Abstract
Compared to adults, adolescents are at heightened risk for drug abuse and dependence. One of the factors contributing to this vulnerability may be age-dependent differences in reward processing, with adolescents approaching reward through stimulus-directed, rather than goal-directed, processes. However, the empirical evidence for this in rodent models of adolescence, particularly those that investigate both sexes, is limited. To address this, male and female rats that were adolescents (P30) or adults (P98) at the start of the experiment were trained in a Pavlovian approach (PA) task and were subsequently tested for the effects of reward devaluation, extinction, and re-acquisition. We found significant interactions between age and sex: females had enhanced acquisition of PA and poorer extinction, relative to males, while adolescents and females were less sensitive to reward devaluation than male adults. These results suggest that females and adolescents exhibit reward behavior that is more stimulus-directed, rather than goal-directed.
Keywords: adolescent, sex, learning, extinction, reward devaluation, rat, Pavlovian approach, reacquisition, reward processing
1. Introduction
Adolescence, the transitional period between childhood and adulthood that is characterized by numerous changes in brain anatomy and function, begins when humans are about twelve years old and may extend through the early- to mid-twenties (Lenroot & Giedd, 2006; Spear, 2000). During this time, sensation seeking, risk taking, and social behavior all tend to increase (Galvan, 2010; Gardner & Steinberg, 2005; Steinberg et al., 2008). Similar changes in behavior have been noted in rodent models of this stage of development, where adolescence has been conservatively defined as beginning around postnatal day (P) 28 and extending to P42 (Spear, 2000) or perhaps as late as P60 (Brenhouse & Andersen, 2011; Tirelli, Laviola, & Adriani, 2003). For example, adolescent rats have been reported to be more responsive towards natural, as well as drug, rewards (Anker, Zlebnik, Navin, & Carroll, 2011; Burton, Noble, & Fletcher, 2011; Friemel, Spanagel, & Schneider, 2010; Shahbazi, Moffett, Williams, & Frantz, 2008; Zakharova, Wade, & Izenwasser, 2009). This enhanced reward-seeking behavior may contribute to increases in vulnerability to drug abuse and addiction during this period (Chambers, Taylor, & Potenza, 2003; Doremus-Fitzwater, Varlinskaya, & Spear, 2010; Spear, 2000).
Debate about the determinants of enhanced reward-seeking behavior during adolescence has often focused on the question of whether adolescents place higher value on rewarding experiences or if they are relatively less sensitive to these rewards (Ernst, Romeo, & Andersen, 2009; Somerville, Jones, & Casey, 2010; Spear, 2000). More recently, however, studies have suggested that adolescents engage in more reward seeking and exhibit enhanced conditioning because their attention for reward relies on stimulus-directed, rather than goal-directed, processes (Ernst, Daniele, & Frantz, 2011). Stimulus-directed behavior refers to behaviors that are guided by exogenous information, such as cues or feedback, while goal-directed behavior refers to those that are guided by endogenous information, such as motivational state (Ernst et al., 2011). A recent study using the Iowa Gambling Task demonstrated that human adolescents were more sensitive to positive feedback than adults (Cauffman et al., 2010). Unfortunately, there have been few studies of appetitive conditioning in humans that directly compare adolescents and adults.
Studies from rodent models that support this hypothesis have focused on conditioned taste aversion (CTA) and extinction, which test for responding in the face of reduced reward value. Reductions in reward value should cause decreases in goal-directed responding through a reduction in the motivational power of the reward. However, if responding is primarily stimulus-directed, (i.e., guided by cues), then reductions in reward value will have less of an impact on behavior. These studies have revealed that adolescents are less sensitive to CTA (Anderson, Varlinskaya, & Spear, 2010; Infurna & Spear, 1979; Shram, Funk, Li, & Lê, 2006) and they are resistant to extinction compared to adults for both drug (Anker & Carroll, 2010; Brenhouse & Andersen, 2008) and food rewards (Andrzejewski et al., 2011; Sturman, Mandell, & Moghaddam, 2010). However, evidence for an enhancement in stimulus-directed reward behavior in adolescents is not yet conclusive. One recent study failed to uncover age differences in extinction and reacquisition for food self-administration (Li & Frantz, 2010). Another recent study found that adults engaged in more sign-tracking, a measure of behavior directed at the stimulus, than adolescents (Doremus-Fitzwater & Spear, 2011). Moreover, CTA is not a pure measure of reward devaluation because it requires the development of an aversion; adolescents are known to be less sensitive to the aversive effects of many drugs (Schramm-Sapyta, Walker, Caster, Levin, & Kuhn, 2009; Spear, 2011). Reward devaluation through reinforcer satiation allows for the reduction of motivational value without the development of aversion, but there has only been one study on the effect of age on this measure (Naneix, Marchand, Di Scala, Pape, & Coutureau, 2012). The currently available research is insufficient to characterize the effects of stimulus-directed and goal-directed behavior on developmental differences in reward seeking.
