Abstract
Chronic inflammatory lesions of the placenta are characterized by the infiltration of the organ by lymphocytes, plasma cells and/or macrophages, and may result from infections (viral, bacterial, parasitic) or be of immune origin (maternal anti-fetal rejection). The three major lesions are villitis (when the inflammatory process affects the villous tree), chronic chorioamnionitis (which affects the chorioamniotic membranes), and chronic deciduitis (which involves the decidua basalis). Maternal cellular invasion is a common feature of the lesions.
Villitis of unknown etiology (VUE) is a destructive villous inflammatory lesion characterized by the infiltration of maternal T cells (CD8+ cytotoxic T cells) into chorionic villi. Migration of maternal T cells into the villi is driven by the production of T cell chemokines in the affected villi. Activation of macrophages in the villi has been implicated in the destruction of the villous architecture. VUE has been reported in association with preterm and term fetal growth restriction, preeclampsia, fetal death and preterm labor. Infants whose placentas have VUE are at risk for death and abnormal neurodevelopmental outcome at the age of 2.
Chronic chorioamnionitis is the most common lesion in late spontaneous preterm birth and is characterized by the infiltration of maternal CD8+ T cells into the chorioamniotic membranes. These cytotoxic T cells can induce trophoblast apoptosis and damage the fetal membranes. The lesion is frequently accompanied by villitis of unknown etiology and evidence of maternal anti-fetal antibodies and deposition of complement in the umbilical vein. Chronic deciduitis consists of the presence of lymphocytes or plasma cells in the basal plate of the placenta. This lesion is more common in pregnancies resulting from egg donation and has been reported in a subset of patients with premature labor. Chronic placental inflammatory lesions are now considered to represent maternal anti-fetal rejection and this process can be associated with the development of a novel form of fetal systemic inflammatory response syndrome characterized by an elevation of the fetal plasma T cell chemokine (CXCL10).
The evidence that maternal anti-fetal rejection may underlie the pathogenesis of many chronic inflammatory lesions of the placenta is reviewed. This article includes figures and histologic examples of all chronic inflammatory lesions of the placenta.
Keywords: allograft, C4d, CD8 T cell, chemokine, chronic chorioamnionitis, chronic deciduitis, complement, CXCL10, fetal death, fetal growth restriction, HLA, massive perivillous fibrin deposition, maternal floor infarction, plasma cells, prematurity, rejection, tolerance, villitis of unknown etiology, VUE
Introduction
The term “chronic inflammation” refers to an inflammatory process characterized by infiltration of lymphocytes, plasma cells, and histiocytes (i.e. tissue macrophages) 1. Chronic placental inflammatory lesions can be present in the villous tree, extraplacental chorioamniotic membranes, chorionic plate, and basal plate of the placenta (Figure 1). The etiology of chronic inflammatory lesions of the placenta is an important challenge 2–4. Infection 5–7 caused by viruses 8–12, bacteria (i.e. Treponema pallidum, Mycobacterium tuberculosis)11,13, and parasites (i.e. Plasmodium spp., Toxoplasma gondii) 8 has been implicated; however, most chronic inflammatory lesions are of unknown etiology (i.e. an infectious agent cannot be identified) 14, and accumulating evidence suggests that an immune process caused by maternal anti-fetal rejection plays a role in the pathogenesis of these conditions 4,15–24.
Figure 1.
The human placenta. Chronic inflammatory lesions can affect different parts of the placenta. Chronic villitis refers to the inflammation involving the villous tree. Chronic chorioamnionitis involves either the extraplacental chorioamniotic membranes or chorionic plate. Chronic deciduitis affects the basal plate. Modified from Benirschke K, Burton GJ, Baergen RN. Infectious Diseases. Pathology of the Human Placenta. Sixth ed. Berlin Heidelberg: Springer; 2012. p. 33.
The placenta and fetus are semi-allografts and a maternal (host) immune response against paternal antigens (expressed in the placenta or fetus) can be considered analogous to allograft rejection 25–34. We will review the evidence in support of the idea that many cases of idiopathic chronic placental inflammation reflect maternal anti-fetal rejection, in which the main effector is the infiltration of maternal CD8+ T cells (cytotoxic lymphocytes) into fetal tissues (Figure 2) 15,16,35. This state is associated with the presence of fetal HLA-specific antibodies in the maternal serum 17,18, 21, and C4d deposition in the umbilical vein 17,22 as well as syncytiotrophoblast24. The presence of fetal HLA specific antibodies in maternal serum was determined by first performing HLA genotyping using fetal genomic DNA, and then assessing whether the maternal HLA antibodies were directed against fetal antigens using a Luminex assay 18,21. Furthermore, the concentrations of T cell chemokine CXCL10 are elevated in different fetal compartments, such as amniotic fluid and fetal plasma 15,16,20. These phenomena resemble those observed in allograft rejection in solid organ transplantation 36–49.
Figure 2.
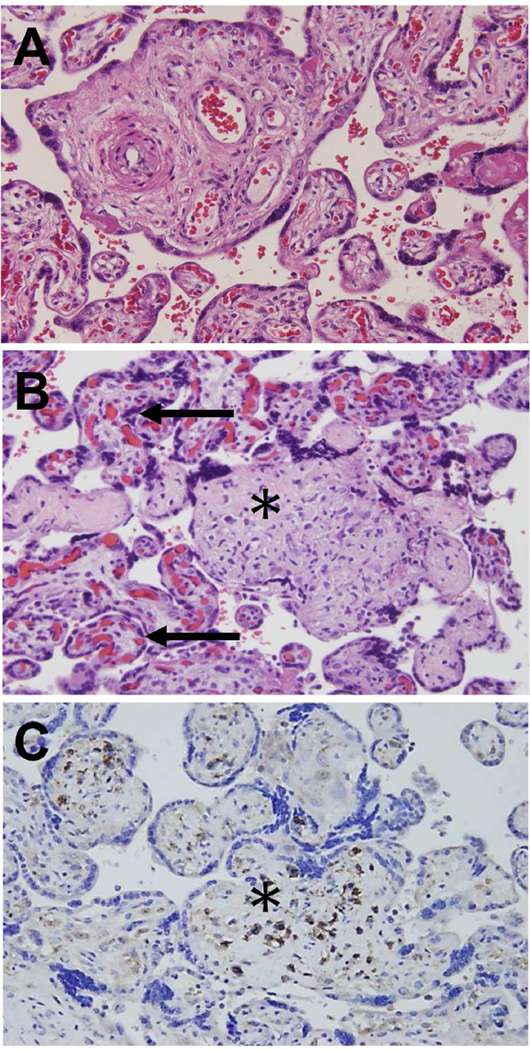
Microscopic findings of chronic nonspecific villitis (villitis of unknown etiology; VUE). (A) Normal chorionic villi showing villous core with fetal vessels and stroma. The intervillous space is shown in white and contains maternal red blood cells. The rest of the image shows cross-sections of the villous tree of the placenta – each chorionic villus is lined with syncytiotrophoblast. Inside the villi, fetal capillaries are observed. (B) Destructive inflammation of the chorionic villus (asterisk). The inflammatory process is diagnosed by the presence of an infiltration of mononuclear cells. Obliteration of the villous capillaries is also seen in comparison with unaffected villi adjacent to the distorted villus (asterisk). Unaffected villi (black arrow). (C) Immunoperoxidase staining for CD8+ T cells. Cells stained in brown express CD8+ on their surface, and are, therefore, cytotoxic lymphocytes. These cells are of maternal origin, and are derived from the intervillous space. A-C, X200.
1.Chronic nonspecific villitis (Villitis of Unknown Etiology; VUE)
1.1 Definition and cellular composition
Villitis of unknown etiology (VUE) is a destructive inflammatory lesion characterized by the infiltration of maternal T cells into the chorionic villi (fetal tissue) 50,51. The frequency of VUE varies among studies, and its prevalence ranges from 2% to 33.8% 7,52–56. The wide range is thought to represent variations in the study population, sampling methods, and diagnostic criteria. The frequency of detection of VUE increases when the number of paraffin blocks from a given placenta increases to four 52.
The main maternal T cell subset infiltrating the chorionic villi is CD8+ cytotoxic T cells 35. The maternal origin of these T cells was demonstrated by Redline et al. 50 using in situ hybridization analysis with X and Y chromosome-specific probes, along with CD3 (a pan-T lymphocyte marker) and CD45 (a leukocyte marker) immunostaining of placental tissues obtained from four male neonates. T lymphocytes infiltrating the villous tree were consistently of maternal origin (as shown by the lack of Y chromosome signals) 50.
Another important cell type in VUE is the macrophage, which is of fetal origin, as demonstrated by using chromogenic in situ hybridization with a Y chromosome-specific probe 35. Placental macrophages, also known as Hofbauer cells, have an activated phenotype in VUE, as shown by expression of CD14 35. Increased CD14 immunoreactivity in VUE cases indicates that there pro-inflammatory activation of placental macrophages. Therefore, VUE is a unique inflammatory process involving T cells and macrophages originating from two different hosts (T cells from the mother and macrophages from the fetus) 35,57. A key feature of VUE is fetal tissue damage by maternal cytotoxic T cells resembling allograft rejection. The relative numbers of T regulatory cells (CD4+ CD25+ FoxP3+) are also higher in placentas with VUE, compared to those without this lesion 58. This is an interesting observation, given that T regulatory cells are implicated in the generation of a tolerogenic state in pregnancy 34,59–63.
The destruction of the villous architecture by the inflammatory process appears to be related to apoptosis, which is more extensive in areas of the placenta with VUE, as demonstrated using transferase dUTP nick end labeling (TUNEL) staining 64. Two mechanisms have been proposed to result in apoptosis: 1) the release of perforin and granzyme B by activated CD8+ T cells, which can induce caspase-dependent cell death in target cells; and 2) complement activation leading to pore formation. Immunohistochemistry studies show expression of perforin and granzyme B, and C5b-9 (membrane attack complex of the complement system) deposition in VUE 64.
1.2 Grading
Histopathological grading of VUE is based on the number of chorionic villi affected, and whether the distribution of villous inflammation is patchy or diffuse. Redline has proposed a grading system using the number of villi affected per focus 54. In low grade lesions less than 10 villi are affected and the lesions can either be focal (only one slide involved) or multifocal (more than one slide involved). High grade lesions are defined as those with more than 10 villi affected per focus, and are divided into patchy and diffuse subgroups 54. The lesion is defined as diffuse when more than 5% of all distal villi are affected (Figure 3). Other grading systems have also been proposed 52,65. The highergrade is the greater the risk of adverse pregnancy outcome 66,67.
Figure 3.
