Abstract
The sequence effect (SE) in Parkinson’s disease (PD) is progressive slowing of sequential movements. It is a feature of bradykinesia, but is separate from a general slowness without deterioration over time. It is commonly seen in PD, but its physiology is unclear. We measured general slowness and the SE separately with a computer-based, modified Purdue pegboard in 11 patients with advanced PD. We conducted a placebo-controlled, four-way crossover study to learn whether levodopa and repetitive transcranial magnetic stimulation (rTMS) could improve general slowness or the SE. We also examined the correlation between the SE and clinical fatigue. Levodopa alone and rTMS alone improved general slowness, but rTMS showed no additive effect on levodopa. Levodopa alone, rTMS alone, and their combination did not alleviate the SE. There was no correlation between the SE and fatigue. This study suggests that dopaminergic dysfunction and abnormal motor cortex excitability are not the relevant mechanisms for the SE. Additionally, the SE is not a component of clinical fatigue. Further work is needed to establish the physiology and clinical relevance of the SE.
Keywords: Parkinson’s disease, sequence effect, fatigue, rTMS, levodopa, Pegboard Test
Bradykinesia generally refers to slowness of movement, but in clinical practice it has been used loosely for different movement disorders (e.g., hypokinesia and akinesia). Such a range of meanings is practical, but can be confounding because the individual aspects may have different mechanisms. There is no correlation between bradykinesia and akinesia;1–3 and the different aspects have different drug responses.3, 4 Initiation of movement may not be specific to a dopaminergic deficit.4, 5 It is useful to separate these several elements in parkinsonian motor abnormalities.6
The sequence effect (SE) is characterized by progressive slowness in speed or a decrease in amplitude of sequential movements, a feature of bradykinesia in Parkinson’s disease (PD),7–9 but its pathophysiology is unknown. The postulated cause is a dopaminergic deficit,8 but it has never been adequately tested. The SE is also observed in parkinsonian gait.7 Medication, attention, and visual cues improved hypokinesia of gait, but only visual cues improved the SE.7 The SE was closely associated with akinesia.9 Results regarding the beneficial effect of levodopa on akinesia are contradictory.5
The SE may be associated with altered cortical excitability, because the basal ganglia (BG) are important for planning movement amplitude;10 the aberrant output from the BG to the motor cortex may produce this abnormality.11 rTMS reverses this abnormal excitability12, 13 and improves motor symptoms.14, 15 Although the exact mechanism of the rTMS effect is mostly unknown, it increased striatal dopamine release, presumably owing to corticostriatal activation.16,17 Cortical stimulation can affect various hypo- or hyperactive distant structures responsible for motor control.18 Motor cortex stimulation can improve both movement time and reaction time simultaneously in PD.15, 19 Cortical stimulation might show different effects from medication.
The SE may be related to fatigue.1, 8 The phenomenon is similar to decreased capacity in fatigue.20 Fatigue is common in PD, occurring in 44% to 56% of patients,21, 22 but the dopaminergic influence is controversial.23, 24 Fatigue is a complex phenomenon,25 and most studies have only used subjective fatigue scales. Objective measurement would be helpful. Measuring the SE is a possible objective measurement of one aspect of fatigue.
On the basis of these reports, we hypothesized that levodopa could mitigate the SE, if the SE was due to a dopaminergic deficit, and that cortical stimulation could alleviate the SE by reversing the influence of BG dysfunction. We also wanted to determine the possible additive effect of rTMS and medication on general slowness and the SE. Previous studies have evaluated rTMS in the off-state. It is more practical to know any additive effect of rTMS, because PD patients usually take medication. We hypothesized that if the SE is one element of fatigue, it might be correlated with clinical fatigue.
To this end, we studied the effect of levodopa and rTMS on the SE in PD patients in a placebo-controlled, four-way crossover study design and examined the correlation between the symptoms of fatigue and the SE.