Gender (or sex) is another important factor that may influence developmental differences in reward-related behavior. For example, self-reported sensation seeking increases from early to late adolescence in males, but remains stable during this time in females (Steinberg et al., 2008). Telescoping, the phenomenon whereby females have a characteristically more rapid onset and worse presentation of addiction compared to males, begins to emerge during adolescence (Kuhn et al., 2010; Randall et al., 1999). In rats, female adults are generally more responsive for drug reward than male adults (Chaudhri et al., 2005; Kosten & Zhang, 2008; Kuhn et al., 2010; Lynch, Roth, Mickelberg, & Carroll, 2001) and there is an interaction between age and sex for cocaine and amphetamine self-administration and conditioned place preference (Anker et al., 2011; Mathews & McCormick, 2007; Shahbazi et al., 2008; Zakharova et al., 2009). The nature of this interaction is not well understood, however, as adolescent age and female sex are additive in some cases (Anker et al., 2011; Mathews & McCormick, 2007), but not in others (Shahbazi et al., 2008; Zakharova et al., 2009). In drug self-administration studies adult females respond more than males throughout extinction (Carroll & Anker, 2010; Kuhn et al., 2010; Perry, Nelson, & Carroll, 2008). This suggests that females, like adolescents, may exhibit behavior that is more stimulus-directed, rather than goal directed. However, sex differences in drug sensitivity may influence these results as females are relatively more sensitive to cocaine and amphetamine and this can vary with estrous cycle (Carroll & Anker, 2010; Festa & Quiñones-Jenab, 2004; Mathews, Waters, & McCormick, 2009).
In the current study we investigated the interaction between age and sex on the expression of stimulus-directed behavior in the context of a simple associative learning paradigm. Rats were trained in a Pavlovian approach (PA) paradigm wherein an auditory conditioned stimulus (CS+) was paired with delivery of a sucrose solution (unconditioned stimulus; US) to a food trough. We assessed the development of food trough entries (conditioned response; CR) during daily training sessions consisting of 8 CS-US pairings, following devaluation of the reward, and during periods of extinction and reacquisition. In light of previous studies with cocaine (Anker et al., 2011; Fuchs, Evans, Mehta, Case, & See, 2005; Kosten & Zhang, 2008) and food reward (Andrzejewski et al., 2011; Anker et al., 2011; Burton et al., 2011; Sturman et al., 2010) suggesting that adolescents and females may have more stimulus-directed behavior, we hypothesized that these groups would exhibit enhanced acquisition of the cue-reward association and decreased sensitivity to manipulations of reward value, such as extinction and reward devaluation.
2. Methods
2.1. Subjects
A total of 51 male and female Sprague-Dawley rats, which were born in our animal facility from breeders originally obtained from Harlan (Indianapolis, IN, USA), were used in these experiments. Seven rats were removed from the study during the initial stages of training because they failed to meet an a priori inclusion criterion of entering the food trough during sucrose delivery in two of the first three training sessions. Of these seven rats, two were adolescent males, two were adult females, and three were adult males. Final group sizes were 10 rats per sex in the adolescent groups and 12 rats per sex in the adult groups.
Rats were weaned on P22 and housed with same-sex littermates in groups of 2–3 per cage for the duration of the experiment. Rats were kept on a 12-h light/dark cycle (lights on at 0800 h) in a temperature-controlled room and water was available ad libitum throughout the study. Food was available ad libitum until 2 days prior to the start of the experiment, which began when rats were adolescents (P30) or adults (P98). At this time, food was given in daily allotments and was limited for each rat so that by the end of the experiment, body weight was 90% of age- and sex-matched controls (adolescents) or 90% of their free-feeding weight (adults). With this procedure, all rats weighed 93–95% of their age-appropriate controls at the midpoint of training and testing. Rats from individual litters were randomly assigned to the adolescent or adult testing groups within an experiment, such that the 8 litters from which these subjects were taken were represented in a nearly equal manner across groups. All procedures were consistent with the `Principles of Laboratory Animal Care' (NIH Publication no. 85-23) and were approved by the IACUC at the University of Illinois, Urbana-Champaign, USA.
2.2. Apparatus
Pavlovian approach (PA) training occurred in standard monitoring chambers (Coulbourn Instruments, Allentown, PA, USA) located within sound attenuating cubicles. Chambers were equipped with a food trough on the front wall, which dispensed liquid via extension of a 0.06 mL dipper cup. Nosepoke ports containing green LEDs were located on either side of the food trough. The food trough and nosepoke ports were equipped with infrared photocells to detect head entries. A white house-light (4W), which was illuminated throughout all behavioral sessions, was located near the top of the chamber on the opposite wall. A tone-emitting speaker (2.9kHz, 80–85dB; Sonalert) was attached to the ceiling of the chamber. Sessions were recorded and analyzed using Graphic State software (Coulbourn Instruments).