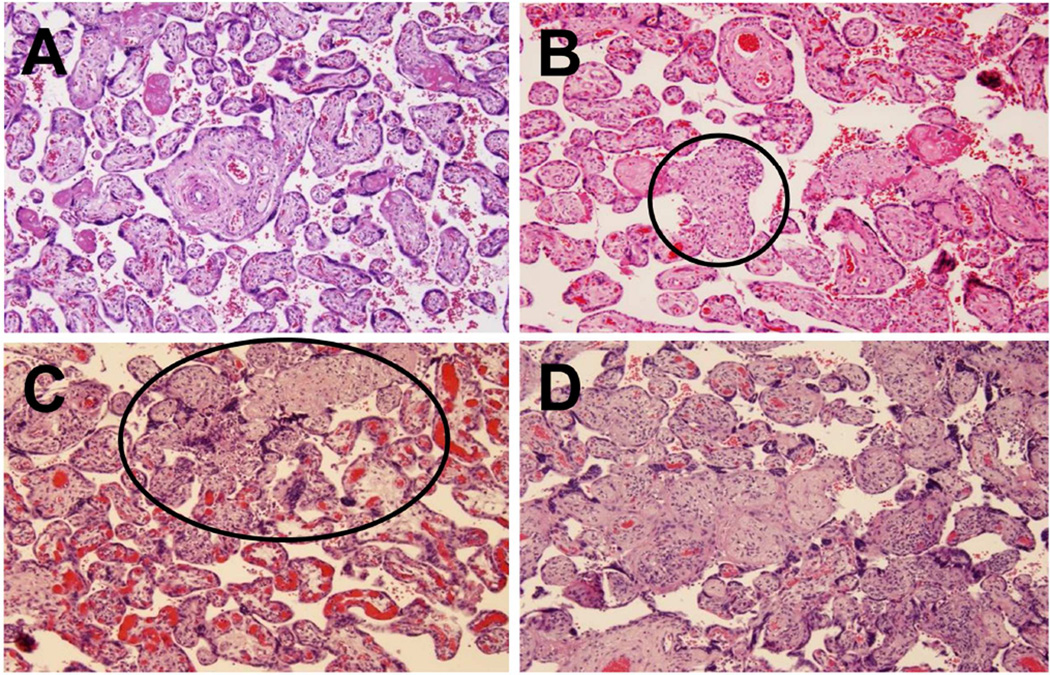
The severity of villitis is assessed by a grading system. (A) Well-preserved chorionic villi in a normal placenta. (B) A low-grade lesion showing involvement of chorionic villi; less than 10 villi are affected. High-grade lesions are shown in 3C and 3D. 3C shows patchy involvement. The villi within the oval are affected, while those outside the oval are unaffected. Most of the villi are involved in 3D – this represents diffuse involvement. A-D, X100.
1.3 Pattern of involvement
Redline proposed that the patterns of VUE involvement be classified as distal, proximal and basal (Figure 4) 54,66. The distal type is the most common (approximately 50% of cases), and is localized to distal villi (terminal and mature intermediate villi). Proximal occurs in 30% of the cases, and involves the proximal stem villi (sometimes the chorionic plate along with the distal villi) 54. This type can be associated with obliterative vasculopathy and fetal vascular thrombo-occlusive disease, resulting in hyalinized avascular villi 54. The third pattern is basal villitis, involving villi anchoring to the basal plate – this type is frequently associated with chronic deciduitis 54.
Figure 4.
Histological description of villitis of unknown etiology (VUE). (A) An illustration showing the placental villous tree. Inflammation of the villous tree is the hallmark of villitis. (i) in Figure 4A is a stem villus. (ii) represents terminal and mature intermediate villi; (iii) represents anchoring villus. The infiltration of lymphocytes at this site represents proximal villitis. (B) The histological demonstration of villitis of the stem villi is shown (asterisk). (C) Distal villitis involving terminal and mature intermediate villi is the most common pattern of VUE (asterisks). (D) Basal villitis involves anchoring villus (asterisk). B-D, X100.
1.4 Pathogenesis
What drives maternal T cells in the intervillous space to infiltrate the chorionic villi? Gene expression profiling of placentas affected by VUE demonstrates overexpression of genes involved in the immune response. For example, there is overexpression of major T cell chemokines and their receptor (i.e. CXCR3). The T cell chemokines CXCL9, CXCL10, and CXCL11 are overexpressed in Hofbauer cells (placental macrophages of fetal origin), stromal and endothelial cells 15. Therefore, a chemotactic gradient could be established between villi and the intervillous space, where maternal lymphocytes circulate. Importantly, maternal CD8+ T cells within the villi express the receptor for the T cell chemokines CXCR3+, indicating that they are able to receive the chemotactic signal 15. Based on this evidence, we propose that activated Hofbauer cells attract maternal T cells into the villous compartment through the production of chemokines. Another potential mechanism facilitating maternal T cell infiltration into the chorionic villi includes increased expression of intercellular adhesion molecule-1 (ICAM-1) both in syncytiotrophoblast and maternal immunocytes 68–71. It is also possible that small breaks in the syncytiotrophoblast contribute to the disease process 72.
In support of an immunological origin of this lesion, we reported that using transcriptomic analysis, placentas with VUE have an enrichment of genes involved in antigen presentation, such as class II major histocompatibility antigens (HLA-DM, -DO, -DP, -DQ, -DR) 15 and overexpression of class I molecules (HLA-B, -C, -G) 15. VUE is characterized by the proliferation of Hofbauer cells within the villi (demonstrated by nuclear immunoreactivity for Ki-67, a protein associated with cell proliferation). In summary, the gene expression profile of VUE resembles that observed in transplant rejection and graft-versus-host disease 73–78.
The immunological changes in VUE are not restricted to the villi, since maternal and fetal plasma concentrations of CXCL9, −10, and −11 are also significantly higher in cases with VUE than in controls 15. Moreover, VUE is histologic evidence of maternal cell trafficking into the fetus, which could lead to the development and establishment of fetal-maternal microchimerism 79. It remains to be established if maternal T cells are grafted into the visceral organs of the fetus. The precise nature of the fetal antigens that induce the maternal immune response has not been established. However, we have evidence that maternal sensitization to fetal specific anti-HLA antigens occurs in patients with VUE (see below).
Placentation provides a unique anatomical context for allograft rejection. The fetus (the placental villi are of fetal origin) differs from typical allografts, such as the kidney and the liver, in that it is a distinct immunologically-competent host (Figure 5).
Figure 5.
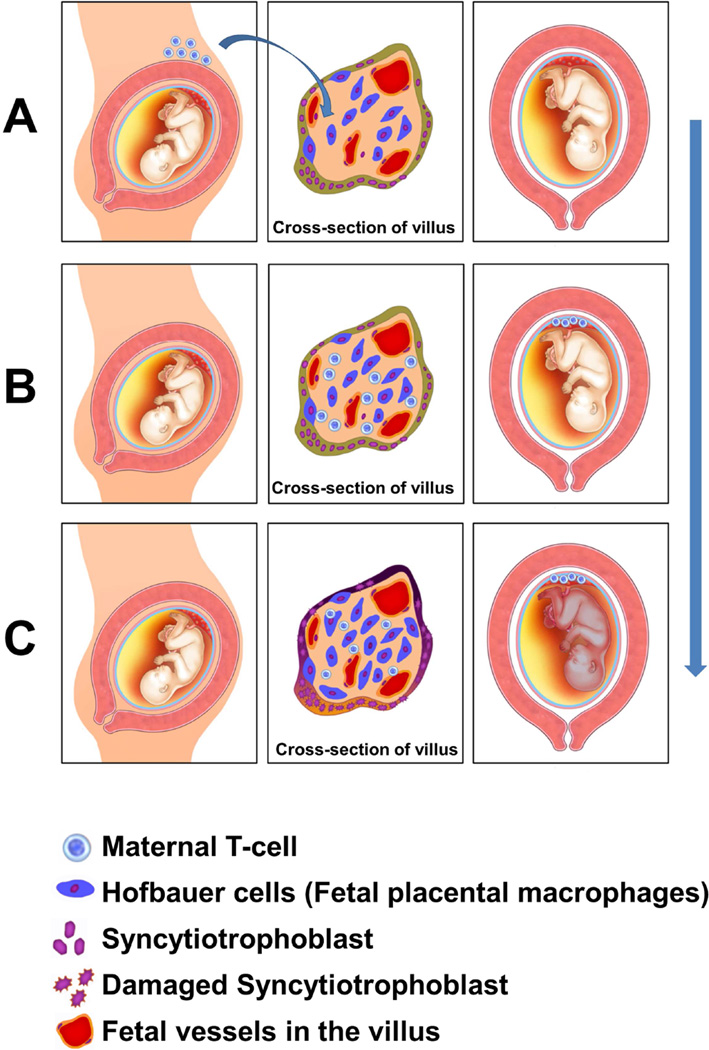
The unique immunological nature of villitis of unknown etiology (VUE). (A, B) Circulating maternal T cells entering the intervillous space can infiltrate the villus. (C) Following maternal T cell infiltration, Hofbauer cells (fetal placental macrophages) are activated. This would be considered semi-allograft rejection, because maternal T cells (recipient of the semi-allograft) infiltrate the placenta (semi-allograft).
1.5 Clinical significance
VUE is generally considered to be a lesion of term pregnancies, and is often subclinical in nature (normal pregnancy outcome) 80. However, extensive involvement of the placenta by VUE has been associated with preterm and term fetal growth restriction 5,6,15,53,65,81–87, spontaneous preterm delivery 15,85,88, small for gestational age (SGA) associated with preeclampsia 15,82,89,90, and fetal death 6,15,52,65,91. The frequency of VUE in the “great obstetrical syndromes” 92–94 is displayed in Figure 6. This lesion is also associated with the occurrence of chronic chorioamnionitis (Figure 6B, and the next section in this review).
Figure 6.
