Abstract
Background
Wilms tumor (WT) is the most common renal neoplasm of childhood. We previously showed that restricted activation of the WNT/β-catenin pathway in renal epithelium late in kidney development is sufficient to induce small primitive neoplasms with features of epithelial WT. Metastatic disease progression required simultaneous addition of an activating mutation of the oncogene K-RAS. Here, we sought to define the molecular pathways activated in this process and their relationship to human renal malignancies.
Methods
Affymetrix expression microarray data from murine kidneys with activation of K-ras, Ctnnb1 (β-catenin), or both restricted to renal epithelium were analyzed and compared to publically available expression data from normal and neoplastic human renal tissue. Target genes were verified by immunoblot and immunohistochemistry.
Results
Mouse kidney tumors with activation of K-ras and Ctnnb1 and human renal malignancies have similar mRNA expression signatures and are associated with activation of networks centered on β-catenin and TP53. Up-regulation of WNT/β-catenin targets (MYC, Survivin, FOXA2, Axin2, Cyclin D1) was confirmed by immunoblotting. K-RAS/β-catenin murine kidney tumors were more similar to human WT than other renal malignancies and demonstrated activation of a TP53 dependent network of genes including the transcription factor E2F1. Up-regulation of E2F1 was confirmed in both murine and human WT samples.
Conclusions
Simultaneous activation of K-RAS and β-catenin in embryonic renal epithelium leads to neoplasms similar to human WT associated with activation of TP53 and up-regulation of E2F1. Further studies to evaluate the role of TP53 and E2F1 in human WT are warranted.
INTRODUCTION
Wilms tumor (WT) is the most common renal neoplasm of childhood1. While current multi-modal management cures the majority of children2, this survival comes at the cost of significant long term treatment related morbidity3. In addition, a substantial number of patients relapse (15%) and half of these tumors resist intensive salvage therapies and progress4. The main challenge in WT research, therefore, is to identify novel therapies to address these deficiencies. Such novel approaches critically rely on an in depth understanding of the activated pathways underlying WT disease progression.
WT are triphasic, embryonic-like tumors that are generally thought to arise from primitive nephrogenic rests derived from the metanephric mesenchyme, a multipotent progenitor pool within the developing kidney. Some of the genetic aberrations underlying this process include inactivating mutations of Wilms tumour 1 (WT1), Wilms tumour gene found on chromosome X (WTX), and stabilizing/activating mutations of β-catenin (CTNNB1)5. The mechanisms by which these differing alterations lead to WT have not been fully elucidated, but all share an association with canonical WNT/β-catenin signaling6, 7.
In addition to WNT/β-catenin, aberrations of the TP53 pathway also play an important role in numerous malignancies, including WT and adult renal cell carcinomas5, 8. However, the specifics by which TP53 and its regulatory network induce malignant transformation and impact disease progression vary across tumor types. In WT, while accumulation of the TP53 protein and several alterations in its gene are associated with anaplasia and treatment resistance, the precise mechanisms underlying its influence on disease progression remain unknown5. One mechanism could be via the E2F family of transcription factors which play an important role in regulating the cell cycle through their interaction with the Rb family of proteins. As with TP53, the E2F-Rb pathway is also disrupted in a wide range of malignancies9. Classically, cell cycle regulation is thought to occur through increased levels of free E2F proteins upon release by phosphorylated Rb, leading to transcription of key target genes involved in cell proliferation. This Rb-E2F pathway interacts with the MDM2-TP53 pathway to regulate the cell cycle, particularly in response to cellular stresses, such as DNA damage or oncogene activation10. Whether these pathways are critical to WT biology has not been fully elucidated.
We showed that constitutive restricted activation of the canonical Wnt/β-catenin pathway in murine renal tubular epithelial cells late in renal development is sufficient to induce small tumors with features of human WT11. Metastatic disease progression required the simultaneous addition of an activating mutation of the G-coupled protein/oncogene K-ras. This was accompanied by increased activation of oncogenic pathways such as PI3K/AKT and activation of canonical Wnt/β-catenin signalling.