2.3. Sucrose pre-exposure and magazine training (P30–33 or P98–101)
Rats were given two daily 60-min sessions where they were introduced to the 20% sucrose solution prior to the start of training. This was accomplished by placing rats individually into cages and giving them free access to a sipper tube filled with 20% sucrose solution. The individual cages were identical to the rats' home cages and each rat used the same cage for sucrose exposure and transportation to the behavioral testing room for the duration of the study. Next, rats underwent two daily sessions of magazine training in the monitoring chambers. During these 40–45 min sessions, the dipper cup filled with 20% sucrose was extended into the food trough for 4–8 s. This occurred on a random time (RT) 30 schedule for a total of 60 presentations. Sucrose pre-exposure and magazine training sessions were conducted between 1100 and 1400 h.
2.4. Conditioning (P34–41 or P102–109)
Each 35–40 min session consisted of 16 trials, which were divided into 8 CS+ and 8 CS− trials that were presented in random order. During CS+ trials, a tone was presented for 10-s, followed immediately by extension of the sucrose-filled dipper cup for 4-s and a variable intertrial interval (ITI) of 90-150-s. During CS− trials a light (illumination of LEDs in the nosepoke ports) was presented for 10-s and was followed immediately by the ITI. Rats received 8 daily sessions of conditioning between 1100 and 1400 h.
2.5. Reward devaluation (P42 or P110)
To assess the effects of reward devaluation on PA, rats were given 60 min of free access to sipper tube containing 20% sucrose. The procedure for this was identical to that used during their pre-exposure to the sucrose solution. Immediately after the free access period, rats were placed in the monitoring chambers for the devaluation session. This session was conducted under extinction conditions, wherein no sucrose solution was available in the dipper cup but all other aspects of the session were identical to conditioning sessions.
2.6. Extinction and Reacquisition (P43–51 or P111–119)
Starting the day after the reward devaluation session, rats received two more daily sessions of conditioning (with sucrose solution present) in order to re-establish PA. They then received 10 twice-daily extinction sessions over the course of five days. During these sessions, which were conducted in the morning (0800-1100 h) and afternoon (1500-1800 h), no sucrose solution was available in the dipper cup but all other aspects of the session were identical to conditioning sessions. Daily food allotments were not given until the conclusion of the afternoon training session.
Following extinction, rats underwent two daily sessions of PA training to examine re-acquisition of the cue-reward association following extinction. These sessions were conducted in a manner identical to those in the conditioning phases of the study.
2.8. Data analysis
In the magazine training sessions the total number of entries into the trough was recorded for each session. In PA sessions trough entries were recorded during the 10-s CS+ and 10-s CS− periods, as well as during the 10-s preceding each CS+ and CS− period. An approach score was calculated by subtracting the number of trough entries during the 10-s before the onset of the CS+ or CS− from the number of CS+ or CS− trough entries. The total number of trough entries (magazine training) and the CS+ and CS− approach scores (PA sessions) were analyzed using a mixed factorial three-way ANOVA with age (adolescent or adult) and sex (male or female) as between-subjects factors and session or day (average of morning and evening sessions for extinction phase) as the repeated measure factor. The effects of age (collapsed across sex) and sex (collapsed across age) were analyzed separately using planned two-way ANOVA with session or day as the second factor. During the reward devaluation phase, sucrose consumption during the free-access period was normalized to individual rats' bodyweights (ml/kg consumed) and devaluation relative to baseline conditioning was assessed by computing the change in approach score from the 8th conditioning day. These variables were then analyzed statistically with between-subjects ANOVA (age × sex). For all statistical analyses, significant interactions were further analyzed with Holm-Sidak post hoc tests.
3. Results
3.1. Initial Learning
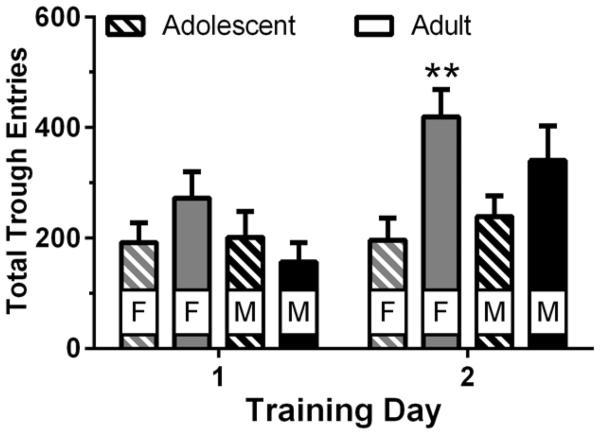
Adults entered the magazine more than adolescents during the magazine training sessions prior to PA training (Fig. 1). Three-way ANOVA of total entries revealed main effects of age (F1,40 = 4.56, p = 0.039) and session (F1,40 = 23.6, p < 0.001), as well as an age × session interaction (F1,40 = 14.2, p < 0.001).
Figure 1.