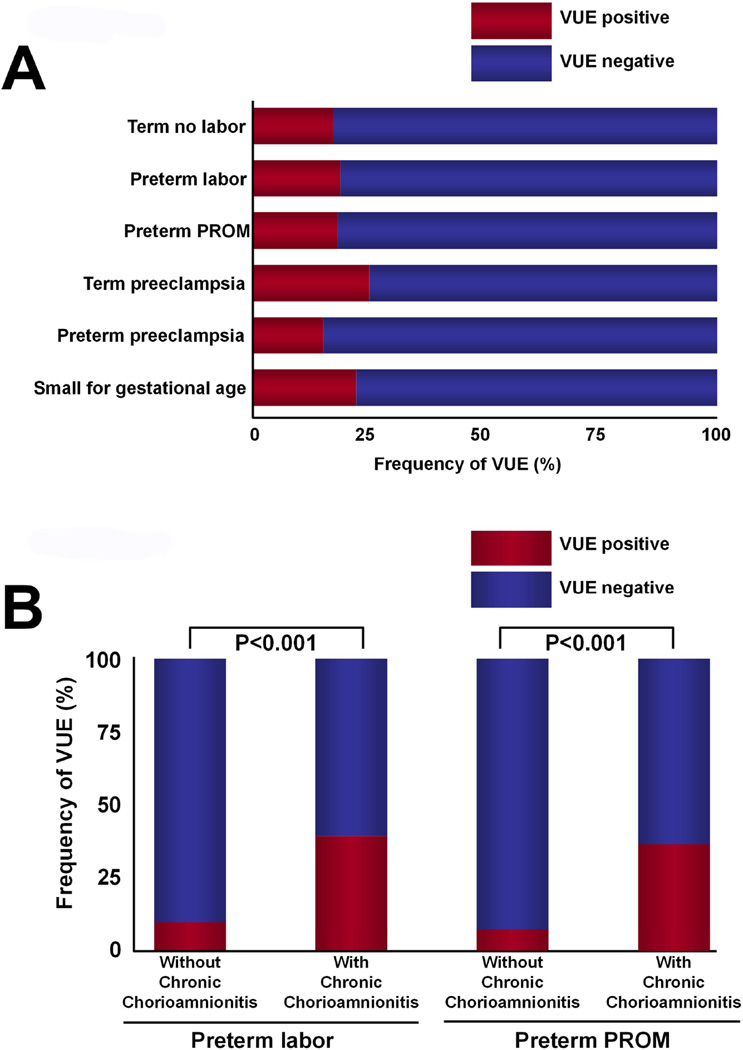
VUE is frequently associated with chronic chorioamnionitis; (A) The frequency of VUE in women at term not in labor, preterm labor with intact membranes, preterm PROM, preeclampsia at term, preterm preeclampsia, and small for gestational age. Modified from Kim CJ et al. Mod Pathol. 2010 Jul;23(7):1000-11; (B) The frequency of VUE is higher in the placenta of patients who had preterm labor with intact membranes and in patients with preterm PROM who had chronic chorioamnionitis than in those who did not. Modified from KIM CJ et al. Mod Pathol. 2010 Jul;23(7):1000-11.
VUE: villitis of unknown etiology; PROM: prelabor rupture of membranes
In a cohort study of 180 neonates with a birthweight below the 10[san]th[san] centile delivered <34 weeks of gestation with abnormal umbilical artery Doppler velocimetry, the presence of villitis was an independent risk factor for infant death (odds ratio 5.7; 95% CI, 1.16–28.1) 95 and VUE was a predictor of necrotizing enterocolitis 95. Moreover, infants from pregnancies with placental villitis were at risk for an abnormal developmental outcome at the age of 2 (odds ratio 3.19; 95% CI 1.26–8.09) 95. The mechanisms whereby VUE may lead to SGA with an abnormal Doppler of the umbilical artery are unknown. Becroft et al. argued that the extent of the placental parenchymal damage by VUE is not sufficient to restrict fetal growth, as only 0.01%–5% of the villous tree would be affected 6. We propose that the most likely mechanism whereby VUE compromises fetal growth is a systemic fetal inflammatory response (a fetal inflammatory response syndrome, type II – see below for details).
Diffuse chronic villitis has been associated with neurologic impairment in other studies 67, 96. VUE is more frequent in the placenta of the smaller twin compared to the larger twin in dichorionic pregnancies 97,98. Interestingly, the incidence of VUE is higher in placentas from patients who had ovum donation 99–101. Pregnancy in cases of ovum donation represents a total allograft rather than a semi-allograft. These results strengthen the case for an immunological origin of VUE.
2. Chronic chorioamnionitis
2.1 Definition and cellular composition
Chronic chorioamnionitis is defined by the infiltration of mononuclear cells into the chorioamniotic membranes or the chorionic plate. The typical lesion of chronic chorioamnionitis shows patchy or diffuse infiltration of maternal CD8+ T cells (Figure 7). The extent and severity of T cell infiltration is less than neutrophilic infiltration in cases of acute chorioamnionitis 102–104. The primary locus of interaction is between maternal CD8+ T cells, and the fetal cells are the choriodecidual border in the chorion laeve 16. Trophoblast damage by CD8+ T cells in the form of apoptosis can be demonstrated using double immunofluorescence staining with antibodies against CD8+ lymphocytes and M30 (a specific antibody against an epitope associated with cleavage of cytokeratin 18 during apoptosis) 105. Figure 8 shows direct contact between CD8+ T cells and trophoblasts expressing M30. Trophoblast apoptosis often leads to thinning of the chorionic trophoblast layer with a “moth-eaten” appearance of the choriodecidual border 16.
Figure 7.
The mechanisms and progression of chronic chorioamnionitis. (A) Increased concentration of the intra-amniotic T cell chemokine CXCL10 (and increased expression of CXCL9, −10, and −11 in the chorioamnionitic membranes) induces migration of CXCR3+ T cells from the decidua (maternal tissue) into the chorioamniotic membranes (fetal tissue). (B) T cells (depicted in with a blue nucleus, originally located in the decidua on the left side of the figure) migrate into the chorioamniotic membranes (“amniotropism”; right side of the figure). The term “amniotropic” implies that the T cells are migrating towards the amnion.
Figure 8.
Chorionic trophoblast apoptosis by cytotoxic T cells. (A) Confocal microscopy with immunofluorescence of the chorioamniotic membranes in a case with chronic chorioamnionitis. Nuclei of all cells are stained blue. Green represents staining with an antibody against CD8. These cells are CD8+ cytotoxic cells of maternal origin which are infiltrating the chorion (fetal). M30 (antibody against an epitope associated with cleavage of cytokeratin 18 during apoptosis) detects trophoblast that has undergone apoptosis; apoptotic cells are depicted in red. Direct contact between cytotoxic CD8+ cells (green) and apoptotic trophoblast (red) is indicated with white arrows – this represents the cytotoxic effect of maternal CD8+ cells in inducing apoptosis of fetal cells. (B) Light microscopic image showing chorionic trophoblast apoptosis with increased cytoplasmic eosinophilia and pyknotic nuclei (arrows). Loss of trophoblasts results in shaggy and irregular choriodecidual border (moth-eaten appearance). Hematoxylin & Eosin, X200.
2.2 Grading
We proposed a grading system to assess the severity of chronic chorioamnionitis 16. Grade 0 indicates the absence of inflammation; grade 1 when there are more than two foci of inflammation or patchy inflammation; and grade 2 when diffuse inflammation is present 16. The stage of inflammation was scored as 1 if amniotropic lymphocytic infiltration was limited to the chorionic trophoblast layer sparing the chorioamniotic connective tissue, and stage 2 if lymphocytic infiltration into the chorioamniotic connective tissue was noted (Figure 9) 16. Importantly, cases with mild inflammatory lesions have significantly higher amniotic fluid CXCL10 concentrations than patients without these lesions16 The substantial increase in the amniotic fluid CXCL10 concentration in cases with low grade, low stage inflammation underscores that the lesion can be associated with a unique form of intra-amniotic inflammation, characterized by an increase in T cell chemokines, but not necessarily amniotic fluid IL-6, which is a marker of acute inflammation 16.
Figure 9.
Microscopic staging of chronic chorioamnionitis. (A) Normal chorioamniotic membranes with amnion, chorion and decidua. (B) Stage 1 chronic chorioamnionitis showing T cell infiltration confined to the chorionic trophoblast layer. The choriodecidual border is studded with lymphocytes, while the chorioamniotic connective tissue is spared. The arrows indicate infiltration of T cells along the choriodecidual border. (C) In stage 2 chronic chorioamnionitis, lymphocytic infiltration into the chorioamniotic connective tissue is clearly seen. The arrows indicate infiltration of T cells into the amniotic connective tissue below the epithelium of the amnion at the top of the picture. A-C, X200
2.3 Pathogenesis
The choriodecidual junction is a large interface between the mother and the fetus (see Figure 1), and maternal immune cells in the decidua can recognize fetal cells, specifically chorionic trophoblasts (i.e. chorion laeve). Under normal circumstances, an inflammatory response in the choriodecidual junction does not occur. The mechanisms responsible for the tolerogenic state in pregnancy include: 1) expression of non-polymorphic HLA-G expression in the trophoblasts 106–109; and 2) T cell chemokine gene silencing in the decidual cells, as shown by Nancy et al. 110. Other mechanisms have been implicated, such as a role for T-regulatory cells 59, 60,111,112, tryptophan catabolism by indolemine 2,3-dioxygenase (IDO) 113, T-cell apoptosis 114, complement 115,116, and co-stimulatory molecules such as the programmed death ligand 1 (PDL 1) 117. The mechanisms implicated in fetomaternal tolerance are shown in Table 1. A full discussion of this subject is outside the scope of this review.
Table 1.
Proposed mechanisms implicated in the tolerogenic state of pregnancy
|
|
Chronic chorioamnionitis was first described by Gersell et al. in a case series of 17 placentas in which the inflammatory infiltrate in the chorioamniotic membranes consisted predominantly of lymphocytes 102. Importantly, an extensive study of amniotic fluid and placental microbiology, as well as immunohistochemical staining and maternal serology, did not show evidence of infection with either bacteria or viruses 102. Patients had no clinical evidence of infection, such as fever, a flu-like syndrome, or a rash, however, preterm birth occurred in 76% (13/17) of patients. Prior to this seminal observation, few reports had documented a chronic inflammatory infiltrate of the fetal membranes in patients with maternal rubella infection 118 and toxoplasmosis 119. Subsequently, Qureshi and Jacques reported 31 cases of chronic chorioamnionitis and emphasized that 71% (22/31) of the cases had VUE, suggesting a common immunological etiology for the two lesions 104. Immunohistochemistry showed that the predominant cells were CD8+ lymphocytes, 39% (12/31) of the cases were associated with a preterm birth, and 16% (5/31) were SGA 104.
The chemotactic gradient favoring migration of T cells from the decidua into the chorioamniotic membranes has been proposed to represent increased concentrations of the chemokine CXCL10 in amniotic fluid, and overexpression of CXCL9, CXCL10 and CXCL11 in the chorioamniotic membranes 16. Therefore, the gradient of T cell chemokine concentrations leads to CXCR3+ T cell chemotaxis into the chorioamniotic membranes and the chorionic plate. This is analogous to the situation of acute chorioamnionitis in that elevated amniotic fluid neutrophil chemokines, such as IL-8 120–129, promote chemotaxis of neutrophils. Chronic chorioamnionitis is also characterized by distinct changes in the amniotic fluid proteome compared to acute chorioamnionitis 130. Among patients with preterm labor, 31 differentially expressed proteins have been identified in chronic chorioamnionitis compared to cases with either acute chorioamnionitis or controls without inflammatory lesions. Of interest is that the amniotic fluid concentration of glycodelin-A is decreased in chronic chorioamnionitis 130. This molecule has been implicated in the maintenance of maternal tolerance against the semi-allogeneic placenta/fetus 131.
2.4 Clinical significance
The prevalence of chronic chorioamnionitis in the “great obstetrical syndromes” 92–94 is displayed in Figure 10. The frequency in patients with spontaneous preterm labor and delivery is 34%, and 39% in patients with preterm PROM 16. In a separate study of consecutive cases of 1,206 preterm deliveries, chronic chorioamnionitis was the most common lesion (20.8%), and was particularly common among late preterm birth, which represents 70% of all preterm deliveries 23. Importantly, we found that 60% of cases of fetal death had evidence of chronic chorioamnionitis, and high amniotic fluid concentrations of CXCL10. This suggests that some cases of unexplained fetal death may represent an extreme form of maternal anti-fetal rejection 19,132.