METHODS
Subjects
Eleven right-handed patients (5 women, 6 men) with predictable symptomatic fluctuations in response to levodopa participated. Their mean (±SD) age was 60.6±9.0 years; the mean (±SD) disease duration was 9.5±5.4 years. The Hoehn and Yahr stages were 2.5 and 3. Mini-Mental State Examination (MMSE), Hamilton Depression Rating Scale (HDRS), Fatigue Severity Scale (FSS), and Multidimensional Fatigue Inventory (MFI) were evaluated (Table 1). We recruited the patients from the NINDS Clinics. Handedness was assessed with the Edinburgh Handedness Inventory.26 All patients gave written informed consent for this research protocol, which was approved by the NINDS Institutional Review Board.
TABLE 1.
Characteristics of patients with Parkinson’s disease
| No. | Age (yr) | Sex | Durationa (yr) | H&Y (on)b | MMSE | UPDRS (on)b | HDRS | FSS | MFI | Medication (mg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | 10 | 3 | 30 | 26 | 2 | 3.78 | 45 | Pramipexole 4.5 |
| 2 | 50 | F | 10 | 2.5 | 29 | 26 | 8 | 5.4 | 49 | Levodopa 600 Slow-release levodopa 100 Pramipexole 4.5 Entacapone 800 |
| 3 | 63 | M | 9 | 3 | 29 | 27 | 16 | 4.67 | 59 | Levodopa 550 Pramipexole 4.5 Entacapone 600 |
| 4 | 70 | F | 3 | 2.5 | 30 | 19 | 2 | 3 | 37 | Levodopa 300 Rasagiline 1 |
| 5 | 61 | M | 9 | 2.5 | 30 | 30 | 5 | 2 | 40 | Levodopa 500 Slow-release levodopa 200 Pramipexole 1 Entacapone 600 |
| 6 | 48 | M | 7 | 2.5 | 30 | 32 | 10 | 3.89 | 48 | Levodopa 400 Slow-release levodopa 800 Ropinirole 24 Entacapone 800 |
| 7 | 59 | F | 7 | 2.5 | 29 | 48 | 26 | 6.7 | 86 | Levodopa 300 Ropinirole 6 Entacapone 600 |
| 8 | 72 | M | 4 | 3 | 28 | 54 | 10 | 2.2 | 48 | Levodopa 300 Amantadine 200 |
| 9 | 51 | F | 7 | 3 | 30 | 41 | 11 | 3.67 | 50 | Levodopa 800 Entacapone 800 |
| 10 | 60 | M | 16 | 2.5 | 29 | 46 | 9 | 2 | 41 | Levodopa 1125 Ropinirole 12 |
| 11 | 58 | F | 22 | 3 | 29 | 32 | 6 | 2.56 | 30 | Levodopa 400 Slow-release levodopa 200 Pramipexole 4 |
Disease duration since diagnosis.
On medication.
Procedures
We assessed the SE as a progressive lengthening of peg movement time for successive peg movements, using a Modified Purdue Pegboard Test and a computer-based device (part of the At-Home Testing Device, Intel, courtesy of the Kinetics Foundation).27 Four interventions were applied to each patient: levodopa and rTMS; levodopa and sham stimulation; placebo and rTMS; placebo and sham stimulation. Before rTMS, the patients received medication in a double-blind fashion. The assignment to rTMS was via open randomization. A replicated four periods scheme and a four intervention-Latin Square design were used for the crossover design. Patients were randomly allocated to a fixed intervention order as determined by the balanced Latin square. Figure 1 shows an example of the order of the interventions. The interventions were separated by a greater than 1-week washout period that was set for rTMS, not medication.28, 29 All participants were asked not to take their antiparkinsonian medication for 12 hours before their visit in the morning.30–32
FIG. 1.

An example of the order of the four interventions:
levodopa and rTMS, levodopa and sham, placebo and rTMS, placebo and sham. The orders of the four interventions were randomly assigned.
For uniform testing conditions, the pegboard test, medication, and stimulation were carried out at the same time of day. The time (mean±SD) from medication to the start of the second pegboard test at each visit was 44.9±4.1 minutes and from medication to rTMS at each visit was 58.8±4.8 minutes; the duration between medication and the start of the peg board test after stimulation at each visit was 102.8±5.7 minutes.