Total trough entries during magazine training sessions for male (M) and female (F) adolescent and adult rats (n = 10–12/group). ** p < 0.01 vs. adolescent females
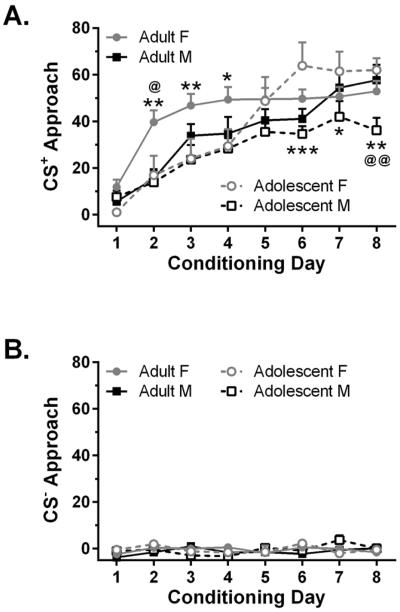
Although three-way ANOVA revealed that all rats developed approach behavior toward the CS+ during the first 8 conditioning sessions (Fig. 2a; session: F7,280 = 24.2, p < 0.001), females displayed enhanced development of approach relative to males (sex: F1,40 = 7.43, p = 0.010). In addition, there were sex × session (F7,280 = 2.45, p = 0.019) and age × sex × session (F7,280 = 2.13, p = 0.040) interactions. Specifically, adult females developed approach more rapidly than adult males or adolescent females; adolescent females responded more than adolescent males in later sessions. Planned two-way ANOVA revealed an age × session interaction in females (F7,140 = 2.62, p = 0.014), as well as a sex × session interaction in both adolescents (F7,126 = 2.11, p = 0.047) and adults (F7,154 = 2.38, p = 0.024). Rats did not develop approach toward the CS− (Fig. 2b; session p > 0.25).
Figure 2.
Approach behavior during the first eight conditioning sessions (n = 10–12/group). Panel A shows CS+ approach, panel B shows CS− approach. The approach score was calculated as a difference score: trough entries during the 8 10-s CS trials - trough entries during the 8 10-s periods preceding each CS trial. * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. adolescent females; @ p < 0.05 and @@ p < 0.01 vs. adult males
3.2. Reward devaluation
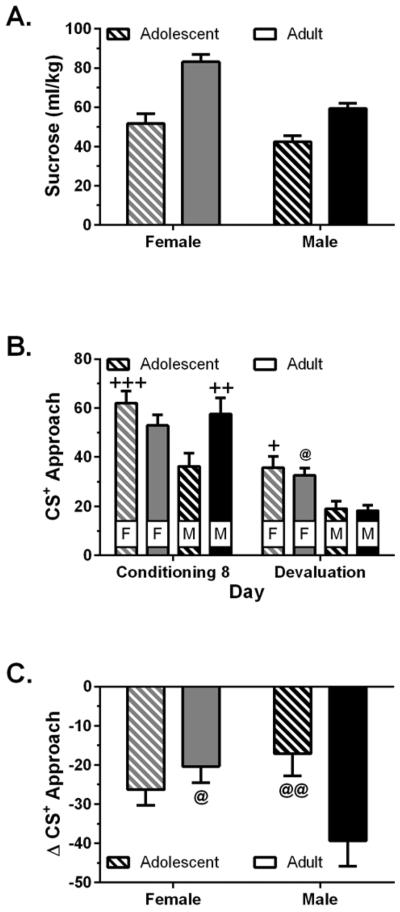
During the period of free access to sucrose, adult females consumed more sucrose per bodyweight than any other group (Fig. 3a). Two-way ANOVA of sucrose consumption revealed main effects of age (F1,40 = 43.8, p < 0.001) and sex (F1,40 = 20.7, p < 0.001), as well as an age × sex interaction that approached statistical significance (p = 0.053). Three-way ANOVA revealed that there was a reduction in approach behavior following the free access period (Fig. 3b; session: F1,40 = 95.31, p < 0.001). Adolescent males exhibited less approach behavior than adult males (sex × age interaction: F1,40 = 5.05, p = 0.030) and females exhibited more approach behavior than males during both conditioning and devaluation (sex: F1,40 = 12.8, p < 0.001). However, the effect of devaluation on approach depended on age and sex (age × sex × session interaction: F1,40 = 7.14, p = 0.011), such that adult males were relatively more affected by devaluation than the other groups. Planned two-way ANOVA revealed an age × session interaction in males (F1,20 = 6.36, p = 0.020) and a sex × session interaction in adults (F1,22 = 6.05, p = 0.022). To control for differences in responding during conditioning session 8, responding during the reward devaluation session was further analyzed by computing the change in approach (Fig. 3c; [CS+ approach during devaluation session - CS+ approach during conditioning session 8]). Two-way ANOVA of these data revealed an age × sex interaction (F1,40 = 7.14, p = 0.011), with adult males showing the greatest reduction in approach following devaluation.
Figure 3.