Here, we sought to define the molecular pathways activated in this process and their relationship to human renal malignancies. Using an expression micro-array, we show that the kidneys from mice with simultaneous activation of K-ras and Ctnnb1 restricted to the renal epithelium induce an expression signature that is similar to human renal malignancies and WT in particular. This is associated with a network of genes regulated by TP53 including up-regulation of the transcription factor E2F1, suggesting that further study of the role of E2F proteins in WT biology is warranted.
METHODS AND MATERIALS
Mice
Mice harboring γGT-Cre recombinase were a kind gift from Eric Neilson (Northwestern University Feinberg School of Medicine)12. Mice with a conditional activating mutation of Ctnnb1 in which exon 3 is flanked by lox sites (Catnblox(ex3)) were a kind gift from Makoto M. Taketo13. Mice with a conditional activating mutation of Kras (LSL-KrasG12D) were obtained from Tyler Jacks (Massachusetts Institute of Technology)14. All mice were bred and housed under an Institutional Animal Care and Use Committee approved protocol. Mice were crossed to obtain mice with genotypes γGT-Cre/Catnb+/lox(ex3) (referred to as CatnbΔex3), γGT-Cre/Kras+/G12D (referred to as KrasG12D), γGT-Cre/Kras+/G12D/Catnb+/lox(ex3) (referred to as KrasG12D/CatnbΔex3) and litter-mate controls as previously described11. For the microarray experiment, mice were sacrificed at age 15–20 weeks and the kidneys flash frozen in liquid nitrogen. RNA was extracted from the kidney of three individual mice from each group (two from KrasG12D/CatnbΔex3) and cDNA created. For immunoblotting and immunohistochemistry, we bred an independent cohort with the same genotypes, harvested the kidneys at 15–20 weeks of age and extracted protein from whole kidneys or fixed and embedded kidneys as described.
WT Tissue Microarray
Briefly, using formalin fixed paraffin embedded renal tumor and adjacent kidney specimens collected prospectively and archived in our IRB-approved laboratory embryonal tumor repository, we created two tissue microarrays (TMA) comprised of 148 total punches (~1 mm in diameter each) derived from 32 consecutive childhood WT’s15. Serial 5 µm sections of these two TMAs were included for the IHC analysis, which was concentrated on the 32 WT specimens.
Antibodies
Antibodies used for immunohistochemistry (IHC) and/or immunoblot were as follows: c-Myc (Epitomics), Actin (Sigma-Aldrich), Axin2 (Abcam), E2F1 (Atlas Antibodies), survivin, and Cyclin D1 (Cell Signaling Technology).
Histology and Immunohistochemistry
Murine kidneys were harvested, fixed in 10% buffered formalin, processed and paraffin embedded. Sections were either stained with hematoxylin and eosin (H&E) or underwent IHC. For IHC, the slides were incubated with primary antibodies then exposed to biotinylated secondary antibody and incubated with an ABC-HRP complex (Vector Laboratories) and then with liquid 3,3’-diaminobenzidine tetrahydrochloride (DAB) (DAKO liquid DAB + substrate chromogen system, cat. #2012-02). Stained sections were photographed and processed using a Zeiss AX10 Imager.M1 microscope and AxioVision Release 4.6 software.
Microarray
Snap frozen murine kidneys were crushed rapidly on ice using a cold mortar and pestle, dissolved in Trizol and sonicated. RNA was extracted with chloroform, centrifugated, pelleted from aqueous phase with isopropanol, washed with 75% ethanol, and re-dissolved in water. After cleaning with RNasin and DNase, RNA samples were further purified using the QIAGEN RNeasy mini kit and the concentration measured using UV/Vis spectrophotometer (Beckman Coulter, Brea, CA). RNA integrity was evaluated by VANTAGE (Vanderbilt Technologies for Advanced Genomics core), and microarray gene expression analysis using the Affymetrix Gene Titan platform was performed by the VANTAGE core according to standardized technique.
Immunoblotting
Snap frozen murine kidneys were crushed rapidly on ice using a cold mortar and pestle, dissolved in lysis buffer (made fresh from 6× stock solution of 2 M Tris-HCl pH 6.8, 20% SDS, glycerol and protease inhibitors) and sonicated. The lysis was cleared by centrifugation. Protein concentration was determined using Bio-Rad protein assay then subjected to SDS-PAGE electrophoresis, transferred to Immobilon-P transfer membranes (Millipore Corporation) and subjected to immunoblotting utilizing standard techniques. All experiments were completed in duplicate with 3–4 animals of each genotype.