Peg Insertion Testing
The Modified Purdue Pegboard Test had a vertical line of eight holes on both the right and the left sides. The task began with pegs on the right side. Patients were asked to transfer each peg from the right side to the line of holes on the left side as quickly as possible (one run). The pegboard could detect the time when each peg was pulled out and inserted. After they moved all pegs to the left side, the patients had to wait for a beep (10 seconds) before transferring the pegs back to the right side. There were six runs, three runs with the right hand first, and then three runs with the left hand. At the first visit, patients practiced the task of the six runs twice before data were recorded. The device sat on a table and was centered in front of the participant’s body. We could adjust the height of the table so that the patient was comfortable, and the angle between the forearm and the upper arm was kept at about 90 degrees during peg movements.
Repetitive Transcranial Magnetic Stimulation
rTMS was applied through a figure-of-eight coil over the left motor cortex. Focal TMS was performed with the first dorsal interosseous (FDI) muscle at rest, as described elsewhere.14 A Magstim Rapid magnetic stimulator (Whitland, Dyfed, UK) was used to deliver four rTMS blocks, each 10 minutes apart. Each block consisted of 15 25-pulse trains of 1-second duration at 25 Hz with an intertrain interval of 10 seconds at 100% resting motor threshold, modified from previous studies.14, 16, 33
Antiparkinsonian Medication and Placebo
We only used Sinemet (levodopa/carbidopa) and Sinemet placebo. We gave patients Sinemet equivalents of their usual morning dose. This was estimated according to previously published information.34–36 We did not estimate Sinemet equivalents for entacapone, rasagiline, and amantadine. Amantadine was withheld for the 3 days preceding the experimental session.
Data Analysis
We analyzed the pegboard test data prior to medication and after rTMS. “General slowness” means slowness of movement without progressive deterioration over time. This definition is necessary to avoid confusion with the all-encompassing phenomenon of bradykinesia. To assess general slowness, total time to move eight pegs from the right side to the left side and from the left side to the right side was measured. (?as meant) Total time to move eight pegs was averaged over three runs for each hand, per pegboard test, and per patient, and the resulting averages were used to calculate relative changes between pre- and post-interventions per patient to assess general slowness as: [(mean value in post-intervention–mean value in pre-intervention)/|mean value in pre-intervention|×100]. A positive value indicated increased total time after intervention and a negative value indicated decreased total time.
To evaluate the SE, differences between the time to move the first four pegs (1st–4th) and the time to move the last four pegs (5th–8th) were calculated for each of the 1st and 3rd runs with the right hand and 4th and 6th runs with the left hand, per pegboard test, and per subject. We did not consider the 2nd run using the right hand and the 5th run using the left hand because the direction was opposite to the other two runs for each hand. The differences were averaged over the two runs, per hand, per pegboard test, and per patient. To know whether the baseline SE in both hands was statistically significant, differences were averaged across the four interventions in the pre-medication condition for each hand and evaluated by means of a paired t-test. To determine whether medication and rTMS improved the SE, (%) relative change of the mean value in each hand between pre- and post-intervention was calculated [(mean value in post-intervention–mean value in pre-intervention)/|mean value in pre-intervention|×100]. A positive mean value indicated that the intervention did not reverse the SE and a negative mean value indicated improved SE after intervention.
Statistical Analysis
The (%) relative changes of general slowness or the SE between the four interventions were analyzed with a repeated-measures analysis of variance (ANOVA) and planned post-hoc comparisons. Since there was a significant interaction between medication condition and status of rTMS on relative change of general slowness, the comparison of the (%) relative change between rTMS and sham was assessed in each condition of medication and placebo by paired t-tests. Parallel analyses were done with nonparametic statistics with similar results and, therefore, not reported.
A Spearman correlation coefficient was used to explore the association between the SE in pre-intervention (the averaged value of the mean difference in four sessions with the right hand) and each of HDRS, FSS, MFI, and five dimensions of MFI. The coefficient was also calculated for comparison of HDRS with FSS, MFI, and five dimensions of MFI.