Approach behavior following reward devaluation (n = 10–12/group). Panel A shows consumption of 20% sucrose solution, relative to bodyweight, during the 60 min free access period. Panel B shows CS+ approach during the 8th conditioning session and the reward devaluation session. Panel C shows the change in CS+ approach following devaluation, calculated as a difference score: devaluation session CS+ approach – conditioning session 8 CS+ approach. + p < 0.05, ++ p < 0.01 and +++ p < 0.001, vs. adolescent males; @ p < 0.05 and @@ p < 0.01, vs. adult males
3.3. Extinction and reacquisition
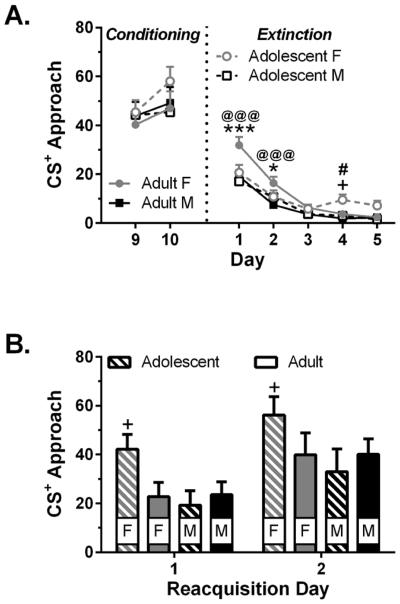
Three-way ANOVA revealed that all rats decreased approach during extinction (Fig. 4a; day: F4,160 = 65.9, p < 0.001). Although females responded more than males during extinction (sex: F1,40 = 13.3, p < 0.001), females did not respond more than males during the two conditioning sessions preceding extinction (p > 0.25). In addition, there were age × day (F4,160 = 4.07, p = 0.004) and sex × age × day (F4,160 = 2.70, p = 0.033) interactions. Relative to adult males and adolescent females, adult females had high levels of approach during the first two days of extinction. Adolescent females, meanwhile, had high approach relative to adolescent males and adult females later in extinction. Planned two-way ANOVA revealed an age × day interaction (F4,80 = 4.14, p = 0.004) in females as well as a main effect of sex (F1,22 = 11.9, p = 0.002) and a sex × day interaction (F4,88 = 3.47, p = 0.011) in adults.
Figure 4.
Extinction and reacquisition (n = 10–12 rats/group). Panel A shows the CS+ approach for the two conditioning sessions prior to extinction, as well as the CS+ approach during each day (average of morning and evening sessions) of extinction. Panel B shows CS+ approach during reacquisition. * p < 0.05 and *** p < 0.001, vs. adolescent females; # p < 0.05 vs. adult females; + p < 0.05 vs. adolescent males; @@@ p < 0.001, vs. adult males
Three-way ANOVA revealed that all rats increased responding from the first to the second session of reacquisition (Fig. 4b; session: F1,40 = 38.5, p < 0.001). Although the three-way ANOVA failed to reach significance for sex (p=0.096) or the age × sex interaction (p=0.083), planned two-way ANOVA revealed an effect of sex (F1,18 = 5.83, p = 0.027) in adolescents. Adolescent females had a greater expression of approach behavior during both sessions of reacquisition relative to adolescent males.
4. Discussion
In the present study we used adolescent and adult rats to examine the interaction between age and sex in a Pavlovian approach task. Performance on the Pavlovian approach task is influenced by goal-directed processes, whereby the representation of the outcome and internal motivation state influence performance of actions directed toward obtaining that outcome, such as trough entries (Dickinson, 1994). However, the acquisition of PA requires stimulus-directed processes as well; Pavlovian approach is defined as behavior elicited by a reward-predictive stimulus. We found that female rats showed enhanced acquisition of the cue-reward association, but were less affected by reward devaluation and extinction, which are processes that reduce the motivational value of the reward (Ostlund & Balleine, 2008). Adolescent rats were less affected by reward devaluation than adults and had a greater degree of reacquisition of the cue-reward association, but these effects were sex-specific. Rather than being additive, the effects of age and sex were such that females had enhanced development of stimulus-directed behavior, while adolescents had weaker expression of goal-directed behavior.
Conditioning
Adults responded significantly more than adolescents during magazine training. This finding is consistent with recent studies in male rats, where adult animals had substantially higher baseline levels of responding for sweetened liquid compared to adolescents (Andrzejewski et al., 2011) and adolescent rats took longer to approach the site of reward delivery (Burton & Fletcher, 2012; Doremus-Fitzwater & Spear, 2011). Trough entries during magazine training provide a measure of initial goal-directed behavior, as rats are developing a representation of the reward associated with trough entry during this period. This baseline difference in activity toward the food trough supports the notion that adolescents have relatively weaker goal-directed attention (Ernst et al., 2011).
Our results suggest that the relationship between goal-directed attention during magazine training and the development of stimulus-directed approach behavior depends on sex. During the initial phase of Pavlovian approach training adult females exhibited more rapid acquisition of approach behavior, compared to adolescent females and adult males. However, adult males did not acquire the association more rapidly than adolescent males. This suggests that the enhanced goal-directed attention in adults enhanced the development of stimulus-directed behavior during the Pavlovian approach task for females, but not for males. This sex difference in the development of approach behavior was seen in adolescent animals as well, though its expression was delayed. Female adolescents reached higher levels of approach by the final sessions of training, relative to male adolescents. The sex difference in acquisition of approach is consistent with studies of cocaine self-administration that show females have enhanced acquisition of this behavior compared to males (Carroll & Anker, 2010). Overall, these results suggest that females exhibit enhanced development of stimulus-directed behavior that is accelerated by enhanced goal-directed attention in adulthood.