Figure 10.
The frequency of chronic chorioamnionitis in “Great Obstetrical Syndromes”. The frequency is notably higher in the order of fetal death, preterm prelabor rupture of membranes (PROM), and preterm labor/delivery. Modified from Kim CJ et al. Mod Pathol. 2010 Jul;23(7):1000-11 and Lee et al. Histopathology 2011:59:928–938.
3. Chronic deciduitis
Chronic deciduitis is diagnosed by the presence of lymphocytes and plasma cells in the basal plate of the placenta (Figure 1 and Figure 11) 133,134. Chronic microbial infection and immune mechanisms have been implicated in the etiology of chronic deciduitis. Originally recognized by Naeye when reviewing the placentas from the Collaborative Perinatal Study of the National Institute of Neurological and Communicative Disorders and Stroke (study of nearly 40,000 pregnancies in which clinical outcome and placentas were collected prospectively), chronic deciduitis was thought to be present in 1–2% of all pregnancies, and to be associated with fetal growth restriction and fetal death 135. However, the original description involved infiltration of chronic inflammatory cells in both the decidua basalis and capsularis; therefore, chronic chorioamnionitis as well as chronic deciduitis of the basal plate may have been included in Naeye’s original report 135.
Figure 11.
Chronic deciduitis. (A) Normal basal plate along with anchoring villus (asterisk). This image has cross-sections of villi, and the intervillous space is in white. The basal plate of the placenta is the horizontal tissue at the bottom of the picture. (B) Dense infiltration of the basal plate of the placenta and anchoring villi with mononuclear and plasma cells. Basal villitis (asterisk) is present. (C) Immunostaining confirms the presence of CD138+ positive plasma cells (brown color) in the basal plate. A-C, × 200.
Bendon and Miller focused attention on a lesion in the basal plate of the placenta whose prominent feature was the infiltration by plasma cells 136. The frequency of the lesion was 4% (25/600) in placentas examined because of complications of pregnancy requiring admission to a neonatal intensive care unit. Bendon and Miller emphasized that plasma cells were not normally seen in the decidua parietalis of the same placentas, and proposed an immune origin for the lesion based upon the proximity of the plasma cells to trophoblast cells 136. Adverse pregnancy outcome was common in these 25 cases, as there were 5 infants with intrauterine growth restriction, 5 fetal deaths, and 3 mothers had systemic lupus erythematosus. Of interest is that recurrent lesions were observed in 3 patients 136.
The precise definition of chronic deciduitis in the basal plate was established by an international collaborative effort reported by Khong et al., in which 30 slides of placental sections were distributed to 6 experienced perinatal/placental pathologists to determine the degree of concordance in the identification of chronic deciduitis 133. Pathologists scored the presence or absence of a lesion, its extent (focal, multi-focal, or diffuse), severity (mild, moderate, or severe), and the presence or absence of plasma cells; however, no prespecified definitions were provided to the pathologists, and the first round of examinations was used to generate a diagnostic tool for defining the disease 133. The conclusion of the study was that the diagnosis should be made on the basis of severe and extensive lymphocyte infiltration and the presence of plasma cells 133. Recently, the scoring system for chronic deciduitis has been proposed 137. The criterion for the diagnosis of chronic deciduitis is the presence of ≥ 50 lymphocytes/per high-power field (Grade 1). Grade 2 and Grade 3 chronic deciduitis are characterized by the presence of lymphocyte in multiple and diffuse foci in at least one slide, respectively 137. This classification correlates well with that of Khong et al 133.
The association between chronic deciduitis and preterm labor without clinical chorioamnionitis was first reported by Edmonson et al. after examining the placentas of 39 patients with idiopathic preterm labor and 39 age-matched control placentas of singleton pregnancies (induced because of fetal congenital anomalies, excluding aneuploidy) 138. The frequency of chronic deciduitis was significantly higher in patients with preterm labor than in a control group (41% vs 15%; p=0.02) 138.
Evidence that chronic deciduitis may have an immune origin has been suggested, as this lesion is significantly associated with basal villitis and has been reported more frequently in the basal plate of the placenta of pregnancies resulting from egg donation vs. non-donor in vitro fertilization (IVF) pregnancies. In one study, the frequency of chronic deciduitis was 42% (14/33) in egg donation pregnancies, and 1.6% (1/60) in the controls (p=0.001) 100. However, in a subsequent study, the frequency was 2.8% after oocyte donation vs. 1.8% in non-oocyte donor IVF (p=0.03) 101.
4. Chronic placental inflammation as maternal anti-fetal rejection
The placenta and fetus express both paternal and maternal antigens; they are semi-allografts 34,139,140. The syncytiotrophoblast is in direct contact with maternal blood, and the chorion laeve is with decidua; thus, the maternal immune system is exposed to paternal antigens expressed by the fetus 141,142. Immune tolerance is a requirement for successful pregnancy 25,26, 31,32,59,143,144. Immune effector cells with fetal specificity are selectively “silenced” during pregnancy by complex mechanisms 31,32. A full discussion of the mechanisms responsible for tolerance during pregnancy is outside the scope of this article; however, the main mechanisms are listed in Table 1.
In solid organ transplantation, breakdown of tolerance leads to rejection of the graft, and ultimately, injury 145–152. Allograft rejection results from a cellular and/or humoral (antibody-mediated) immune response by the recipient of a graft 145–152. The major histocompatibility complex (MHC) class I and II molecules include human leukocyte antigens (HLA), and this system is implicated in the rejection of solid organs 153–157 as well as bone marrow 158. We have proposed that disruption of the tolerogenic state of normal pregnancy leads to maternal anti-fetal rejection, placental damage, and complications of pregnancy (i.e. fetal growth restriction and spontaneous preterm labor). The extreme form of graft failure in organ transplantation is extensive damage of the transplanted organ – the equivalent to this in pregnancy would be fetal death caused by maternal anti-fetal rejection 19,132.
The two mechanisms of allograft rejection are cell- (T-cell) and antibody-mediated 145–152. In the context of pregnancy, the histopathologic manifestations of cell-mediated rejection are VUE and chronic chorioamnionitis. In the former, the battleground for rejection is the chorionic villi, where maternal T-cells invade the villous tree; in the latter, the battleground is the extraplacental choriodecidual interface, where maternal T-cells infiltrate the chorioamniotic membranes. A cellular mediated feature of maternal anti-fetal rejection can be detected in maternal systemic circulation in patients with chronic chorioamnionitis 159. The proportion of CD300a+, cytotoxic T lymphocytes, is significantly increased in patients with chronic chorioamnionitis than in those without this lesion 159. Moreover, we have found an increased number of CD CD300a+CD8+ T cells, which overexpress mRNA of granzyme genes (GZMA, GZMB, and GZMK), granulysin (GNLY), and perforin (PRF1) indicated that CD300a+CD8+ T lymphocytes have more cytotoxic phenotype 159.
Antibody-mediated rejection has been reported in both VUE 24,64 and chronic chorioamnionitis 17,18,21. The effector mechanism for rejection in antibody-mediated allograft damage involves complement activation. Deposition of complement C4d in the umbilical vein endothelium in mothers with complications of pregnancy and detectable maternal anti-fetal antibodies has been demonstrated in patients with both chronic chorioamnionitis 17 and VUE 22, 24.The most frequent alloantigens involved in transplant rejection are encoded by the major histocompatibility complex (MHC) genes, or HLA in humans 160–162. The degree of HLA mismatch between the donor and the recipient is a risk factor for rejection, as is the presence of existing donor-specific antibodies in the recipient 163–171. Sensitization of mothers to fetal-specific HLA antigens occurs frequently during pregnancy, increases with gestational age and parity, and has traditionally been considered benign 172–175. However, we have recently demonstrated that maternal HLA sensitization diagnosed in the midtrimester is a risk factor for spontaneous preterm delivery (OR 2.8; p=0.01) 21, and the strength of the association increases (OR 5.9; 95% CI 1.6–21.83, p=0.008) when patients present with spontaneous preterm labor 17.
Maternal HLA sensitization is necessary, but not sufficient, for antibody-mediated maternal anti-fetal rejection. Antibodies specific to fetal antigens must also cross the placenta, activate complement, and damage the semi-allograft (placenta and/or fetus) – we have reported that this occurs in cases of chronic chorioamnionitis and massive perivillous fibrin deposition, also known as maternal floor infarction 176. In both conditions, the following evidence supports maternal anti-fetal rejection: 1) maternal HLA sensitization; 2) fetal HLA-specific antibodies; 3) complement deposition in the umbilical vein; and 4) chronic chorioamnionitis or VUE 20. We consider preterm labor/delivery 21, fetal growth restriction, and fetal death 19,132 as the clinical manifestations of graft injury. Similar findings have been reported in cases of maternal floor infarction with fetal death 177. Why some sensitized mothers develop maternal anti-fetal rejection and others do not requires further investigation.