RESULTS
Figure 2 shows the time to move the first four pegs (open circles) and the time to move the last four pegs (closed circles) with the right and left hands in pre-intervention over four interventions. There was progressive slowing (SE), as well as increased speed, during movement of the last four pegs. (?as meant) A paired-t-test showed that the SE in pre-intervention was significant with the right hand, but did not reach a level of significance with the left hand (right hand, 7342.9±147.1 ms. vs. 7551.5±189.1 ms, p=0.04; left hand, 7893.9±250.9 ms. vs. 8045.8±252.4 ms, p = 0.28). A paired-t-test did not show the SE in pre-intervention with more and less affected hands.
FIG. 2.
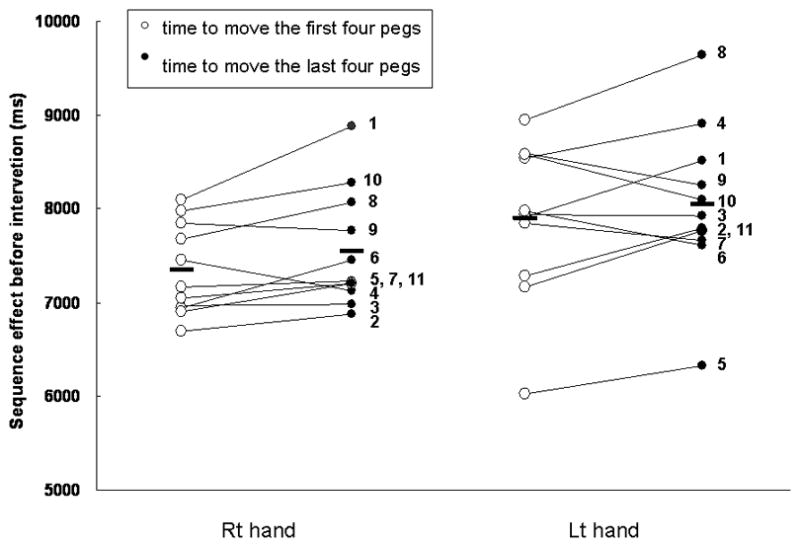
Sequence effect (SE) in 11 patients pre-intervention over four interventions.
Circles indicate time to move the first four pegs (1st–4th) (open circles) and time to move the last four pegs (5th–8th) (closed circles) (individual data as circles, group averages as bars). Increased mean value in closed circles indicates a SE (progressive slowing during peg movements) and decreased mean value indicates the opposite (speeding up during peg movements). The SE was significant in peg movements with the right hand, but did not reach a level of significance with the left hand (right hand, p=0.04; left hand, p=0.28; paired t-test).
Medication and rTMS improved general slowness, but rTMS had no additive effect on medication. Comparison of differences in medication condition between rTMS (−1.1±11.2 %) and sham stimulation (−3.5±6.9%) showed no significant mean (±SEM) difference of (%) relative change in the peg movements with the right hand (p=0.273). Comparison of differences in placebo condition between rTMS (2.8±12.3 %) and sham stimulation (6.9±9.8 %) showed a significant mean (±SEM) difference of (%) relative change in peg movements with the right hand (p<0.05; Figure 3).
FIG. 3.

(%) Relative change of general slowness in the right hand of PD patients.
General slowness is defined as total time for eight peg movements. The y-axis shows the value (mean±SEM) of (%) relative change for the general slowness between pre- and post-intervention in 11 patients. A positive value indicates increased total time after intervention; a negative value indicates decreased total time after intervention. There was a significant improvement of (%) relative change in peg movements between rTMS and sham stimulation in the placebo condition (*p<0.05).
Medication and rTMS did not improve the SE. The mean (±SEM) value of (%) relative change decreased more in the group with medication and rTMS (−504.5±1843.5 %) compared with medication and sham stimulation (−5.9±167.2%); the mean (±SEM) value of (%) relative change was lower in the group with placebo and rTMS (43.5±310.6%) compared with placebo and sham stimulation (100.5±205.7 %). However, there was no significant difference among the four interventions (p>0.1; Figure 4). The improvement with medication and rTMS appeared to be significantly better than the other interventions, but the large SD defeated statistical significance.