We did not find evidence for enhanced development of stimulus-directed behavior during adolescence. In fact, adult males had higher levels of approach than adolescent males in the final conditioning sessions. This result is consistent with a recent study of instrumental conditioning where adult males responded more than adolescent males with repeated training (Sturman et al., 2010). The results in male and female animals indicate that the reduction in goal-directed behavior in adolescents may have a negative effect on the acquisition of approach behavior. The results from the magazine training and conditioning phases suggest that sex mediates the development of stimulus-directed behavior, while age seems to mediate expression of goal-directed behavior.
Reward devaluation
Because reward devaluation reduces the internal motivation for reward, we predicted that adolescent and female animals would be less sensitive to its effects (Dickinson, 1994; Ostlund & Balleine, 2008). We found that both adolescents and females were relatively less affected by reward devaluation than adult males, consistent with the hypothesis that stimulus-directed processes have a greater contribution than goal-processes to reward behavior in adolescents and females. Though we have asserted that adolescents initially had weaker goal-directed attention, adolescent females did not differ from adult females in their sensitivity to reward devaluation. Adult females may have been overtrained during the conditioning phase, as their behavior was stable after the 3rd session. Overtraining would cause a decrease in the contribution of goal-directed processes to reward behavior (Ostlund & Balleine, 2008), thereby making adolescent and adult females more similar, with respect to goal-directed reward processing, by the end of conditioning. Adult females also consumed more sucrose than adolescent females. This is in contrast to a recent study which reported that adolescents consumed more sweetened liquid than adults during 24-h of free access (Friemel et al., 2010). Adolescents may consume smaller quantities of reward at a time, but for a longer duration than adult rats, as rats in this study received only 1-h of free access. If this is the case, the free access period may have had a greater satiating effect on adults.
Female adults were less affected by reward devaluation than male adults despite consuming more sucrose during the free-access period than any other group. Females may be relatively less sensitive to the effects of reinforcer-specific satiety. We have shown that females consume more sucrose than males when given ad libitum access to food and water (Sherrill, Berthold, Koss, Juraska, & Gulley, 2011; Sherrill, Koss, Foreman, & Gulley, 2011); females have also been shown to maintain high levels of food intake following sucrose access, while males reduce intake (Kanarek, Homoleski, & Wiatr, 2000). This idea is consistent with a recent study in humans (Wang et al., 2009) that suggested females are less able to exert top-down control over the neural response to appetitive stimuli.
Although there was no effect of age in females, adolescent males were less sensitive to the effects of reward devaluation than adult males. The relative insensitivity to reward devaluation in adolescent males is consistent with the hypothesis that adolescents favor stimulus-directed, rather than goal-directed, processes (Ernst et al., 2011). This may be related to developmental changes within the prefrontal cortex, which is thought to have relatively impaired functioning during adolescence (Cohen et al., 2010; Ernst, Phillips, & Hardin, 2006; Galvan et al., 2006; Koss, Franklin, & Juraska, 2011; Paul & Cox, 2012; Van Leijenhorst et al., 2010). Specifically, developmental alterations within the orbitofrontal cortex, which is a subregion of the prefrontal cortex involved with updating the expected value of outcomes (Everitt & Robbins, 2005; Galvan et al., 2006; Ostlund & Balleine, 2007; Overman, 2004; Pickens et al., 2003; Schoenbaum & Roesch, 2005; Sturman et al., 2010; Van Leijenhorst et al., 2010), could lead to impairment in both reward devaluation and extinction learning. Though we found an effect of age on outcome devaluation in males, this effect was not observed in a recent study which found that adolescents were relatively less sensitive to contingency degradation, but not to outcome devaluation (Naneix et al., 2012). Differences in the behavioral paradigm employed may explain the inconsistency with our results. In the Naneix et al. study, rats underwent operant conditioning and devaluation via a brief two-choice extinction test, whereas our extinction test was identical to the training session except for the absence of sucrose solution. In addition, rats in the Naneix et al. study underwent a greater number of training sessions, which may have resulted in overtraining that in turn diminished age differences in goal-directed behavior, as discussed previously. Though adult males in this study responded more during conditioning, we do not believe that unequal training was responsible for the effect of age on devaluation. If adult males were overtrained, we would expect them to be less sensitive to reward devaluation relative to adolescent males, rather than more sensitive.