RT-PCR
cDNA was synthesized using Superscript reverse transcription (RT) reagents (Life Technologies, Grand Island, NY). Real time PCR was performed using LuminoCt® SYBR® Green qPCR ReadyMix™ (Sigma-Aldrich, St.Louis, MO) and CFX96 Touch Real Time System (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. The primers were as follows: mouse GAPDH 5’ CTGGCATGGCCTTCCGTG 3’ and 5’ GAAATGAGCTTGACAAAG 3’; mouse E2F1 5' GTGAAACGGAGGCTGGATCT 3' and 5' CGTTTCTGCACCTTCAGC AC 3'. For GAPDH annealing t was 60°C and 25 ng cDNA used per reaction, for E2F1 annealing t was 67°C and 50 ng cDNA used per reaction. Comparisons of expression by RT-PCR across groups was done using the 2−ΔΔCT16. Analysis was completed using GraphPad Prism v5.02 (La Jolla).
Generation of Renal Cancer Signatures
Gene-expression patterns were determined at the genome level among four biological groups using Principal component analysis (PCA) and unsupervised clustering analysis with the open-source R scripts, version 2.10.1 (www.rproject.org). Mouse renal signatures were derived by comparing four mouse groups (KrasG12D/CatnbΔex3, KrasG12D, CatnbΔex3, and controls). For statistical analysis, we used the Significance Analysis of Microarray (SAM) software package implemented in TIGR MeV program17. Based on practical considerations, the SAM false discovery rates were adjusted to obtain approximately equal numbers of significant genes (ca. 500) for various mouse signatures. The three significant gene lists were generated from KrasG12D/CatnbΔex3 mice compared to the other three genotypes. NCBI Gene and NCBI HomoloGene databases were used to translate mouse signature genes to human orthologous gene symbols to generate the human versions of these signatures.
The murine derived human orthologous signatures were used as queries against publically available human cancer signature databases from GEO using the EXALT (Expression AnaLysis Tool) program18. The public human renal cancer dataset GSE11024 was downloaded from NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) including expression data from 79 human renal tissue samples. GSE11024 has six sample groups of 27 Wilms tumors (WT), 40 adult renal neoplasms (10 clear cell carcinoma, 17 papillary renal cell carcinoma, 6 chromophobe renal cell carcinoma, 7 oncocytoma), and 12 normal kidney tissue samples19. We identified three homologous human renal cancer signatures from this GEO data set, showing significant signature similarities to murine derived human orthologous signatures.
Identification of a Novel Renal Cancer Meta-Signature
Three human signatures in GSE11024 were correspondingly designated by EXALT as renal cancer sig3424 (Normal vs. Renal cancer), WT sig3711 (Normal vs. WT) tumors, and WT sig2724 (WT vs. Adult renal neoplasm). Each human signature formed a signature cluster with the three human orthologous signatures based on their data similarities such as identical gene symbols, concordance in the directions of gene expression changes, and significant confidence scores20. Signature genes within each cluster were combined, and overlapping genes across four signatures were then compiled as a meta-signature by data similarity-driven meta-analysis (EXALT) of transcriptional profiles21. In so doing, a human renal cancer signature and three murine derived human orthologous signatures derived from KrasG12D/CatnbΔex3 generated a meta-signature. Functional annotation networks of the three meta-signatures were generated using Ingenuity Pathway Analysis (IPA; Ingenuity Systems) software.