FIG. 4.

(%) Relative change of sequence effect (SE) in the right hand of PD patients.
The y-axis shows (mean±SEM value of the (%) relative change in the SE between pre- and post-intervention in 11 patients. A positive value indicates that the intervention did not reverse the SE; A negative value indicates improvement of the SE after intervention. There was no significant difference among the four interventions (p>0.1).
There was no correlation of the SE in pre-intervention with HDRS, FSS, MFI, and five dimensions of MFI. HDRS correlated with MFI (r=0.883, p=0.001) and showed a tendency to correlate with FSS (r=0.449, p=0.166). Among five dimensions of MFI, HDRS correlated with general fatigue (r=0.838, p=0.001) and physical fatigue (r=0.690, p=0.019).
DISCUSSION
Neither levodopa nor rTMS improved the SE in PD patients. The SE was not correlated with the subjective assessment of fatigue. Levodopa and rTMS were of benefit in general slowness, but rTMS did not show additive improvement in the on-medication state. A study of gait showed similar results.7 In a study of arm movement,37 in which patients repetitively squeezed a rubber bulb, performance rapidly deteriorated and was reversed by motivation, but not by anticholinergics. Levodopa was not tried, since the experiment was done in the pre-dopa era.
Defective cue production of the BG may lead to the SE.7 Despite this possible relationship, no interventions alleviated the SE in our study. Not all motor symptoms respond to these interventions. Reaching movements did not improve with either dopaminergic medication or deep brain stimulation.38–40 Non-dopaminergic medications such as zonisamide and alpha-2 antagonists can improve some motor symptoms.41, 42 A non-dopaminergic system may be related to the SE.
The non-primary motor cortex might be a better candidate for the SE. The BG is linked to the premotor area in a cortico-basal ganglia-thalamo-cortical circuit. In fMRI studies, sequential movements induced more activation in the premotor area in PD patients compared with healthy controls.43, 44 PD patients with frontal lobe dysfunction showed the SE.45 The supplementary motor area might be a good target because of its role in preparing and maintaining motor tasks.46, 47 The more affected side may be a reasonable target, but we stimulated the left motor cortex of right-handed patients. We thought that handedness might affect the performance of a complex motor task, since it is related to functional and structural hemispheric asymmetry.48, 49 Functional lateralization becomes larger with sequential movements.50, 51 Although it is likely that the more affected side may be correlated with the severity of the SE, it has so far not been investigated, and previous studies showed the SE involving the right hand regardless of the more severely affected side. For these reasons, we selected right-handed patients and applied TMS over the left motor cortex. The TMS was targeted to the distal hand muscle representation in the motor cortex. There were two components to the movement, elbow and finger movements. Optimized targeting of the proximal muscles might produce a different result. Targeting distal hand muscle was our main consideration, although the task demanded both proximal and distal muscles activation.15, 52 Because proximal and distal muscles are intermixed in the primary motor cortex53 and TMS activates a relatively large area, we certainly influenced proximal as well as distal muscles.
The severity of clinical fatigue was not correlated with the degree of the SE. It is possible that it is not one of the responsible elements of fatigue, but subjective assessment might not properly detect the SE. We can call the SE a type of central fatigue because of its relation to central nervous system dysfunction. It is characterized by a failure of physical tasks that demand self-motivation without obvious cognitive dysfunction or motor weakness.54 Some authors have suggested that central fatigue should be assessed by objective tools, because patients cannot perceive it. They suggested that it could occur under an unconscious (automatic) link between a sequential motor or cognitive task and sensory input.54 This concept is quite reasonable because the SE appears to be more apparent in an over-learned task (e. g., gait, writing) for which patients do not require much attention.
We found a statistically significant SE only in the right hand at the pre-intervention stage. Handedness is determined by the asymmetry of the proficiency of both hands,49 which suggests a difference of motor learning abilities. When the dominant hand is used for a repetitive task, there could be more automaticity, making the hand more subject to the SE. More attention for each movement might be needed for the non-dominant hand, and this might negate a SE.