Extinction and reinstatement
Behavior that is primarily stimulus-directed is less sensitive to the effects of extinction. We found that females responded more during extinction than males, but the effect of sex depended on age. Relative to males, adult females responded at high levels during the first two days of extinction training, while adolescent females had higher levels of responding later. The finding that adult females have weaker extinction learning than adult males is in line with the sex differences seen in extinction from drugs of abuse (Carroll & Anker, 2010; Kuhn et al., 2010; Perry et al., 2008). Estrous cycling may have affected these results, as extinction responding for cocaine has been shown to be enhanced in female estrous-stage rats, relative to female non-estrous stage and male rats (Kerstetter, Aguilar, Parrish, & Kippin, 2008). In the current study, estrous stage was not analyzed, so it is unclear whether this might have influenced our results. Future studies will need to examine whether estrous cycling affects extinction responding for non-drug rewards. It is noteworthy, however, that the effect of sex was diminished in adolescents, relative to adults. It is possible that sex differences are more pronounced in adulthood due to the sex-specific development of the dopamine system throughout adolescence. For example, adult females have much greater drug-induced NAc dopamine release than adult males, but this sex difference is not present in adolescents (Kuhn et al., 2010; Walker & Kuhn, 2008; Walker, Ray, & Kuhn, 2006).
In the current experiment, there was no effect of age on male animals, while the effect of age on female animals was session-dependent. There is evidence for weaker extinction of drug reward in male adolescents (Anker & Carroll, 2010; Brenhouse & Andersen, 2008; Li & Frantz, 2009), but results have not been consistent with food reward (Andrzejewski et al., 2011; Li & Frantz, 2010; Sturman et al., 2010). Among female animals we found that adolescents initially had lower levels of responding, but maintained slightly higher levels of responding by the final extinction sessions. This may indicate that adolescent females did not extinguish the association as completely as adult females.
During reacquisition we found that adolescent females responded more than adolescent males, but there was no effect of sex in adult animals. The effect of sex on reacquisition of approach behavior for food has not been analyzed previously. Adult females do have enhanced reinstatement of drug responding when given a priming injection of the drug (Carroll & Anker, 2010; Lynch, Roth, & Carroll, 2002; Perry et al., 2008), however the effect of sex on cue-induced reinstatement is unclear (Fuchs et al., 2005; Kerstetter et al., 2008). Our finding that adult females do not reacquire faster than adult males or adolescent females is not consistent with our initial finding of faster acquisition in adult females. In fact, adolescent females responded more than adult females during reacquisition. This is difficult to interpret given the role of conditioning and extinction in the expression of reacquisition (Ricker & Bouton, 1996). The enhanced reacquisition in adolescent females may have resulted from the incomplete extinction of the cue-reward association, given that adolescent females had higher levels of extinction responding than both adolescent males and adult females. Our hypothesis that female sex is associated with an enhanced contribution of stimulus-directed processes to reward behavior, while adolescent age is associated with a diminished contribution of goal-directed behavior, could explain the incomplete extinction and enhanced reacquisition in female adolescents.
Conclusion
The literature suggests that adolescent age and female sex are each independently associated with relatively more stimulus-directed behavior, relative to goal-directed behavior (Andrzejewski et al., 2011; Anker et al., 2011; Burton et al., 2011; Fuchs et al., 2005; Kosten & Zhang, 2008; Sturman et al., 2010). The results of the current experiment are consistent with the hypothesis that adolescence is associated with a reduced contribution of goal-directed processes to reward behavior, as measured through outcome devaluation (Ernst et al., 2011). We also found that female sex is associated with an enhanced development of stimulus-directed behavior. Enhanced development of stimulus-directed behavior in females may be an important contributing factor to the vulnerability of females to developing compulsive behaviors like addiction, as well as the phenomenon of telescoping. Our results are consistent with studies conducted using drugs of abuse (Anker & Carroll, 2010; Anker et al., 2011; Carroll & Anker, 2010; Kosten & Zhang, 2008; Lynch, 2008; Perry et al., 2008) and suggest that age and sex differences in response to drug reward are likely due to both differences in reward processing and specific drug effects.
Acknowledgements
This study was supported by a grant from the National Institute on Drug Abuse (R01 DA029815). We thank Alex Waldman, Olubankole Aladesuyi Arogundade, Sapan Shah, Nick Senese and Rakesh Marreddy for technical assistance.
