancer can be determined. The conventional belief that the blood–brain barrier offers immune privilege no longer seems accurate, suggesting that immunotherapy could have benefits in CNS cancer. However, the relevance of this finding to drug delivery must be considered. The existence of multiple co-stimulatory and co-inhibitory pathways provides numerous targets for immunomodulatory agents, but current clinical evidence for their efficacy in glioblastoma is lacking.
Much research activity has focused on the discovery of prognostic and predictive biomarkers that can identify patients who are most likely to benefit from therapy, but the clinical utility of these markers is yet to be confirmed. Challenges in the design and conduct of clinical trials for immunotherapies are numerous, particularly in trials involving patients with glioblastoma: different measures of response are required for checkpoint inhibitors, and the management of immune-related adverse events in the CNS are a concern. No standardized and validated assays to measure immune response exist, and the current standard of care for glioblastoma—radiotherapy, chemotherapy and supportive steroid use—can have immunosuppressive effects that could counteract the stimulatory effects of checkpoint inhibitors and thereby confound findings.
Conversely, certain glioblastoma therapies might have synergies that could be exploited, though the clinical application of combination strategies will require careful consideration of several factors, including dosage regimens, concurrent versus sequential administration, and the potential for increased toxicities. Other areas of interest will be the potential for targeting co-stimulatory pathways as well as co-inhibitory ones, and combining immunotherapeutics with other novel treatment modalities.
The introduction of immune checkpoint inhibitors has dramatically changed the prognosis for some advanced tumours. We hope that with careful attention to the particular issues associated with CNS tumours, and rational and ethical approaches to the design and execution of clinical trials, the clinical utility of immune checkpoint inhibitors in patients with glioblastoma will be confirmed, thus providing new treatment options for this devastating disease.
Key points.
The prognosis for glioblastoma patients is poor, with median overall survival of approximately 15–17 months
Immunotherapy has clinical benefits in other advanced tumours, such as melanoma and lung cancer, for which conventional therapies have had limited success
The blood–brain barrier prevents macromolecules from entering the CNS, but readily allows traffic of activated lymphocytes; thus, communication occurs between the CNS and the immune system
The success of immunotherapy in other cancers, and the current understanding of the interaction between the brain and the immune system provide a rationale for exploration of immune checkpoint inhibitors in glioblastoma
Tumour progression could involve multiple immunosuppressive mechanisms, making combination of immunotherapeutic agents that target different pathways a promising approach
Clinical trials evaluating immune checkpoint inhibitors in glioblastoma patients are ongoing
Acknowledgements
M. Daniels from inScience Communications in Philadelphia, PA, USA, provided editorial support for writing this article, comprising compilation of tables, management of the reference database, and editing of the manuscript text before submission. The editorial support was funded by Bristol-Myers Squibb. D.A.H. has received the National Multiple Sclerosis Society Collaborative Research Centre Award CA1061-A-18, NIH grants P01 AI045757, U19 AI046130, U19 AI070352, and P01 AI039671, and research support from the Nancy Taylor Foundation for Chronic Diseases and the Penates Foundation. J.H.S. has received the NIH grants R01-CA177476-02, R01-NS086943-01, P50-CA190991-01, and 2R25-NS065731-06, and research support from the Accelerate Brain Cancer Cure and Pediatric Brain Tumor Foundation.
Footnotes
Author contributions
All authors contributed to researching data for the article, substantial contribution to discussing of content, writing, editing and reviewing of the article.
Competing interests
M.P. has received honoraria, research support (unrestricted grants), and travel support (to scientific meetings) from Bristol-Myers Squibb, Böhringer-Ingelheim, GlaxoSmithKline, Mundipharma and Roche. M.L. has received research support from Agenus, Arbor Pharmaceuticals, Bristol-Myers Squibb, Celldex Therapeutics, and ImmunoCellular Therapeutics, and has served as a consultant for Bristol-Myers Squibb. D.A.H. has consulted for Allergan Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Genzyme Sanofi-Aventis, MedImmune, Mylan Pharmaceuticals, Novartis Pharmaceuticals, Questcor and Teva Neuroscience, and has received grant support from Bristol-Myers Squibb. D.A.R. has received financial compensation for participation in advisory boards for Amgen, Cavion, Genentech/Roche, Midatech Pharma, Momenta Pharmaceuticals, Novartis and Stemline Therapeutics, served as a member of speakers’ bureaus for Genentech/Roche and Merck, and received research support from Celldex Therapeutics and Incyte. J.H.S. declares no competing interests.