Our study has limitations. The number of trials in individual patients to measure the SE was small, which might increase intrasubject variability. Another possible limitation is that the task might be inadequate to measure the SE. One reason is that considering the definition of the SE, the task chosen here can only capture speed changes, not amplitude changes, because the amplitude was predetermined in this study. However, it is unlikely to substantially affect our results, because the SE was demonstrated with similar tasks in several studies.8, 55, 56 Another reason is that the brain might produce the eight movements as eight separate motor plans. Sequential movements (a motor plan) consist of several submovements (motor programs). Performing these submovements may be normal, whereas automatic execution of the motor plan may be impaired due to defective cueing in PD.57 The selection of each peg may prevent patients from counting the eight-peg movements as a motor plan. Another limitation is the 2-hour experiments, which might have been too long. In the placebo condition and sham stimulation, the general slowness was longer than in pre-intervention, suggesting that motor function deteriorated over time. The aggravation of overall motor function could affect the SE, making it more difficult to reverse.
Acknowledgments
H&Y, Hoehn and Yahr; MMSE, Mini-Mental State Examination; UPDRS, Unified Parkinson’s Disease Rating Scale; HDRS, Hamilton Depression Rating Scale; FSS, Fatigue Severity Scale; MFI, Multidimensional Fatigue Inventory. This work was supported by the intramural research program of the NINDS at the NIH and the American Parkinson Disease Association. We appreciate the Kinetics Foundation (Ken Kubota, BS) and Intel Inc. (William DeLeeuw, BSEE and David Wolff, BS) for providing the at-home testing device and technical support. We thank two anonymous reviewers for their valuable comments and suggestions, and Devera G. Schoenberg, MSc, and B.J. Hessie, BSc, ELS, for skillful editing.
Footnotes
AUTHOR ROLES:
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript: A. Writing of the first draft, B. Review and Critique;
Suk Yun Kang: 1A, 1B, 1C, 2A, 2B, 3A, 3B
Toshiaki Wasaka, Ejaz A. Shamim, Yoshino Ueki, Grisel J Lopez, Tetsuo Kida, Seung-Hyun Jin, Nguyet Dang: 1C
Sungyoung Auh: 2A, 2B, 2C
Mark Hallett: 1A, 1B, 2C, 3B
Financial disclosure: The authors report no conflicts of interest or have nothing to disclose regarding any financial interest.
FULL FINANCIAL DISCLOSURES OF ALL AUTHORS FOR THE PAST YEAR:
Suk Yun Kang, Toshiaki Wasaka, Sungyoung Auh, Yoshino Ueki, Grisel J. Lopez, Tetsuo Kida, Seung-Hyun Jin, Nguyet Dang: None.
Ejaz A. Shamim has medically related stocks, Amgen, Pfizer, and Medtronics; serves on the speakers bureau of Allergen; has received financial support from the Kinetics Foundation.
Mark Hallett serves as Chair of the Medical Advisory Board for and receives funding for travel from the Neurotoxin Institute; serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation and Chair of the Medical Advisory Board of the International Essential Tremor Foundation; has received honoraria and/or funding for travel for lectures or educational activities not funded by industry; serves on Editorial Advisory Boards for Clinical Neurophysiology, Brain, Acta Neurologica Scandinavica, Journal of Clinical Neurophysiology, Italian Journal of Neurological Sciences, Medical Problems of Performing Artists, Annals of Neurology, Neurology and Clinical Neurophysiology, The Cerebellum, NeuroRx, Current Trends in Neurology, Faculty of 1000 Biology, European Neurology, Faculty of 1000 Medicine, Brain Stimulation, Journal of Movement Disorders (Korea), and World Neurology; may accrue revenue on US Patent #6,780,413 B2 (issued: August 24, 2004): immunotoxin (MAB-Ricin) for the treatment of focal movement disorders; US Patent #7,407,478 (issued: August 5, 2008): coil for magnetic stimulation and methods for using the same; receives royalties from publishing from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier; receives research support from Ariston Pharmaceuticals, NIH/NINDS (Intramural Program) and the US Department of Defense (Army); has received license fee payments from the NIH (from Brainsway) for licensing the patent for the H-coil; and with his spouse held stock in Agilent Technologies, Amgen, Amylin Pharmaceuticals, Merck & Co., Monsanto Co New Del, Sanofi-aventis, Coventry Health Care Inc., Sigma Aldrich Corp., Warner Chilcott Ltd., Pfizer Inc., Genentech, Inc., United Health Group, St. Jude Medical, and Eli Lilly and Company. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds come from the US Army via the Henry Jackson Foundation, Ariston Pharmaceutical Company via a Cooperative Research and Development Agreement (CRADA) with the NIH, and the Kinetics Foundation via a Clinical Trials Agreement (CTA) with the NIH.