References
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcoholism: Clinical and Experimental Research. 2010;34:2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. doi:10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. doi:10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology. 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. doi:10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215:785–99. doi: 10.1007/s00213-011-2181-z. doi:10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behavioral Neuroscience. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. doi:10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. doi:10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behavioural Brain Research. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. doi:10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Burton CL, Noble K, Fletcher PJ. Enhanced Incentive Motivation for Sucrose-Paired Cues in Adolescent Rats: Possible Roles for Dopamine and Opioid Systems. Neuropsychopharmacology. 2011;36:1631–1643. doi: 10.1038/npp.2011.44. doi:10.1038/npp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and Behavior. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. doi:10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46:193–207. doi: 10.1037/a0016128. doi:10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041. doi: 10.1176/appi.ajp.160.6.1041. doi:10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180:258–66. doi: 10.1007/s00213-005-2152-3. doi:10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13:1–19. doi: 10.1038/nn.2558. doi:10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Motivational control of goal-directed action. Animal Learning & Behavior. 1994;22:1–18. [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–7. doi: 10.1037/a0023763. doi:10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. doi:10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Daniele T, Frantz KJ. New perspectives on adolescent motivated behavior: attention and conditioning. Developmental Cognitive Neuroscience. 2011;1:377–389. doi: 10.1016/j.dcn.2011.07.013. doi:10.1016/j.dcn.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Phillips AG, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. doi:10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacology, Biochemistry, and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. doi:10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–9. doi: 10.1038/nn1579. doi:10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quiñones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Hormones and Behavior. 2004;46:509–19. doi: 10.1016/j.yhbeh.2004.04.009. doi:10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Behavioral Neuroscience. 2010;4:1–10. doi: 10.3389/fnbeh.2010.00039. doi:10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. a, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179:662–72. doi: 10.1007/s00213-004-2080-7. doi:10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. doi:10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. doi:10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–35. doi: 10.1037/0012-1649.41.4.625. doi:10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacology, Biochemistry, and Behavior. 1979;11:31–5. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Homoleski BA, Wiatr C. Intake of a palatable sucrose solution modifies the actions of spiradoline, a kappa opioid receptor agonist, on analgesia and feeding behavior in male and female rats. Pharmacology, Biochemistry, and Behavior. 2000;65:97–104. doi: 10.1016/s0091-3057(99)00181-1. [DOI] [PubMed] [Google Scholar]
- Kerstetter K. a, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. doi:10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Koss W. a, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Developmental Psychobiology. 2011;53:724–31. doi: 10.1002/dev.20543. doi:10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Kosten T, Zhang XY. Sex differences in non-reinforced responding for cocaine. The American Journal of Drug and Alcohol Abuse. 2008;34:473–488. doi: 10.1080/00952990802082206. doi:10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Hormones and Behavior. 2010;58:122–37. doi: 10.1016/j.yhbeh.2009.10.015. doi:10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. doi:10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology. 2009;204:725–33. doi: 10.1007/s00213-009-1502-y. doi:10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Time-dependent increases in cue-induced reinstatement of sucrose seeking after sucrose self-administration in adolescence. Behavioural Brain Research. 2010;213:109–12. doi: 10.1016/j.bbr.2010.04.011. doi:10.1016/j.bbr.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–46. doi: 10.1007/s00213-007-1028-0. doi:10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. doi:10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology, Biochemistry, and Behavior. 2001;68:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behavioural Pharmacology. 2007;18:641–50. doi: 10.1097/FBP.0b013e3282effbf5. doi:10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Waters P, McCormick CM. Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Developmental Psychobiology. 2009;51:417–28. doi: 10.1002/dev.20381. doi:10.1002/dev.20381. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand a. R., Di Scala G, Pape J-R, Coutureau E. Parallel Maturation of Goal-Directed Behavior and Dopaminergic Systems during Adolescence. Journal of Neuroscience. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. doi:10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. The Journal of Neuroscience. 2007;27:4819–25. doi: 10.1523/JNEUROSCI.5443-06.2007. doi:10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discovery Today: Disease Models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. doi:10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55:134–47. doi: 10.1016/S0278-2626(03)00279-3. doi:10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Paul K, Cox C. Age-dependent Actions of Dopamine on Inhibitory Synaptic Transmission in Superficial Layers of Mouse Prefrontal Cortex. Journal of Neurophysiology. 2012;109:1323–32. doi: 10.1152/jn.00756.2012. doi:10.1152/jn.00756.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Experimental and Clinical Psychopharmacology. 2008;16:165–77. doi: 10.1037/1064-1297.16.2.165. doi:10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. The Journal of Neuroscience. 2003;23:11078–84. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol. 1999;60:252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Ricker ST, Bouton ME. Reacquisition following extinction in appetitive conditioning. Animal Learning and Behavior. 1996;24:423–436. [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–6. doi: 10.1016/j.neuron.2005.07.018. doi:10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. doi:10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology. 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. doi:10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss W. a., Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural Brain Research. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. doi:10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss W. a, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behavioural Brain Research. 2011;216:569–75. doi: 10.1016/j.bbr.2010.08.048. doi:10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. doi:10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. doi:10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. doi:10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44:1764. doi: 10.1037/a0012955. doi:10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behavioral Neuroscience. 2010;124:16–25. doi: 10.1037/a0018463. doi:10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience & Biobehavioral Reviews. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. doi:10.1016/S0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–9. doi: 10.1093/cercor/bhp078. doi:10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicology and Teratology. 2008;30:412–8. doi: 10.1016/j.ntt.2008.04.002. doi:10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–202. doi: 10.1038/sj.npp.1300915. doi:10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1249–54. doi: 10.1073/pnas.0807423106. doi:10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacology, Biochemistry, and Behavior. 2009;92:131–4. doi: 10.1016/j.pbb.2008.11.002. doi:10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]