RESULTS
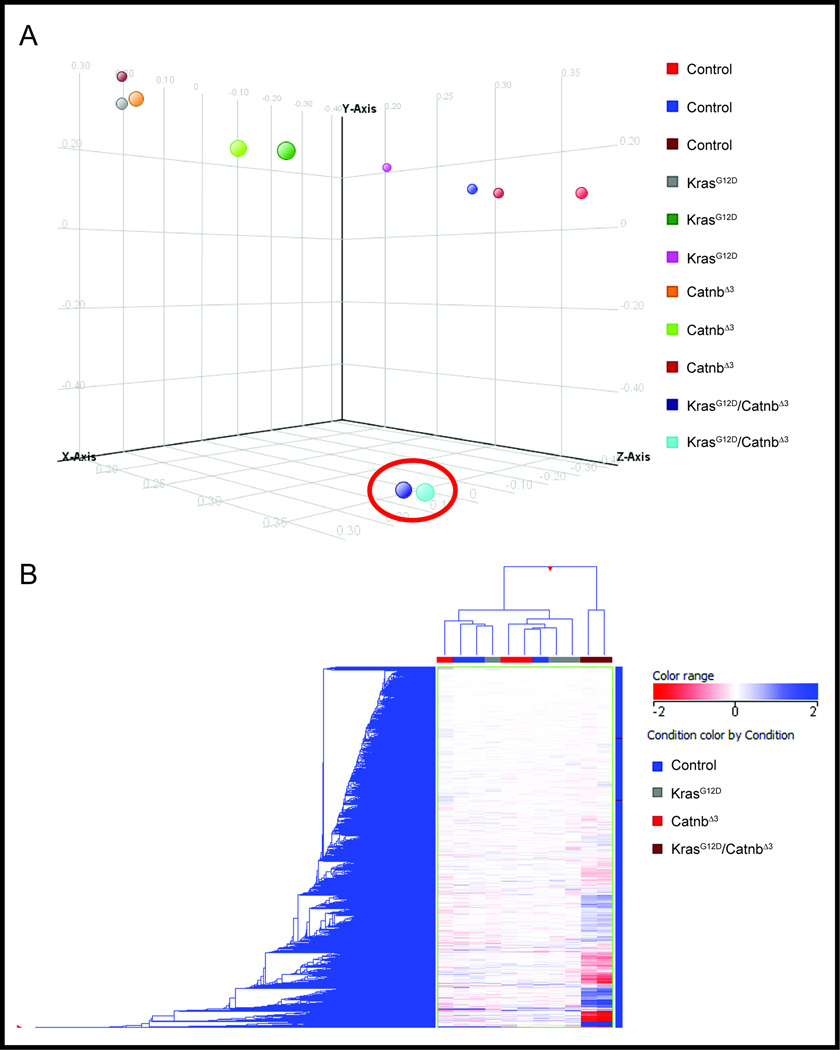
We showed that simultaneous activation of both K-ras and Ctnnb1 is required for rapid, metastatic progression of primitive renal malignancies such as WT11, however the predominant signaling pathways activated were not identified. We therefore compared gene expression from mice with activation of both the K-RAS and canonical Wnt/β-catenin pathway in the renal epithelium of the kidney (KrasG12D/CatnbΔex3) to either pathway alone (KrasG12D and CatnbΔex3), and controls. Using the TITAN Affymetrix platform we found by both principal components analysis (unsupervised cluster analysis, Figure 1A) and supervised hierarchical clustering (Figure 1B), that the expression profile of kidneys from KrasG12D/CatnbΔex3 mice was significantly different from the KrasG12D, CatnbΔex3, and control mice, which all shared a similar expression profile. We used Genespring to interrogate the molecular pathways up-regulated in KrasG12D/CatnbΔex3 mice and found the top two pathways, and three of the top ten, involved Wnt signalling networks (Table). Up-regulation of the Wnt targets c-Myc, survivin, Cyclin D1, Axin2, and FoxA2 in KrasG12D/CatnbΔex3 mice was confirmed by immunoblotting of proteins from whole kidney lysates (Figure 2).
Figure 1. Gene expression from tumor bearing murine KrasG12D/CatnbΔex3 kidneys is significantly different than that of KrasG12D, CatnbΔex3, or control kidneys.
The microarray gene-expression data were analyzed among the four biological groups using Principal component analysis (A) and unsupervised hierarchical clustering analysis (B) with the open-source R scripts, version 2.10.1 (www.r-project.org). The KrasG12D/CatnbΔex3 kidneys are indicated by the red circle for principal component analysis and are at the far right two columns in the hierarchical clustering analysis. In both analyses they are significantly different than the other three genotypes, which are similar to each other.
Table.