5. Fetal Inflammatory Response Syndrome, type 2
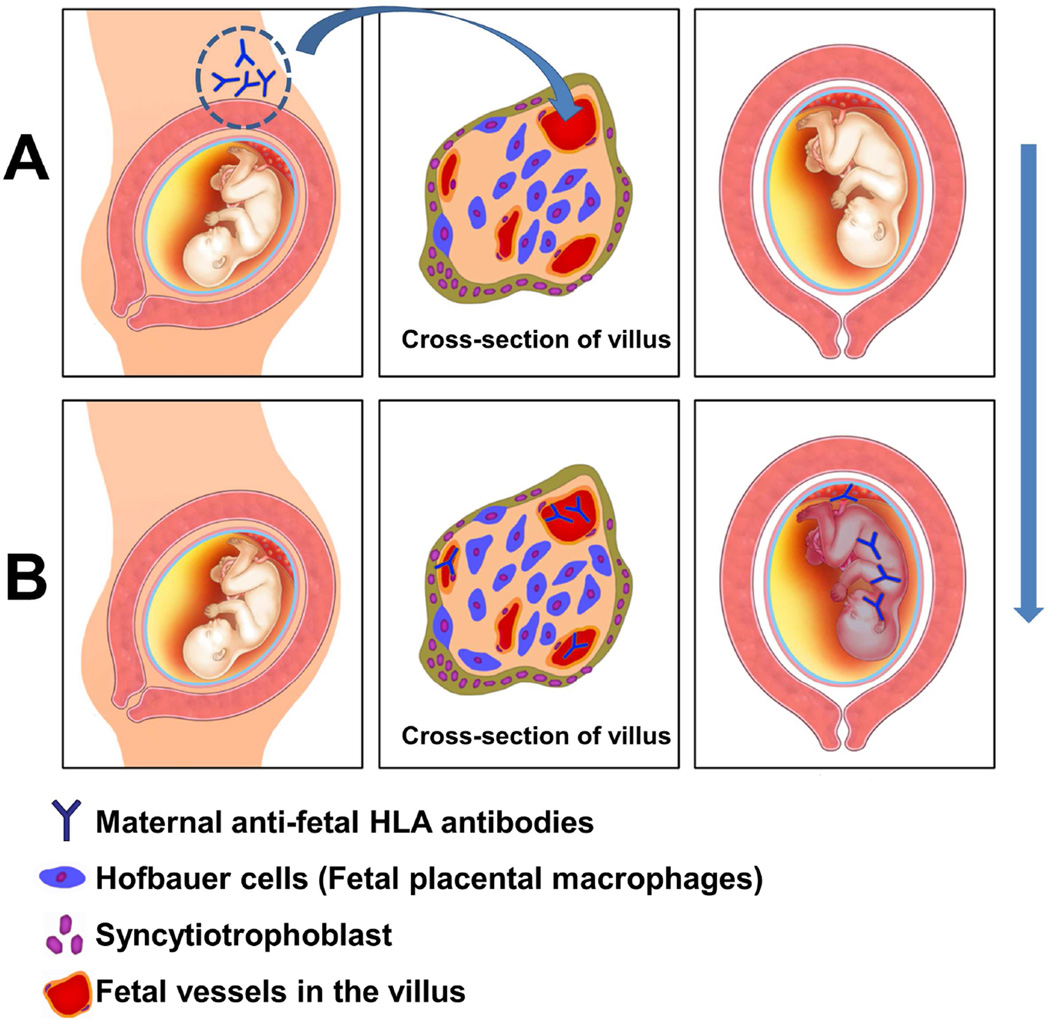
When maternal anti-fetal antibodies cross the placenta and induce an alloimmune reaction (Figure 12), this can lead to a systemic fetal inflammatory response; however, this process is different from fetal systemic inflammation observed in the context of intrauterine infection 20,178–182. In microbial invasion of the amniotic cavity, bacteria lead to activation of the innate immune system and the production of neutrophil chemokines (e.g. IL-8), which generate a chemotactic gradient attracting neutrophils from the decidua into the chorioamniotic membranes, leading to acute histologic chorioamnionitis 120–129. Fetal systemic inflammation in the context of intra-amniotic infection leads to an elevation of the cytokine IL-6 in fetal plasma – we refer to this condition as the fetal inflammatory response syndrome (FIRS) type 1, and the histologic hallmark is funisitis and chorionic vasculitis 183. A full description of FIRS type 1 and details about its biology and long-term consequences are described in a companion article in this issue of the Journal 184. Fetuses born to mothers whose placentas have evidence of VUE and/or chronic chorioamnionitis frequently have elevated concentrations of the chemokine CXCL10 15, 20. This is also the case in amniotic fluid; however, fetal plasma concentrations of IL-6 are generally not elevated in these fetuses, suggesting a different form of systemic inflammatory process 20. CXCL10, a ligand for CXCR3, is chemotactic for activated T-cells, macrophages, and NK cells 185–187. Notably, CXCL10 is one of the most commonly expressed chemokines during allograft rejection and graft-versus-host disease 47,49,188–191. An elevated intra-graft CXCL10 is associated with renal 36,38–42,46, lung 44,192, and cardiac allograft rejection 37,43,193 (akin to the overexpression of CXCL10 in the chorionic membranes in cases with chorioamnionitis, or in the placenta in cases with VUE). An elevated CXCL10 concentration before organ transplantation is predictive of poor allograft outcome 38,41,43,44,191.
Figure 12.
The mechanism whereby an antibody-mediated alloimmune reaction leads to a fetal inflammatory response. (A) Maternal anti-fetal HLA antibodies (depicted as “Y” shape) are present in the maternal circulation, can cross the placenta, and enter the fetal circulation. This process is represented by the blue arrow. (B) Antibodies in the fetal circulation (in the lumen of the fetal vessels in the villus) gain access to the fetus and illicit a systemic inflammatory response, illustrated by the different color of the fetus. Antibodies can lead to complement deposition in the fetal endothelium.
Studies of the white blood cell transcriptome and plasma proteome of fetuses with evidence of maternal anti-fetal rejection identified stereotypic changes in both 15. The maternal anti-fetal rejection phenotype is defined when the patients/fetuses met two or more of the following criteria: 1) presence of chronic placental inflammation; 2) ≥80% of maternal HLA class I panel reactive antibodies (PRA) positivity; and 3) fetal serum CXCL10 concentration >75th percentile 20. Gene ontology analysis showed enrichment of 24 biological processes, such as “response to other organism” and “killing by host of symbiont cells”. A set of differentially-expressed genes discovered by microarray experiments were subsequently confirmed using qRT-PCR 20. We found universal down-regulation of mRNA expression of genes for neutrophil granule proteins and the polymorphonuclear leukocyte surface marker, CD66b 20. All the evidence indicates that there is another type of systemic fetal inflammatory response associated with maternal anti-fetal rejection and an elevated fetal plasma concentration of CXCL10. We propose this as a second type of FIRS (FIRS type 2) (Figure 13).
Figure 13.
Fetal inflammatory response syndrome (FIRS): type 1 vs. type 2. (A) Systemic fetal inflammatory response associated with intra-amniotic infection is FIRS type 1, while the one associated with maternal anti-fetal rejection is FIRS type 2. In FIRS type 1 and type 2, there is elevation of amniotic fluid IL-6 and CXCL10, respectively. (B) Fetal serum CXCL10 concentration is significantly elevated in cases with chronic placental inflammation, while there is no such change in those with acute chorioamnionitis. (C) In acute chorioamnionitis cases, there is significant elevation of fetal serum IL-6 concentration, while CXCL10 concentration does not change. ACA: acute chorioamnionitis, CCA/VUE/CDP: chronic chorioamnionitis/villitis of unknown etiology/chronic deciduitis with plasma cells. Modified from Lee J Am J Reprod Immunol 2013 70:265-80
6. Summary and conclusions
A large body of evidence now supports that idiopathic chronic placental inflammation (villitis of unknown etiology/chronic chorioamnionitis/chronic deciduitis) represents the histological manifestations of maternal anti-fetal cellular rejection, and is common in human pregnancies. It is possible to screen for anti-fetal antibody-mediated rejection by identification of maternal serum antibodies against fetal HLA, and determine whether they are specific for the fetus in the index pregnancy (fetal HLA profile). Further investigation is required to develop and implement tools for the diagnosis of maternal anti-fetal rejection during pregnancy.”
Maternal anti-fetal rejection is significantly associated with late preterm birth and other obstetrical complications 23. Epidemiologic studies show an association between late preterm birth and clinical adverse outcomes in childhood, adolescence, and adulthood 194–200. A recent report of an association between late preterm birth and impaired neurocognitive performance in late adulthood in a Finnish birth cohort underscores the potential importance of this mechanism of disease201. Therefore, further clinical and molecular phenotyping of maternal anti-fetal rejection is needed so that effective monitoring and management of pathological maternal anti-fetal rejection during pregnancy be feasible. Once methods for the identification and diagnosis of maternal anti-fetal rejection become available, clinical trials would be required to determine if interventions such as steroids or immunosuppressive agents could improve pregnancy outcome. The major questions to be answered include the mechanistic explanation of preterm labor associated with maternal anti-fetal rejection, and the solution of the riddle for seemingly normal pregnancy outcome with substantial evidence of maternal anti-fetal rejection.
Acknowledgement
This work was supported, in part, by the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy HS. Inflammation. In: Strayer DS, Rubin E, editors. Rubin’s Pathology. Sixth ed. China: Lippincott Williams & Wilkins; 2012. pp. 47–82. [Google Scholar]
- 2.Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. Sixth ed. Berlin Heidelberg: Springer; 2012. Infectious Diseases; pp. 557–656. [Google Scholar]
- 3.Fox H, Sebire NJ. Pathology of the Placenta. Third ed. China: ELSEVIER; 2007. Infections and Inflammatory Lesions of the Placenta; pp. 303–354. [Google Scholar]
- 4.Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol. 2015;39(1):20–26. doi: 10.1053/j.semperi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Altshuler G, Russell P. The human placental villitides: a review of chronic intrauterine infection. Curr Top Pathol. 1975;60:64–112. [PubMed] [Google Scholar]
- 6.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol. 2005;192(1):264–271. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 7.Boog G. Chronic villitis of unknown etiology. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):9–15. doi: 10.1016/j.ejogrb.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Benirschke K, Coen R, Patterson B, Key T. Villitis of known origin: varicella and toxoplasma. Placenta. 1999;20(5–6):395–399. doi: 10.1053/plac.1999.0405. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AG, Basso NG, Fonseca ME, Zuardi JA, Outanni HN. Enterovirus associated placental morphology: a light, virological, electron microscopic and immunohistologic study. Placenta. 1991;12(5):533–547. doi: 10.1016/0143-4004(91)90029-f. [DOI] [PubMed] [Google Scholar]
- 10.Euscher E, Davis J, Holzman I, Nuovo GJ. Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstet Gynecol. 2001;98(6):1019–1026. doi: 10.1016/s0029-7844(01)01625-8. [DOI] [PubMed] [Google Scholar]
- 11.Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol. 2004;35(5):536–545. doi: 10.1016/j.humpath.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 12.O’malley A, Gillan JE. The incidence of viral infection causing villitis. Placenta. 2005;26:A38. [Google Scholar]
- 13.Redline RW. Recurrent villitis of bacterial etiology. Pediatr Pathol Lab Med. 1996;16(6):995–1001. doi: 10.1080/15513819609168723. [DOI] [PubMed] [Google Scholar]
- 14.Ernst LM, Crouch J, Rinder H, Howe JG. Bacterial etiology for chronic villitis is not supported by polymerase chain reaction for 16S rRNA DNA. Pediatr Dev Pathol. 2005;8(6):647–653. doi: 10.1007/s10024-005-0412-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, Lajeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182(6):3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23(7):1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6(2):e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66(6):510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59(5):928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70(4):265–284. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Romero R, Xu Y, Miranda J, Yoo W, Chaemsaithong P, et al. Detection of anti-HLA antibodies in maternal blood in the second trimester to identify patients at risk of antibody-mediated maternal anti-fetal rejection and spontaneous preterm delivery. Am J Reprod Immunol. 2013;70(2):162–175. doi: 10.1111/aji.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KA, Kim YW, Shim JY, Won HS, Lee PR, Kim A, et al. Distinct patterns of C4d immunoreactivity in placentas with villitis of unknown etiology, cytomegaloviral placentitis, and infarct. Placenta. 2013;34(5):432–435. doi: 10.1016/j.placenta.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kim JS, Park JW, Park CW, Park JS, Jun JK, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34(8):681–689. doi: 10.1016/j.placenta.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Rudzinski E, Gilroy M, Newbill C, Morgan T. Positive C4d immunostaining of placental villous syncytiotrophoblasts supports host-versus-graft rejection in villitis of unknown etiology. Pediatr Dev Pathol. 2013;16(1):7–13. doi: 10.2350/12-05-1195-OA.1. [DOI] [PubMed] [Google Scholar]
- 25.Erlebacher A. Why isn’t the fetus rejected? Curr Opin Immunol. 2001;13(5):590–593. doi: 10.1016/s0952-7915(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 26.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 27.Leslie M. Immunology Fetal immune system hushes attacks on maternal cells. Science. 2008;322(5907):1450–1451. doi: 10.1126/science.322.5907.1450b. [DOI] [PubMed] [Google Scholar]