References
- 1.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 2.Evarts EV, Teravainen H, Calne DB. Reaction time in Parkinson’s disease. Brain. 1981;104:167–186. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD. Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1986;49:1273–1279. doi: 10.1136/jnnp.49.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velasco F, Velasco M. A quantitative evaluation of the effects of L-dopa on Parkinson’s disease. Neuropharmacology. 1973;12:89–99. doi: 10.1016/0028-3908(73)90079-8. [DOI] [PubMed] [Google Scholar]
- 5.Jahanshahi M, Brown RG, Marsden CD. The effect of withdrawal of dopaminergic medication on simple and choice reaction time and the use of advance information in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55:1168–1176. doi: 10.1136/jnnp.55.12.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niedermeyer E. Akinesia and the frontal lobe. Clin EEG Neurosci. 2008;39:39–42. doi: 10.1177/155005940803900112. [DOI] [PubMed] [Google Scholar]
- 7.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006;21:1419–1424. doi: 10.1002/mds.20998. [DOI] [PubMed] [Google Scholar]
- 8.Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- 9.Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson’s disease. Brain. 1987;110(Pt 2):361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 10.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- 11.Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain. 1996;119(Pt 1):71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- 12.Mally J, Farkas R, Tothfalusi L, Stone TW. Long-term follow-up study with repetitive transcranial magnetic stimulation (rTMS) in Parkinson’s disease. Brain Res Bull. 2004;64:259–263. doi: 10.1016/j.brainresbull.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 14.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21:325–331. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson’s disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44:892–898. doi: 10.1212/wnl.44.5.892. [DOI] [PubMed] [Google Scholar]
- 16.Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): insights into the treatment of Parkinson’s disease by cortical stimulation. Neurophysiol Clin. 2006;36:125–133. doi: 10.1016/j.neucli.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1171–1174. doi: 10.1136/jnnp.2003.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churchill’s illustrated medical dictionary. 1. New York: Churchill Livingstone; 1989. Churchill’s illustrated medical dictionary. [Google Scholar]
- 21.Karlsen K, Larsen JP, Tandberg E, Jorgensen K. Fatigue in patients with Parkinson’s disease. Mov Disord. 1999;14:237–241. doi: 10.1002/1531-8257(199903)14:2<237::aid-mds1006>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59:408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JH, Brown RG, Comella C, et al. Fatigue in Parkinson’s disease: a review. Mov Disord. 2007;22:297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- 24.Schifitto G, Friedman JH, Oakes D, et al. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. 2008;71:481–485. doi: 10.1212/01.wnl.0000324862.29733.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Goetz CG, Stebbins GT, Wolff D, et al. Testing objective measures of motor impairment in early Parkinson’s disease: Feasibility study of an at-home testing device. Mov Disord. 2009;24:551–556. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T, Ohnishi T, Okabe S, et al. Long-term effect of motor cortical repetitive transcranial magnetic stimulation [correction] Ann Neurol. 2004;56:77–85. doi: 10.1002/ana.20151. [DOI] [PubMed] [Google Scholar]
- 29.Khedr EM, Rothwell JC, Ahmed MA, Shawky OA, Farouk M. Modulation of motor cortical excitability following rapid-rate transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:140–145. doi: 10.1016/j.clinph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Evans AH, Pavese N, Lawrence AD, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 31.Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson’s disease. Cereb Cortex. 2006;16:64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- 32.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 33.Hallett M, Wassermann EM, Pascual-Leone A, Valls-Sole J. Repetitive transcranial magnetic stimulation. The International Federation of Clinical Neurophysiology Electroencephalogr Clin Neurophysiol Suppl. 1999;52:105–113. [PubMed] [Google Scholar]