Top ten altered molecular pathways comparing KrasG12D/CatnbΔ3 murine kidneys to control, KrasG12D alone, and CatnbΔ3 alone by Genespring.
| Pathway | p-value | Matched Entities |
Pathway Entities |
|---|---|---|---|
| Wnt Signaling Pathway & Pluripotency | 2.74E-07 | 15 | 97 |
| Wnt Signaling Pathway NetPath | 8.40E-06 | 14 | 109 |
| TGF-Beta Signaling Pathway | 3.16E-05 | 9 | 52 |
| Myometrial Relaxation & Contraction Pathways | 1.27E-04 | 15 | 157 |
| EGFR1 Signaling Pathway | 1.48E-04 | 16 | 176 |
| Matrix Metalloproteinases | 2.43E-04 | 6 | 29 |
| Prostaglandin Synthesis & Regulation | 3.58E-04 | 6 | 31 |
| Cell cycle | 3.68E-04 | 10 | 88 |
| miRNA regulation of DNA Damage Response | 4.18E-04 | 9 | 91 |
| Wnt Signaling Pathway | 4.98E-04 | 8 | 60 |
Figure 2. Canonical Wnt/β-catenin target genes are up-regulated in KrasG12D/CatnbΔex3 kidneys.
Immunoblot demonstrating higher levels of the Wnt targets FoxA2, survivin, Axin2, and c-Myc in KrasG12D/CatnbΔex3 kidneys compared to the other three groups.
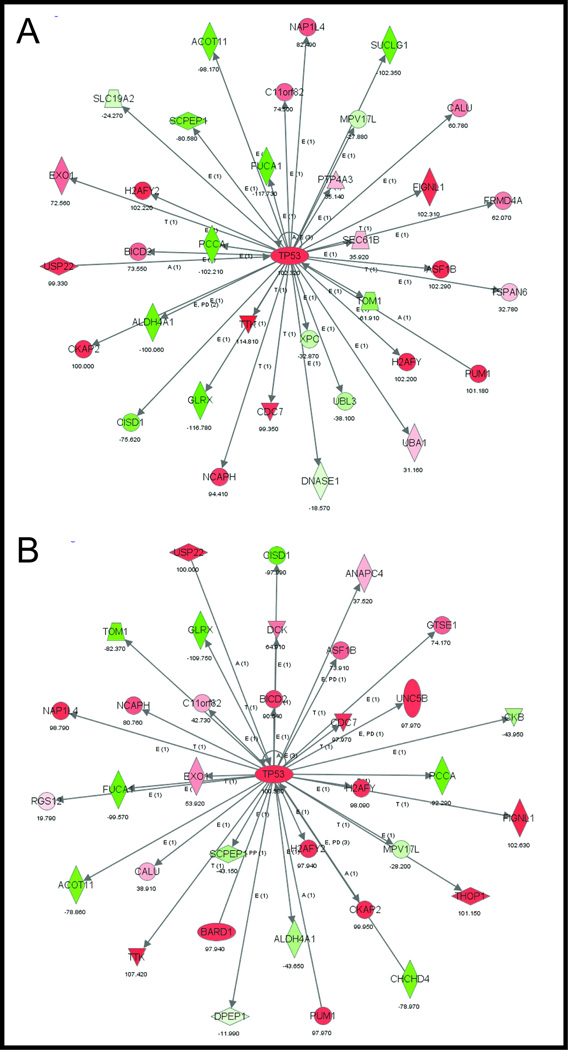
Since simultaneous activation of both ras and Ctnnb1 (β-catenin) leads to metastatic, progressive renal tumors in mice, we tested whether the associated expression changes were also observed in human renal tumors by comparing the mouse gene expression signature that distinguished KrasG12D/CatnbΔex3 with gene signatures characterizing human renal tumors in a publically available GEO dataset (GSE11024) of adult and pediatric renal neoplasms and normal controls19. Using the comparative analysis tool EXALT we demonstrated that murine KrasG12D/CatnbΔex3 kidney tumors were similar to adult renal tumors and identified a concordant gene signature of 2,482 genes that reliably distinguished these murine tumors and human renal neoplasms from the normal human and murine kidney tissues and controls. Ingenuity Pathway Analysis (IPA) on this concordant gene signature demonstrated two networks, one centered on CTNNB1 (Figure 3A), which is consistent with our previous results showing up-regulation of the canonical Wnt/β-catenin pathway (Table and Figure 2), while the second centered around TP53 (Figure 3B).
Figure 3. The gene signature that distinguishes murine KrasG12D/CatnbΔex3 kidneys and human renal malignancies from normal/control tissues are centered on CTNNB1 and TP53.