- 28.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burlingham WJ. A lesson in tolerance--maternal instruction to fetal cells. N Engl J Med. 2009;360(13):1355–1357. doi: 10.1056/NEJMcibr0810752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluestone JA. Mechanisms of tolerance. Immunol Rev. 2011;241(1):5–19. doi: 10.1111/j.1600-065X.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 31.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betz AG. Immunology: Tolerating pregnancy. Nature. 2012;490(7418):47–48. doi: 10.1038/490047a. [DOI] [PubMed] [Google Scholar]
- 33.Williams Z. Inducing tolerance to pregnancy. N Engl J Med. 2012;367(12):1159–1161. doi: 10.1056/NEJMcibr1207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52(4):457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, et al. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am J Kidney Dis. 2001;37(3):518–531. [PubMed] [Google Scholar]
- 37.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, Mccarthy PM, et al. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75(1):72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 38.Rotondi M, Rosati A, Buonamano A, Lasagni L, Lazzeri E, Pradella F, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4(9):1466–1474. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 39.Tatapudi RR, Muthukumar T, Dadhania D, Ding R, Li B, Sharma VK, et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65(6):2390–2397. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 40.Segerer S, Bohmig GA, Exner M, Kerjaschki D, Regele H, Schlondorff D. Role of CXCR3 in cellular but not humoral renal allograft rejection. Transpl Int. 2005;18(6):676–680. doi: 10.1111/j.1432-2277.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 41.Lazzeri E, Rotondi M, Mazzinghi B, Lasagni L, Buonamano A, Rosati A, et al. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation. 2005;79(9):1215–1220. doi: 10.1097/01.tp.0000160759.85080.2e. [DOI] [PubMed] [Google Scholar]
- 42.Schaub S, Nickerson P, Rush D, Mayr M, Hess C, Golian M, et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant. 2009;9(6):1347–1353. doi: 10.1111/j.1600-6143.2009.02645.x. [DOI] [PubMed] [Google Scholar]
- 43.Crescioli C, Buonamano A, Scolletta S, Sottili M, Francalanci M, Giomarelli P, et al. Predictive role of pretransplant serum CXCL10 for cardiac acute rejection. Transplantation. 2009;87(2):249–255. doi: 10.1097/TP.0b013e3181919f5d. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao Y, Wang M, Zhou Q, Jin J, Wang Y, Peng W, et al. CXCL10 and CXCL13 Expression were highly up-regulated in peripheral blood mononuclear cells in acute rejection and poor response to anti-rejection therapy. J Clin Immunol. 2011;31(3):414–418. doi: 10.1007/s10875-010-9500-8. [DOI] [PubMed] [Google Scholar]
- 46.Lo DJ, Weaver TA, Kleiner DE, Mannon RB, Jacobson LM, Becker BN, et al. Chemokines and their receptors in human renal allotransplantation. Transplantation. 2011;91(1):70–77. doi: 10.1097/TP.0b013e3181fe12fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romagnani P, Crescioli C. CXCL10: a candidate biomarker in transplantation. Clin Chim Acta. 2012;413(17–18):1364–1373. doi: 10.1016/j.cca.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med. 2013;369(1):20–31. doi: 10.1056/NEJMoa1215555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Liu YF, Su ZX, Shi LP, Chen YH. Serum fractalkine and interferon-gamma inducible protein-10 concentrations are early detection markers for acute renal allograft rejection. Transplant Proc. 2014;46(5):1420–1425. doi: 10.1016/j.transproceed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993;143(2):473–479. [PMC free article] [PubMed] [Google Scholar]
- 51.Myerson D, Parkin RK, Benirschke K, Tschetter CN, Hyde SR. The pathogenesis of villitis of unknown etiology: analysis with a new conjoint immunohistochemistry-in situ hybridization procedure to identify specific maternal and fetal cells. Pediatr Dev Pathol. 2006;9(4):257–265. doi: 10.2350/08-05-0103.1. [DOI] [PubMed] [Google Scholar]
- 52.Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta. 1984;5(5):395–402. doi: 10.1016/s0143-4004(84)80019-3. [DOI] [PubMed] [Google Scholar]
- 53.Redline RW, Abramowsky CR. Clinical and pathologic aspects of recurrent placental villitis. Hum Pathol. 1985;16(7):727–731. doi: 10.1016/s0046-8177(85)80159-3. [DOI] [PubMed] [Google Scholar]
- 54.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38(10):1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 55.Feeley L, Mooney EE. Villitis of unknown aetiology: correlation of recurrence with clinical outcome. J Obstet Gynaecol. 2010;30(5):476–479. doi: 10.3109/01443611003802339. [DOI] [PubMed] [Google Scholar]
- 56.Tamblyn JA, Lissauer DM, Powell R, Cox P, Kilby MD. The immunological basis of villitis of unknown etiology - review. Placenta. 2013;34(10):846–855. doi: 10.1016/j.placenta.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Tang Z, Abrahams VM, Mor G, Guller S. Placental Hofbauer cells and complications of pregnancy. Ann N Y Acad Sci. 2011;1221:103–108. doi: 10.1111/j.1749-6632.2010.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katzman PJ, Murphy SP, Oble DA. Immunohistochemical analysis reveals an influx of regulatory T cells and focal trophoblastic STAT-1 phosphorylation in chronic villitis of unknown etiology. Pediatr Dev Pathol. 2011;14(4):284–293. doi: 10.2350/10-09-0910-OA.1. [DOI] [PubMed] [Google Scholar]
- 59.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 60.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85(1):71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63(6):445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 63.Schumacher A, Zenclussen AC. Regulatory T cells: regulators of life. Am J Reprod Immunol. 2014;72(2):158–170. doi: 10.1111/aji.12238. [DOI] [PubMed] [Google Scholar]
- 64.Ito Y, Matsuoka K, Uesato T, Sago H, Okamoto A, Nakazawa A, et al. Increased expression of perforin, granzyme B, and C5b-9 in villitis of unknown etiology. Placenta. 2015 doi: 10.1016/j.placenta.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Russell P. Inflammatory lesions of the human placenta III. The histopathology of villitis of unknown aetiology. Placenta. 1980;1(3):227–244. doi: 10.1016/s0143-4004(80)80005-1. [DOI] [PubMed] [Google Scholar]
- 66.Redline RW, O’riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124(12):1785–1791. doi: 10.5858/2000-124-1785-PLAWCP. [DOI] [PubMed] [Google Scholar]
- 67.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192(2):452–457. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 68.Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am J Pathol. 1997;150(5):1845–1860. [PMC free article] [PubMed] [Google Scholar]
- 69.Juliano PB, Blotta MH, Altemani AM. ICAM-1 is overexpressed by villous trophoblasts in placentitis. Placenta. 2006;27(6–7):750–757. doi: 10.1016/j.placenta.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Labarrere CA, Bammerlin E, Hardin JW, Dicarlo HL. Intercellular adhesion molecule-1 expression in massive chronic intervillositis: implications for the invasion of maternal cells into fetal tissues. Placenta. 2014;35(5):311–317. doi: 10.1016/j.placenta.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Egal ES, Mariano FV, Blotta MH, Pina AR, Montalli VA, Almeida OP, et al. ICAM-1 expression on immune cells in chronic villitis. Placenta. 2014;35(12):1021–1026. doi: 10.1016/j.placenta.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Nelson DM. Apoptotic changes occur in syncytiotrophoblast of human placental villi where fibrin type fibrinoid is deposited at discontinuities in the villous trophoblast. Placenta. 1996;17(7):387–391. doi: 10.1016/s0143-4004(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 73.Akalin E, Hendrix RC, Polavarapu RG, Pearson TC, Neylan JF, Larsen CP, et al. Gene expression analysis in human renal allograft biopsy samples using high-density oligoarray technology. Transplantation. 2001;72(5):948–953. doi: 10.1097/00007890-200109150-00034. [DOI] [PubMed] [Google Scholar]
- 74.Gimino VJ, Lande JD, Berryman TR, King RA, Hertz MI. Gene expression profiling of bronchoalveolar lavage cells in acute lung rejection. Am J Respir Crit Care Med. 2003;168(10):1237–1242. doi: 10.1164/rccm.200305-644OC. [DOI] [PubMed] [Google Scholar]
- 75.Ichiba T, Teshima T, Kuick R, Misek DE, Liu C, Takada Y, et al. Early changes in gene expression profiles of hepatic GVHD uncovered by oligonucleotide microarrays. Blood. 2003;102(2):763–771. doi: 10.1182/blood-2002-09-2748. [DOI] [PubMed] [Google Scholar]
- 76.Flechner SM, Kurian SM, Head SR, Sharp SM, Whisenant TC, Zhang J, et al. Kidney transplant rejection and tissue injury by gene profiling of biopsies and peripheral blood lymphocytes. Am J Transplant. 2004;4(9):1475–1489. doi: 10.1111/j.1600-6143.2004.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugerman PB, Faber SB, Willis LM, Petrovic A, Murphy GF, Pappo J, et al. Kinetics of gene expression in murine cutaneous graft-versus-host disease. Am J Pathol. 2004;164(6):2189–2202. doi: 10.1016/S0002-9440(10)63776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6(1):150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 79.Jeanty C, Derderian SC, Mackenzie TC. Maternal-fetal cellular trafficking: clinical implications and consequences. Curr Opin Pediatr. 2014;26(3):377–382. doi: 10.1097/MOP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romero R, Korzeniewski SJ, Chaiwaropongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Histologic Features of Placentas Delivered by Low-Risk Women with Singleton Term Gestations. Am J Obstet Gynecol (in preparation) 2015 [Google Scholar]
- 81.Labarrere C, Althabe O, Telenta M. Chronic villitis of unknown aetiology in placentae of idiopathic small for gestational age infants. Placenta. 1982;3(3):309–317. doi: 10.1016/s0143-4004(82)80007-6. [DOI] [PubMed] [Google Scholar]
- 82.Labarrere C, Althabe O. Chronic villitis of unknown etiology and maternal arterial lesions in preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 1985;20(1):1–11. doi: 10.1016/0028-2243(85)90077-2. [DOI] [PubMed] [Google Scholar]
- 83.Althabe O, Labarrere C. Chronic villitis of unknown aetiology and intrauterine growth-retarded infants of normal and low ponderal index. Placenta. 1985;6(4):369–373. doi: 10.1016/s0143-4004(85)80047-3. [DOI] [PubMed] [Google Scholar]
- 84.Salafia CM, Vintzileos AM, Silberman L, Bantham KF, Vogel CA. Placental pathology of idiopathic intrauterine growth retardation at term. Am J Perinatol. 1992;9(3):179–184. doi: 10.1055/s-2007-999316. [DOI] [PubMed] [Google Scholar]
- 85.Redline RW, Patterson P. Patterns of placental injuryCorrelations with gestational age, placental weight, and clinical diagnoses. Arch Pathol Lab Med. 1994;118(7):698–701. [PubMed] [Google Scholar]
- 86.Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173(4):1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 87.Derricott H, Jones RL, Heazell AE. Investigating the association of villitis of unknown etiology with stillbirth and fetal growth restriction - a systematic review. Placenta. 2013;34(10):856–862. doi: 10.1016/j.placenta.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Salafia CM, Vogel CA, Vintzileos AM, Bantham KF, Pezzullo J, Silberman L. Placental pathologic findings in preterm birth. Am J Obstet Gynecol. 1991;165(4 Pt 1):934–938. doi: 10.1016/0002-9378(91)90443-u. [DOI] [PubMed] [Google Scholar]
- 89.Veerbeek JH, Nikkels PG, Torrance HL, Gravesteijn J, Post Uiterweer ED, Derks JB, et al. Placental pathology in early intrauterine growth restriction associated with maternal hypertension. Placenta. 2014;35(9):696–701. doi: 10.1016/j.placenta.2014.06.375. [DOI] [PubMed] [Google Scholar]