References
- 1.Ostrom QT. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl. 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert MR, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temodar (temozolomide) [prescribing information] Merck & Co., Inc.; 2014. http://www.merck.com/product/usa/pi_circulars/t/temodar_capsules/temodar_pi.pdf. [Google Scholar]
- 4.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol. Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 6.Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Curr. Opin. Neurol. 2014;27:666–674. doi: 10.1097/WCO.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 7.Weller M, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Day SJ, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opdivo (nivolumab) [prescribing information] Bristol-Myers Squibb Company; 2014. http://www.opdivo.bmscustomerconnect.com/gateway. [Google Scholar]
- 16.Keytruda (pembrolizumab) [prescribing information] Merck & Co., Inc.; 2015. http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. [Google Scholar]
- 17.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol. Rev. 2009;229:126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin. Dev. Immunol. 2011;2011:1–21. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakdash G, Sittig SP, van Dijk T, Figdor CG, de Vries IJ. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front. Immunol. 2013;4:53. doi: 10.3389/fimmu.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joller N, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastings WD, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarhini AA, Iqbal F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 2010;3:15–25. doi: 10.2147/ott.s4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolin K, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 26.Queirolo P, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J. Neurooncol. 2014;118:109–116. doi: 10.1007/s11060-014-1400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yervoy (ipilimumab) [prescribing information] Bristol-Myers Squibb Company; 2013. http://www.yervoy.com. [Google Scholar]
- 28.Di Giacomo AM, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879–886. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 29.Luke JJ, Ott P. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 2015;6:3479–3492. doi: 10.18632/oncotarget.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 32.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2014;32(15 Suppl.):8113. [Google Scholar]
- 34.Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J. Clin. Oncol. 2014;32(15 Suppl.):8023. [Google Scholar]
- 35.Gettinger SN, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and correlation of outcomes with PD-L1 status. J. Clin. Oncol. 2014;32(15 Suppl.):8024. [Google Scholar]
- 36.Rizvi NA, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J. Clin. Oncol. 2014;32(15 Suppl.):8022. [Google Scholar]
- 37.Garon EB, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2014;32(15 Suppl.):8020. [Google Scholar]
- 38.Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J. Exp. Med. 2008;205:2699–2701. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2014;2:393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 40.Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin. Ther. Targets. 2011;15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 41.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downey SG, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur. J. Endocrinol. 2012;167:1–5. doi: 10.1530/EJE-12-0167. [DOI] [PubMed] [Google Scholar]
- 44.Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am. Soc. Clin. Oncol. Educ. Book. 2012:174–177. doi: 10.14694/EdBook_AM.2012.32.79. [DOI] [PubMed] [Google Scholar]
- 45.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dranoff G. Immunotherapy at large: balancing tumor immunity and inflammatory pathology. Nat. Med. 2013;19:1100–1101. doi: 10.1038/nm.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13:3–13. doi: 10.1093/neuonc/noq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raizer J. Issues in developing drugs for primary brain tumors: barriers and toxicities. Toxicol. Pathol. 2011;39:152–157. doi: 10.1177/0192623310391482. [DOI] [PubMed] [Google Scholar]
- 50.Taube JM, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin. Dev. Immunol. 2010:2010. doi: 10.1155/2010/689171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. doi: 10.1038/nature14432. http://dx.doi.org/10.1038/nature14432. [DOI] [PMC free article] [PubMed]
- 55.Reardon DA, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16:1441–1458. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller C, et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 2014;6:247ra101. doi: 10.1126/scitranslmed.3009095. [DOI] [PubMed] [Google Scholar]
- 57.Preusser M. Neuro-oncology: a step towards clinical blood biomarkers of glioblastoma. Nat. Rev. Neurol. 2014;10:681–682. doi: 10.1038/nrneurol.2014.208. [DOI] [PubMed] [Google Scholar]
- 58.Tran TT, et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9:259–270. doi: 10.1215/15228517-2007-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wainwright DA, et al. Durable therapeutic efficacy utilizing combinatorial blockade against, IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 61.Fecci PE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 62.Ooi YC, et al. The role of regulatory T-cells in glioma immunology. Clin. Neurol. Neurosurg. 2014;119:125–132. doi: 10.1016/j.clineuro.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Mirghorbani M, Van Gool S, Rezaei N. Myeloid-derived suppressor cells in glioma. Expert Rev. Neurother. 2013;13:1395–1406. doi: 10.1586/14737175.2013.857603. [DOI] [PubMed] [Google Scholar]