Using the comparative analysis tool EXALT the gene expression of murine KrasG12D/CatnbΔex3 kidneys and human renal malignancies were shown to be similar to each other and significantly different from normal tissues/controls. The concordant gene signature that defined this difference was generated and Ingenuity pathway analysis (IPA) of this signature shows gene networks that are centered on CTNNB1 (A) and TP53 (B).
Tumors from KrasG12D/CatnbΔex3 kidneys histologically resemble human WT11 and both CTNNB1 and TP53 have been implicated in human WT. We therefore tested, again using EXALT, whether the gene expression signature from these kidneys was able to specifically distinguish human WT from normal renal tissue or other renal neoplasms. We showed this was the case and generated a concordant gene signature that distinguished murine KrasG12D/CatnbΔex3 kidneys and human WT from normal human renal tissue, while a second gene signature reliably distinguished these from other human renal neoplasms. These signatures indicate that the gene expression of murine KrasG12D/CatnbΔex3 kidneys is more consistent with that seen in human WT when compared to other renal neoplasms or normal tissues. IPA analysis of these gene signatures demonstrated several affected canonical and functional pathways, particularly those involving cellular metabolism and cell survival (Supplementary Tables 1 and 2), and the network associated with each of these signatures was again centered on TP53 (Figure 4A and B). Somewhat surprisingly, this was no longer the case for CTNNB1.
Figure 4. The gene signatures that distinguish murine KrasG12D/CatnbΔex3 kidneys and human WT from other renal malignancies or normal/control tissues are centered on TP53.
Using the comparative analysis tool EXALT the gene expression of murine KrasG12D/CatnbΔex3 and human WT were found to be similar to each other and significantly different from normal tissues/controls. The concordant gene signature defining this association was generated and IPA of this signature shows a gene network centered TP53 (A). The same approach showed the gene expression of murine KrasG12D/CatnbΔex3 kidneys and human WT were more similar to each other that other renal neoplasms. IPA of the corresponding concordant gene signature again showed a gene network centered TP53 (B).
The E2F protein family is intimately involved in a complex interconnected network with the TP53 pathway and signaling through E2F3 is associated with aggressive WT10, 19, 22. In addition, E2F’s have been shown to be elevated in adult renal malignancies23, 24. We therefore interrogated other members of the E2F gene family and found E2F1 is part of the concordant gene signature shared by human WT’s and murine KrasG12D/CatnbΔex3 tumor bearing kidneys. We validated its expression in both mouse and human tissues and confirmed by RT-PCR it is upregulated in KrasG12D/CatnbΔex3 mice compared to KrasG12D, CatnbΔex3, or controls (Figure 5A). Elevated E2F1 protein expression was confirmed by immunoblotting on whole protein extracts (Figure 5B) and by immunohistochemistry in mouse kidneys (Figure 5C–F). Finally, in order to confirm its relevance in human WT, IHC was performed on a tissue microarray of 32 cases of WT, which demonstrated marked nuclear expression of E2F1 in 30 of 32 (94%) cases, including in blastemal, epithelial, and stromal elements (representative images in Figure 6).
Figure 5. E2F1 is up regulated in murine KrasG12D/CatnbΔex3 kidneys.
Mice were bred and sacrificed at 15–20 weeks of age. Kidneys were snap frozen and RNA extracted and RT-PCR performed for E2F1 (A). Results are shown compared to kidneys from control mice and analyzed across groups using the 2−ΔΔCT method with * indicating p value <0.0516. Whole protein lysates were extracted and tested by immunoblot (B) and FFPE kidneys were tested for E2F1 expression by IHC (C–F).
Figure 6. E2F1 is expressed at high levels in the majority of human WT’s.
IHC was performed for E2F1 on a TMA of 32 human WT’s. Strong nuclear staining was seen in 30/32 (94%) WT’s and seen in blastemal elements (A, low power, B high power), stromal elements (* in B), and epithelial elements (C, low power, D high power).
DISCUSSION
In this study, we show that the gene expression signature from murine kidneys with simultaneous activation of K-ras and Ctnnb1, which leads to rapidly progressive, metastatic primitive renal malignancies11, resembles human renal malignancies in general and WT in particular. This is consistent with our previous observation that these tumors have histologic and immunohistochemical staining characteristics of human WT11 and further supports the potential utility of this transgenic model in studying human WT pathobiology.