- 90.Benzon S, Zekic Tomas S, Benzon Z, Vulic M, Kuzmic Prusac I. Involvement Of T Lymphocytes In The Placentae With Villitis Of Unknown Etiology From Pregnancies Complicated With Preeclampsia. J Matern Fetal Neonatal Med. 2015:1–21. doi: 10.3109/14767058.2015.1032239. [DOI] [PubMed] [Google Scholar]
- 91.Redline RW, Zaragoza M, Hassold T. Prevalence of developmental and inflammatory lesions in nonmolar first-trimester spontaneous abortions. Hum Pathol. 1999;30(1):93–100. doi: 10.1016/s0046-8177(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 92.Romero R. Prenatal medicine: the child is the father of the man 1996. J Matern Fetal Neonatal Med. 2009;22(8):636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 93.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22(8):633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 94.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torrance HL, Bloemen MC, Mulder EJ, Nikkels PG, Derks JB, De Vries LS, et al. Predictors of outcome at 2 years of age after early intrauterine growth restriction. Ultrasound Obstet Gynecol. 2010;36(2):171–177. doi: 10.1002/uog.7627. [DOI] [PubMed] [Google Scholar]
- 96.Scher MS, Trucco GS, Beggarly ME, Steppe DA, Macpherson TA. Neonates with electrically confirmed seizures and possible placental associations. Pediatr Neurol. 1998;19(1):37–41. doi: 10.1016/s0887-8994(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 97.Eberle AM, Levesque D, Vintzileos AM, Egan JF, Tsapanos V, Salafia CM. Placental pathology in discordant twins. Am J Obstet Gynecol. 1993;169(4):931–935. doi: 10.1016/0002-9378(93)90029-i. [DOI] [PubMed] [Google Scholar]
- 98.Jacques SM, Qureshi F. Chronic villitis of unknown etiology in twin gestations. Pediatr Pathol. 1994;14(4):575–584. doi: 10.3109/15513819409023332. [DOI] [PubMed] [Google Scholar]
- 99.Styer AK, Parker HJ, Roberts DJ, Palmer-Toy D, Toth TL, Ecker JL. Placental villitis of unclear etiology during ovum donor in vitro fertilization pregnancy. Am J Obstet Gynecol. 2003;189(4):1184–1186. doi: 10.1067/s0002-9378(03)00577-5. [DOI] [PubMed] [Google Scholar]
- 100.Perni SC, Predanic M, Cho JE, Baergen RN. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J Perinat Med. 2005;33(1):27–32. doi: 10.1515/JPM.2005.004. [DOI] [PubMed] [Google Scholar]
- 101.Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010;93(2):397–404. doi: 10.1016/j.fertnstert.2008.12.144. [DOI] [PubMed] [Google Scholar]
- 102.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10(3):217–229. [PubMed] [Google Scholar]
- 103.Gersell DJ. Chronic villitis, chronic chorioamnionitis, and maternal floor infarction. Semin Diagn Pathol. 1993;10(3):251–266. [PubMed] [Google Scholar]
- 104.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29(12):1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 105.Krol J, Mengele K, Ottl-Mantchenko I, Welk A, Wasilewitsch I, Von Steinburg SP, et al. Ex vivo detection of apoptotic trophoblast cells applying flow cytofluorometry and immunocytochemistry using M30 antibody directed to the cytokeratin 18 neo-epitope. Int J Mol Med. 2005;16(3):415–420. [PubMed] [Google Scholar]
- 106.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, Demars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 107.Mcmaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, Demars R, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154(8):3771–3778. [PubMed] [Google Scholar]
- 108.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171(3):1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 109.Hunt JS, Petroff MG, Mcintire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19(7):681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 110.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336(6086):1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 113.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 114.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158(9):4122–4128. [PubMed] [Google Scholar]
- 115.Holmes CH, Simpson KL, Wainwright SD, Tate CG, Houlihan JM, Sawyer IH, et al. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990;144(8):3099–3105. [PubMed] [Google Scholar]
- 116.Hsi BL, Hunt JS, Atkinson JP. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J Reprod Immunol. 1991;19(3):209–223. doi: 10.1016/0165-0378(91)90036-p. [DOI] [PubMed] [Google Scholar]
- 117.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202(2):231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garcia AG, Marques RL, Lobato YY, Fonseca ME, Wigg MD. Placental pathology in congenital rubella. Placenta. 1985;6(4):281–295. doi: 10.1016/s0143-4004(85)80038-2. [DOI] [PubMed] [Google Scholar]
- 119.Garcia AG. Congenital toxoplasmosis in two successive sibs. Arch Dis Child. 1968;43(232):705–710. doi: 10.1136/adc.43.232.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165(4 Pt 1):813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 121.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169(5):1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 122.Puchner T, Egarter C, Wimmer C, Lederhilger F, Weichselbraun I. Amniotic fluid interleukin-8 as a marker for intraamniotic infection. Arch Gynecol Obstet. 1993;253(1):9–14. doi: 10.1007/BF02770627. [DOI] [PubMed] [Google Scholar]
- 123.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22(2):281–342. [PubMed] [Google Scholar]
- 124.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177(4):825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 125.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78(1):5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 126.Hsu CD, Meaddough E, Aversa K, Hong SF, Lu LC, Jones DC, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol. 1998;179(5):1267–1270. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 127.Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L-selectin, GRO-alpha, and interleukin-8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol. 1998;178(3):428–432. doi: 10.1016/s0002-9378(98)70414-4. [DOI] [PubMed] [Google Scholar]
- 128.Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18(4):241–247. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 129.Yoneda S, Shiozaki A, Ito M, Yoneda N, Inada K, Yonezawa R, et al. Accurate Prediction of the Stage of Histological Chorioamnionitis before Delivery by Amniotic Fluid IL-8 Level. Am J Reprod Immunol. 2015;73(6):568–576. doi: 10.1111/aji.12360. [DOI] [PubMed] [Google Scholar]
- 130.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223(4):553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alok A, Mukhopadhyay D, Karande AA. Glycodelin A, an immunomodulatory protein in the endometrium, inhibits proliferation and induces apoptosis in monocytic cells. Int J Biochem Cell Biol. 2009;41(5):1138–1147. doi: 10.1016/j.biocel.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 132.Lannaman K, Romero R, Chaemsaithong P, Ahmed A, Yeo L, Hassan S, et al. Fetal death: an extreme form of maternal anti-fetal rejection (Abstract 497) Am J Obstet Gynecol. 2015;212(supplement 1):S251. [Google Scholar]
- 133.Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31(3):292–295. doi: 10.1016/s0046-8177(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 134.Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM. Placental Pathology: Atlas of Nontumor Pathology (First Series, Fascicle 3) Washington DC: American Registry of Pathology; 2004. Inflammation and infection; pp. 75–115. [Google Scholar]
- 135.Naeye RL. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol. 1987;18(7):680–691. doi: 10.1016/s0046-8177(87)80239-3. [DOI] [PubMed] [Google Scholar]
- 136.Bendon RW, Miller M. Routine pathological examination of placentae from abnormal pregnancies. Placenta. 1990;11(4):369–370. doi: 10.1016/s0143-4004(05)80227-9. [DOI] [PubMed] [Google Scholar]
- 137.Maroun LL, Mathiesen L, Hedegaard M, Knudsen LE, Larsen LG. Pathologic evaluation of normal and perfused term placental tissue. Pediatr Dev Pathol. 2014;17(5):330–338. doi: 10.2350/12-08-1243-OA.1. [DOI] [PubMed] [Google Scholar]
- 138.Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009;12(1):16–21. doi: 10.2350/07-04-0270.1. [DOI] [PubMed] [Google Scholar]
- 139.Koch CA, Platt JL. Natural mechanisms for evading graft rejection: the fetus as an allograft. Springer Semin Immunopathol. 2003;25(2):95–117. doi: 10.1007/s00281-003-0136-0. [DOI] [PubMed] [Google Scholar]
- 140.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63(6):624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 141.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 142.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117(5):1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szekeres-Bartho J. Immunological relationship between the mother and the fetus. Int Rev Immunol. 2002;21(6):471–495. doi: 10.1080/08830180215017. [DOI] [PubMed] [Google Scholar]
- 144.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 145.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73(9):1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 146.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5(10):807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 147.Alegre ML, Florquin S, Goldman M. Cellular mechanisms underlying acute graft rejection: time for reassessment. Curr Opin Immunol. 2007;19(5):563–568. doi: 10.1016/j.coi.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 148.Kim IK, Bedi DS, Denecke C, Ge X, Tullius SG. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation. 2008;86(7):889–894. doi: 10.1097/TP.0b013e318186ac4a. [DOI] [PubMed] [Google Scholar]
- 149.Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189–220. doi: 10.1146/annurev.pathmechdis.3.121806.151508. [DOI] [PubMed] [Google Scholar]
- 150.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 151.Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93(1):1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 152.Ali JM, Bolton EM, Bradley JA, Pettigrew GJ. Allorecognition pathways in transplant rejection and tolerance. Transplantation. 2013;96(8):681–688. doi: 10.1097/TP.0b013e31829853ce. [DOI] [PubMed] [Google Scholar]
- 153.Mckenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation. 2000;69(3):319–326. doi: 10.1097/00007890-200002150-00001. [DOI] [PubMed] [Google Scholar]
- 154.Ho EK, Vlad G, Colovai AI, Vasilescu ER, Schwartz J, Sondermeijer H, et al. Alloantibodies in heart transplantation. Hum Immunol. 2009;70(10):825–829. doi: 10.1016/j.humimm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 155.Howell WM, Carter V, Clark B. The HLA system: immunobiology, HLA typing, antibody screening and crossmatching techniques. J Clin Pathol. 2010;63(5):387–390. doi: 10.1136/jcp.2009.072371. [DOI] [PubMed] [Google Scholar]
- 156.Ayala Garcia MA, Gonzalez Yebra B, Lopez Flores AL, Guani Guerra E. The major histocompatibility complex in transplantation. J Transplant. 2012;2012:842141. doi: 10.1155/2012/842141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahdi BM. A glow of HLA typing in organ transplantation. Clin Transl Med. 2013;2(1):6. doi: 10.1186/2001-1326-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Buckley RH. 27, Transplantation immunology: organ and bone marrow. J Allergy Clin Immunol. 2003;111(2 Suppl):S733–S744. doi: 10.1067/mai.2003.142. [DOI] [PubMed] [Google Scholar]