- 64.Vlahovic G, Fecci PE, Reardon D, Sampson JH. Programmed death-ligand 1(PD-L1) as an immunotherapy target in patients with glioblastoma. Neuro Oncol. 2015;17:1043–1045. doi: 10.1093/neuonc/nov071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berghoff AS, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2014;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloch O, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013;19:3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velcheti V, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doucette T, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol. Res. 2013;1:112–122. doi: 10.1158/2326-6066.CIR-13-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhat KP, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avril T, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2, 3-dioxygenase (IDO) on tumour-specific T cell functions. J. Neuroimmunol. 2010;225:22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Wintterle S, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 72.Magnus T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J. Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berghoff AS, et al. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL) Clin. Neuropathol. 2014;33:42–49. doi: 10.5414/np300698. [DOI] [PubMed] [Google Scholar]
- 74.Berghoff AS, et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66:289–299. doi: 10.1111/his.12537. [DOI] [PubMed] [Google Scholar]
- 75.Berghoff AS, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunol. 2015 doi: 10.1080/2162402X.2015.1057388. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han S, et al. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yue Q, et al. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J. Neurooncol. 2014;116:251–259. doi: 10.1007/s11060-013-1314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rutledge WC, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin. Cancer Res. 2013;19:4951–4960. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kjaergaard J, et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 80.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 2007;13:2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 81.Reardon DA, et al. Immune checkpoint blockade for glioblastoma: Preclinical activity of single agent and combinatorial therapy. J. Clin. Oncol. 2014;32(15 Suppl.):2084. [Google Scholar]
- 82.Zeng J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belcaid Z, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schumacher T, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 85.Schuster J, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17:854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther. Deliv. 2015;6:353–369. doi: 10.4155/tde.14.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sims JS, Ung TH, Neira JA, Canoll P, Bruce JN. Biomarkers for glioma immunotherapy: the next generation. J. Neurooncol. 2015;3:359–372. doi: 10.1007/s11060-015-1746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 90.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 91.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin. Cancer Res. 2009;15:7116–7118. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 92.Wolchok J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann. Oncol. 2012;23(Suppl. 8):viii15–viii21. doi: 10.1093/annonc/mds258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishino M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin. Cancer Res. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 95.Papadatos-Pastos D, Soultati A, Harries M. Targeting brain metastases in patients with melanoma. Biomed. Res. Int. 2013;2013:186563. doi: 10.1155/2013/186563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 2014;15:927–943. doi: 10.3390/ijms15010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frey B, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation – implications for cancer therapies. Curr. Med. Chem. 2012;19:1751–1764. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 98.Grossman SA, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grimaldi AM, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicholas S, et al. Current trends in glioblastoma multiforme treatment: radiation therapy and immune checkpoint inhibitors. Brain Tumor Res. Treat. 2013;1:2–8. doi: 10.14791/btrt.2013.1.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stupp R, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 102.Zitvogel L, et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampson JH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchez-Perez LA, et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS ONE. 2013;8:e59082. doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Avastin (bevacizumab) [prescribing information] Genentech, Inc.; 2014. http://www.gene.com/download/pdf/avastin_prescribing.pdf. [Google Scholar]
- 107.Mansfield AS, Nevala WK, Lieser EA, Leontovich AA, Markovic SN. The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma. Oncoimmunology. 2013;2:e24436. doi: 10.4161/onci.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terme M, et al. Modulation of immunity by antiangiogenic molecules in cancer. Clin. Dev. Immunol. 2012;2012:8. doi: 10.1155/2012/492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS. Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J. Immunother. Cancer. 2014;2:40. doi: 10.1186/s40425-014-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schaff LR, et al. Ipilimumab for recurrent glioblastoma (GBM) J. Clin. Oncol. 2014;32(15 Suppl.):32, e13026. [Google Scholar]
- 111.Chinot OL, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 112.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr. Opin. Oncol. 2004;16:593–600. doi: 10.1097/01.cco.0000142076.52721.b3. [DOI] [PubMed] [Google Scholar]
- 113.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer. 2002;10:322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 114.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 2011;164:303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]