- 34.Alonso-Frech F, Zamarbide I, Alegre M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- 35.Fine J, Duff J, Chen R, et al. Long-term follow-up of unilateral pallidotomy in advanced Parkinson’s disease. N Engl J Med. 2000;342:1708–1714. doi: 10.1056/NEJM200006083422304. [DOI] [PubMed] [Google Scholar]
- 36.Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- 37.Schwab RS, England AC, Peterson E. Akinesia in Parkinson’s disease. Neurology. 1959;9:65–72. doi: 10.1212/wnl.9.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Bastian AJ, Kelly VE, Perlmutter JS, Mink JW. Effects of pallidotomy and levodopa on walking and reaching movements in Parkinson’s disease. Mov Disord. 2003;18:1008–1017. doi: 10.1002/mds.10494. [DOI] [PubMed] [Google Scholar]
- 39.Benice TS, Lou JS, Eaton R, Nutt J. Hand coordination as a quantitative measure of motor abnormality and therapeutic response in Parkinson’s disease. Clin Neurophysiol. 2007;118:1776–1784. doi: 10.1016/j.clinph.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Melvin KG, Doan J, Pellis SM, Brown L, Whishaw IQ, Suchowersky O. Pallidal deep brain stimulation and L-dopa do not improve qualitative aspects of skilled reaching in Parkinson’s disease. Behav Brain Res. 2005;160:188–194. doi: 10.1016/j.bbr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Murata M, Hasegawa K, Kanazawa I. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68:45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 42.Schapira AH. Future directions in the treatment of Parkinson’s disease. Mov Disord. 2007;22:S385–S391. doi: 10.1002/mds.21679. [DOI] [PubMed] [Google Scholar]
- 43.Mallol R, Barros-Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–271. doi: 10.1016/j.brainres.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 44.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 45.Rogers MA, Phillips JG, Bradshaw JL, Iansek R, Jones D. Provision of external cues and movement sequencing in Parkinson’s disease. Motor Control. 1998;2:125–132. doi: 10.1123/mcj.2.2.125. [DOI] [PubMed] [Google Scholar]
- 46.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 47.Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- 48.Amunts K, Schlaug G, Schleicher A, et al. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 49.Herve PY, Mazoyer B, Crivello F, Perchey G, Tzourio-Mazoyer N. Finger tapping, handedness and grey matter amount in the Rolando’s genu area. NeuroImage. 2005;25:1133–1145. doi: 10.1016/j.neuroimage.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 50.Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 51.Serrien DJ, Spape MM. The role of hand dominance and sensorimotor congruence in voluntary movement. Exp Brain Res. 2009;199:195–200. doi: 10.1007/s00221-009-1998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord. 2006;21:2201–2205. doi: 10.1002/mds.21089. [DOI] [PubMed] [Google Scholar]
- 53.Devanne H, Cassim F, Ethier C, Brizzi L, Thevenon A, Capaday C. The comparable size and overlapping nature of upper limb distal and proximal muscle representations in the human motor cortex. Eur J Neurosci. 2006;23:2467–2476. doi: 10.1111/j.1460-9568.2006.04760.x. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 55.Agostino R, Berardelli A, Formica A, Stocchi F, Accornero N, Manfredi M. Analysis of repetitive and nonrepetitive sequential arm movements in patients with Parkinson’s disease. Mov Disord. 1994;9:311–314. doi: 10.1002/mds.870090305. [DOI] [PubMed] [Google Scholar]
- 56.Berardelli A, Accornero N, Argenta M, Meco G, Manfredi M. Fast complex arm movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1986;49:1146–1149. doi: 10.1136/jnnp.49.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsden CD. Function of the basal ganglia as revealed by cognitive and motor disorders in Parkinson’s disease. Can J Neurol Sci. 1984;11:129–135. doi: 10.1017/s031716710004628x. [DOI] [PubMed] [Google Scholar]