- 159.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, et al. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67(3):184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li XC, Raghavan M. Structure and function of major histocompatibility complex class I antigens. Curr Opin Organ Transplant. 2010;15(4):499–504. doi: 10.1097/MOT.0b013e32833bfb33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petersdorf EW. The major histocompatibility complex: a model for understanding graft-versus-host disease. Blood. 2013;122(11):1863–1872. doi: 10.1182/blood-2013-05-355982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Petersdorf EW. Genetics of graft-versus-host disease: the major histocompatibility complex. Blood Rev. 2013;27(1):1–12. doi: 10.1016/j.blre.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2(7465):662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 164.Sumitran-Holgersson S. HLA-specific alloantibodies and renal graft outcome. Nephrol Dial Transplant. 2001;16(5):897–904. doi: 10.1093/ndt/16.5.897. [DOI] [PubMed] [Google Scholar]
- 165.Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88(4):568–574. doi: 10.1097/TP.0b013e3181b11b72. [DOI] [PubMed] [Google Scholar]
- 166.Gloor JM, Winters JL, Cornell LD, Fix LA, Degoey SR, Knauer RM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10(3):582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 167.Montgomery RA, Cozzi E, West LJ, Warren DS. Humoral immunity and antibody-mediated rejection in solid organ transplantation. Semin Immunol. 2011;23(4):224–234. doi: 10.1016/j.smim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 168.Focosi D, Vistoli F, Boggi U. Rejection of the kidney allograft. N Engl J Med. 2011;364(5):485–486. doi: 10.1056/NEJMc1012440. author reply 6. [DOI] [PubMed] [Google Scholar]
- 169.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 170.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23(12):2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thaunat O. Humoral immunity in chronic allograft rejection: puzzle pieces come together. Transpl Immunol. 2012;26(2–3):101–106. doi: 10.1016/j.trim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 172.Honger G, Fornaro I, Granado C, Tiercy JM, Hosli I, Schaub S. Frequency and determinants of pregnancy-induced child-specific sensitization. Am J Transplant. 2013;13(3):746–753. doi: 10.1111/ajt.12048. [DOI] [PubMed] [Google Scholar]
- 173.Jones M, Jeal H, Harris JM, Smith JD, Rose ML, Taylor AN, et al. Association of maternal anti-HLA class II antibodies with protection from allergy in offspring. Allergy. 2013;68(9):1143–1149. doi: 10.1111/all.12213. [DOI] [PubMed] [Google Scholar]
- 174.Masson E, Vidal C, Deschamps M, Bongain S, Thevenin C, Dupont I, et al. Incidence and risk factors of anti-HLA immunization after pregnancy. Hum Immunol. 2013;74(8):946–951. doi: 10.1016/j.humimm.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 175.Lashley EE, Meuleman T, Claas FH. Beneficial or harmful effect of antipaternal human leukocyte antibodies on pregnancy outcome? A systematic review and meta-analysis. Am J Reprod Immunol. 2013;70(2):87–103. doi: 10.1111/aji.12109. [DOI] [PubMed] [Google Scholar]
- 176.Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70(4):285–298. doi: 10.1111/aji.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70(4):285–298. doi: 10.1111/aji.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 179.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183(5):1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 180.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 181.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 182.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 184.Kim CJ, Romero R, Chaemsaithong P, Kim YM. Acute chorionitis and funisitis: Definition, Pathologic features, and clinical significance. Am J Obstet Gynecol (in preparation) 2015 doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177(6):1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155(8):3877–3888. [PubMed] [Google Scholar]
- 187.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25(4):201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(1):109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- 190.Tan J, Zhou G. Chemokine receptors and transplantation. Cell Mol Immunol. 2005;2(5):343–349. [PubMed] [Google Scholar]
- 191.Romagnani P. From basic science to clinical practice: use of cytokines and chemokines as therapeutic targets in renal diseases. J Nephrol. 2005;18(3):229–233. [PubMed] [Google Scholar]
- 192.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, Mccarthy PM, et al. Chemokine and receptor-gene expression during early and late acute rejection episodes in human cardiac allografts. Transplantation. 2003;75(12):2044–2047. doi: 10.1097/01.TP.0000069601.73079.94. [DOI] [PubMed] [Google Scholar]
- 194.Kamath BD, Marcotte MP, Defranco EA. Neonatal morbidity after documented fetal lung maturity in late preterm and early term infants. Am J Obstet Gynecol. 2011;204(6):518. doi: 10.1016/j.ajog.2011.03.038. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Teune MJ, Bakhuizen S, Gyamfi Bannerman C, Opmeer BC, Van Kaam AH, Van Wassenaer AG, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374. doi: 10.1016/j.ajog.2011.07.015. e1–9. [DOI] [PubMed] [Google Scholar]
- 196.Taylor HG. Outcomes of late preterm birth: who is at risk and for what? Am J Obstet Gynecol. 2012;206(3):181–182. doi: 10.1016/j.ajog.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 197.Lipkind HS, Slopen ME, Pfeiffer MR, Mcveigh KH. School-age outcomes of late preterm infants in New York City. Am J Obstet Gynecol. 2012;206(3):222. doi: 10.1016/j.ajog.2012.01.007. e1–6. [DOI] [PubMed] [Google Scholar]
- 198.Zanardo V, Straface G, Trevisanuto D. Future learning abilities of late preterm infants. Am J Obstet Gynecol. 2012;207(1):e17. doi: 10.1016/j.ajog.2012.03.017. author reply e-8. [DOI] [PubMed] [Google Scholar]
- 199.Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209(4):p368. doi: 10.1016/j.ajog.2013.05.041. e1–8. [DOI] [PubMed] [Google Scholar]
- 200.Berry KA, Baron IS, Weiss BA, Baker R, Ahronovich MD, Litman FR. In vitro fertilization and late preterm preschoolers’ neuropsychological outcomes: the PETIT study. Am J Obstet Gynecol. 2013;209(4):356. doi: 10.1016/j.ajog.2013.06.041. e1–6. [DOI] [PubMed] [Google Scholar]
- 201.Heinonen K, Eriksson JG, Lahti J, Kajantie E, Pesonen AK, Tuovinen S, et al. Late preterm birth and neurocognitive performance in late adulthood: a birth cohort study. Pediatrics. 2015;135(4):e818–e825. doi: 10.1542/peds.2014-3556. [DOI] [PubMed] [Google Scholar]
- 202.Larsen MH, Hviid TV. Human leukocyte antigen-G polymorphism in relation to expression, function, and disease. Hum Immunol. 2009;70(12):1026–1034. doi: 10.1016/j.humimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 203.Uckan D, Steele A, Cherry Wang BY, Chamizo W, Koutsonikolis A, et al. Trophoblasts express Fas ligand: a proposed mechanism for immune privilege in placenta and maternal invasion. Mol Hum Reprod. 1997;3(8):655–662. doi: 10.1093/molehr/3.8.655. [DOI] [PubMed] [Google Scholar]
- 204.Kudo Y, Boyd CA. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochim Biophys Acta. 2000;1500(1):119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 205.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2(1):64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 206.Mellor AL, Chandler P, Lee GK, Johnson T, Keskin DB, Lee J, et al. Indoleamine 2,3-dioxygenase, immunosuppression and pregnancy. J Reprod Immunol. 2002;57(1–2):143–150. doi: 10.1016/s0165-0378(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 207.Kudo Y. The role of placental indoleamine 2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 2013;56(4):209–216. doi: 10.5468/ogs.2013.56.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179(8):5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 209.D’addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187(9):4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Holmes CH, Simpson KL, Okada H, Okada N, Wainwright SD, Purcell DF, et al. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55) Eur J Immunol. 1992;22(6):1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- 211.Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J Immunol. 1993;151(3):1562–1570. [PubMed] [Google Scholar]
- 212.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287(5452):498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 213.Girardi G, Bulla R, Salmon JE, Tedesco F. The complement system in the pathophysiology of pregnancy. Mol Immunol. 2006;43(1–2):68–77. doi: 10.1016/j.molimm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 214.Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol Invest. 2008;37(5):645–659. doi: 10.1080/08820130802191615. [DOI] [PubMed] [Google Scholar]
- 215.Stites DP, Bugbee S, Siiteri PK. Differential actions of progesterone and cortisol on lymphocyte and monocyte interaction during lymphocyte activation--relevance to immunosuppression in pregnancy. J Reprod Immunol. 1983;5(4):215–228. doi: 10.1016/0165-0378(83)90237-1. [DOI] [PubMed] [Google Scholar]
- 216.Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. Progesterone and maintenance of pregnancy: is progesterone nature’s immunosuppressant? Ann N Y Acad Sci. 1977;286:384–397. doi: 10.1111/j.1749-6632.1977.tb29431.x. [DOI] [PubMed] [Google Scholar]
- 217.Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31(1–2):81–95. doi: 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- 218.Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, et al. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151(11):5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- 219.Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011;187(4):1778–1787. doi: 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mincheva-Nilsson L, Nagaeva O, Chen T, Stendahl U, Antsiferova J, Mogren I, et al. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol. 2006;176(6):3585–3592. doi: 10.4049/jimmunol.176.6.3585. [DOI] [PubMed] [Google Scholar]
- 221.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63(6):520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 222.Mangeney M, Renard M, Schlecht-Louf G, Bouallaga I, Heidmann O, Letzelter C, et al. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci U S A. 2007;104(51):20534–20539. doi: 10.1073/pnas.0707873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tolosa JM, Schjenken JE, Clifton VL, Vargas A, Barbeau B, Lowry P, et al. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2012;33(11):933–941. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 224.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56(5–6):345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 225.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176(3):1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 226.Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183(1):340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 227.Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2(11):1018–1024. doi: 10.1038/ni719. [DOI] [PubMed] [Google Scholar]
- 228.Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005;11(1):35–41. doi: 10.1093/molehr/gah129. [DOI] [PubMed] [Google Scholar]
- 229.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191(11):5515–5523. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]