We further demonstrate that specific signatures can distinguish KrasG12D/CatnbΔex3 murine kidneys and human renal malignancies from other groups and are associated with activation of the canonical Wnt/β-catenin pathway and up-regulation of downstream target genes. This finding is consistent with a large body of work showing an important role for activation of the canonical Wnt/β-catenin pathway in both adult renal cell carcinomas as well as WT. In WT, this oncogenic pathway is thought to play a critical role in its tumorigenesis and is activated in up to 75% of WT’s25. Importantly, adult renal cell carcinomas have also demonstrated enhanced activation of the Wnt/β-catenin pathway that is associated with disease progression and oncologic outcomes26. These findings combined with our results here showing enhanced pathway activation in KrasG12D/CatnbΔex3 murine kidneys and human renal tumors suggest this oncogenic pathway plays an important role across most renal malignancies.
In addition to the Wnt/β-catenin pathway, we show here that the signatures defining human WT and KrasG12D/CatnbΔex3 kidneys also include networks with TP53 at their center. We go on to show that this is associated with elevation of E2F1 in both human WT and KrasG12D/CatnbΔex3 tumor bearing kidneys. In addition to their role in regulating the cell cycle through their interaction with Rb and MDM2-TP53, the E2F proteins have also been shown to be involved in other cellular processes including apoptosis, cellular differentiation, and RNA processing9, 27. These responses are in a complex crosstalk with the TP53 pathway and, depending on the cellular context, E2F protein activation can act as an oncogene in promoting cellular proliferation or a tumor suppressor through increasing apoptosis. This strongly implies that tight regulation of E2F proteins is critical to normal cellular homeostasis.
The precise mechanism driving up-regulation of E2F1 in human WT and murine KrasG12D/CatnbΔex3 kidneys remains unclear. However, activation of RAS is known to induce increased E2F1 mRNA and protein expression28, which may explain how E2F1 is elevated in KrasG12D/CatnbΔex3 kidneys despite the constitutive activation of the canonical Wnt/β-catenin pathway that normally would down-regulate E2F129. In addition, this study and our prior work11 have shown elevated Myc in experimental tumors of KrasG12D/CatnbΔex3 kidneys compared to the kidneys from KrasG12D, CatnbΔex3, or controls (Figure 2 and11). Myc has also been shown to transcriptionally up-regulate E2F130. Thus, marked elevation in Myc may also explain why elevated E2F1 is noted in KrasG12D/CatnbΔex3 but not from KrasG12D kidneys. These observations require further study in order to determine the dominant mechanisms leading to E2F1 elevation in this unique model of metastatic WT.
Multiple studies have confirmed elevated expression of E2F family members in renal malignancies. In adult clear cell renal cell carcinoma (ccRCC), E2F1 has been shown to be elevated in several studies23, 24, which is consistent with the results presented here. This is likely due, at least in part, to the observation that the protein product of the VHL gene (pVHL) inhibits E2F1 expression23. Therefore the loss of pVHL seen in familial and sporadic forms of ccRCC would be expected to lead to increased E2F1 levels. In WT, E2F3 and its associated downstream target genes have been shown to be activated in and associated with more aggressive features19, 22. Here we show for the first time that E2F1 is also elevated in both human WT and a mouse model of progressive, metastatic WT. Collectively, these observations strongly imply that the E2F family of proteins play an important role in WT pathobiology and deserve further study.
CONCLUSIONS
The kidneys from mice with simultaneous activation of K-ras and Ctnnb1 (β-catenin) in renal tubular epithelium have an expression signature that is similar to renal malignancies in general, and WT in particular. The expression profile of KrasG12D/CatnbΔex3 and WT’s are associated with upregulation of the canonical Wnt/β-catenin pathway and its target genes and as well TP53 signaling networks. Here, we have shown that changes in these critical signaling networks are associated with increased expression of the transcription factor E2F1 in both a murine WT model and human WT’s, suggesting further study of the role of the family of E2F proteins in WT biology is warranted.
Supplementary Material
Acknowledgments
Funding Support: The project described was supported in part by Award Numbers K08 CA113452(PEC), P30 DK079341(PEC pilot fund),
References
- 1.Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80:2321. doi: 10.1002/(sici)1097-0142(19971215)80:12<2321::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Sonn G, Shortliffe LM. Management of Wilms tumor: current standard of care. Nat Clin Pract Urol. 2008;5:551. doi: 10.1038/ncpuro1218. [DOI] [PubMed] [Google Scholar]
- 3.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19:1926. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 4.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 5.Huff V. Wilms' tumours: about tumour suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer. 2011;11:111. doi: 10.1038/nrc3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koesters R, Ridder R, Kopp-Schneider A, et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 1999;59:3880. [PubMed] [Google Scholar]
- 7.Ruteshouser EC, Robinson SM, Huff V. Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer. 2008;47:461. doi: 10.1002/gcc.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 9.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- 11.Clark PE, Polosukhina D, Love H, et al. beta-Catenin and K-RAS synergize to form primitive renal epithelial tumors with features of epithelial Wilms' tumors. Am J Pathol. 2011;179:3045. doi: 10.1016/j.ajpath.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terzi F, Burtin M, Hekmati M, et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest. 2000;106:225. doi: 10.1172/JCI8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce J, Murphy AJ, Panzer A, et al. SIX2 Effects on Wilms Tumor Biology. Transl Oncol. 2014;7:800. doi: 10.1016/j.tranon.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Saeed AI, Sharov V, White J, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Qiu Q, Xie L, et al. Web-based interrogation of gene expression signatures using EXALT. BMC Bioinformatics. 2009;10:420. doi: 10.1186/1471-2105-10-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kort EJ, Farber L, Tretiakova M, et al. The E2F3-Oncomir-1 axis is activated in Wilms' tumor. Cancer Res. 2008;68:4034. doi: 10.1158/0008-5472.CAN-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi Y, Li C, Miller C, et al. Strategy for encoding and comparison of gene expression signatures. Genome Biol. 2007;8:R133. doi: 10.1186/gb-2007-8-7-r133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu Q, Lu P, Xiang Y, et al. A data similarity-based strategy for meta-analysis of transcriptional profiles in cancer. PLoS One. 2013;8:e54979. doi: 10.1371/journal.pone.0054979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An Q, Wang Y, An R, et al. Association of E2F3 expression with clinicopathological features of Wilms' tumors. J Pediatr Surg. 2013;48:2187. doi: 10.1016/j.jpedsurg.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Mans DA, Vermaat JS, Weijts BG, et al. Regulation of E2F1 by the von Hippel-Lindau tumour suppressor protein predicts survival in renal cell cancer patients. J Pathol. 2013;231:117. doi: 10.1002/path.4219. [DOI] [PubMed] [Google Scholar]
- 24.Ma X, Gao Y, Fan Y, et al. Overexpression of E2F1 promotes tumor malignancy and correlates with TNM stages in clear cell renal cell carcinoma. PLoS One. 2013;8:e73436. doi: 10.1371/journal.pone.0073436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su MC, Huang WC, Lien HC. Beta-catenin expression and mutation in adult and pediatric Wilms' tumors. APMIS. 2008;116:771. doi: 10.1111/j.1600-0463.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 26.Krabbe LM, Westerman ME, Bagrodia A, et al. Dysregulation of beta-Catenin is an Independent Predictor of Oncologic Outcomes in Patients with Clear Cell Renal Cell Carcinoma. J Urol. 2013 doi: 10.1016/j.juro.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 27.Putzer BM, Engelmann D. E2F1 apoptosis counterattacked: evil strikes back. Trends Mol Med. 2013;19:89. doi: 10.1016/j.molmed.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Berkovich E, Ginsberg D. Ras induces elevation of E2F-1 mRNA levels. J Biol Chem. 2001;276:42851. doi: 10.1074/jbc.M103596200. [DOI] [PubMed] [Google Scholar]
- 29.Abramova MV, Zatulovskiy EA, Svetlikova SB, et al. e2f1 Gene is a new member of Wnt/beta-catenin/Tcf-regulated genes. Biochem Biophys Res Commun. 2010;391:142. doi: 10.1016/j.bbrc.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Leung JY, Ehmann GL, Giangrande PH, et al. A role for Myc in facilitating transcription activation by E2F1. Oncogene. 2008;27:4172. doi: 10.1038/onc.2008.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.