Abstract
The present study used the context preexposure facilitation effect (CPFE) to examine long-term retention of incidental context learning in periweanling, adolescent and adult rats. The CPFE is a variant of contextual fear conditioning in which encoding the context representation, associating this representation with shock, and expressing the context–shock association each occur on separate occasions. Experiment 1 manipulated the retention interval—1 d, 8 d, 15 d, or 22 d—between context preexposure and training with immediate shock to determine how long the encoded context could be remembered (testing always occurred 24 h following training). The other factors were age—postnatal day (PND) 24 vs 31—and training group—Preexposed to the training context (Pre) vs. an alternate context (Alt-Pre).At both ages, significantly more freezing was evident in the Pre vs. Alt Pre Groups at the 24 h, 8 d and 15 d retention intervals but not at the 22 d interval, indicating that juvenile– adolescent rats remember the context for up to 15 d. In contrast, context memory persists for 22 days in adult rats (Experiment 2); and is not evident after 24 h, 8 d, or 15 d retention intervals in PND 17 rats (Experiment 3). The present study illustrates the value of the CPFE paradigm for investigations of long-term context memory in developing rats. Implications for the neurobiology of infantile amnesia are discussed.
Keywords: Contextual fear, Spatial learning, Infantile amnesia, Prefrontal cortex, Hippocampus, Amygdala
1. Introduction
Infantile amnesia, the inability of adults to recall events learned in early childhood, has been studied for many years in both animal models [10] and humans [28–31]. The idea that infantile amnesia has a biological basis is also longstanding [10]. However, understanding of the neural mechanisms of the ontogeny of long-term memory remains poorly developed [2,8,21].
Recent proposals attribute poor long-term memory in infancy to hippocampal neurogenesis [2] altered neocortical storage [21] and/or retrieval [18]. Recent work on this problem has used hippocampus-dependent tasks, such as contextual fear conditioning [1,2] although standard contextual fear conditioning (sCFC), in which context exposure is directly paired with foot shock, only depends on the hippocampus under certain conditions [32,40]. In contrast, a variant of sCFC known as the context preexposure facilitation effect (CPFE) cannot be learned without a conjunctive representation of the context [20,34] and therefore always requires a functional hippocampus [27,32,33,36]. The CPFE procedure consists of the three phases that typically occur at 24-h intervals—context exposure, training, and testing. Context learning occurs on the first day, a retrieved representation of the context is associated with immediate-shock on the second day, and this results in enhanced freezing (relative to non-preexposed controls) on the test day [13]. Thus, in the CPFE, acquisition of the context representation and the association of the context with shock occur on different occasions, making it easier to manipulate and analyze these two components of context fear learning independently.
The CPFE has been used to probe the different components of context learning in rats during adulthood [13,35] and over the course of development [20,36]. Recently, data featuring the retention of context memory have shown that PND 17 mice retain sCFC for one day, with partial forgetting at 7 or 14 days, and no memory evident at 28 days [2]. In contrast, adult mice trained at PND 60 show full retention of sCFC at 28 days. A similar effect was observed in this same study using the CPFE where infant mice showed increased levels of forgetting relative to the adults at the 7, 14 and 28 day intervals. Currently, there is a lack of data concerning the ontogeny of long-term context memory in rats.
The CPFE emerges between PND 17 and PND 24 in rats in parallel with standard contextual fear conditioning, depends on hippocampal NMDA-receptor function [36] and requires preexposure to the combined elements of the context and not just its elemental features [20]. The present study used the CPFE to examine the ontogeny of long-term retention of context learning in Long Evans (LE) rats. The interval between preexposure and training was manipulated whereas the interval between training and testing was always 24 h. Rats were preexposed on either PND 24 or PND 31 with a 24 h, 8 day, 15 day, or 22 day retention interval between preexposure and training (Experiment 1). These ages were chosen because they show comparable levels of context fear with a 24-h retention interval [36] but long-term retention of context learning is likely to increase over this period [9]. In order to confirm the ability of adult rats to retain the context memory under the specific parameters of our CPFE paradigm, and to replicate previous reports of adult context memory retention [35], rats were also preexposed on PND 52 with only a 24-h retention interval or the longest interval (22 days) at which PND 24 and PND 31 failed to show the CPFE (Experiment 2). Finally, we preexposed pre-weanling rats on PND 17 and tested them with a 24-h, 8 day and 15 day retention interval (Experiment 3) to explore age differences in retention in pre-weanlings that might be masked by their inability to express the CPFE after 24-h [14]. Taken together these studies sought to determine whether there are ontogenetic differences in context memory retention that would result in differential expression of conditioned fear at testing after extended retention intervals between preexposure and training.
2. General methods
2.1. Subjects
Subjects were Long–Evans rats born in the animal colony at the University of Delaware and moved to in-lab colony rooms on PND 2. On PND 3, litters were culled to 8 pups (typically 4 males and 4 females) and weaned on PND 21, except where noted. Dams and their litters were housed in polypropylene cages measuring 8 in. high × 18 in. long × ×9 in. wide in an animal colony maintained on a 12:12 h light/dark schedule and in accordance with the NIH guidelines. Following weaning, pups were housed with 2–4 same-sex littermates and provided ad libitum food and water throughout the entire course of the experiment. No more than 1 same-sex littermate was assigned to a given experimental condition.
2.2. Apparatus
The apparatus has been previously described [7,36]. Conditioning occurred in 1 of 4 identical Plexiglas conditioning chambers connected to a grid-floor shock generator [36] situated under a fume hood, which provided the only source of overhead lighting and low-level background noise. There was also a white opaque sheet covering any adjacent walls between two chambers so that the animals could not see each other. The alternate context consisted of wire mesh cages housed within BRS-LVE sound-attenuating shells used for eyeblink conditioning [6,36]. Preexposure sessions occurred in one of these two sets of chambers which were situated in two different rooms. Training and testing always occurred in the Plexiglas chambers (see below).
2.3. Data analysis
Conditioned fear was assessed by measuring freezing during the contextual fear tests. Freezing was defined as the cessation of all visible movement except for respiration. The data were analyzed using FreezeFrame software (Actimetrics, Wilmette, IL) as previously described [7]. Data were analyzed via ANOVA and post hoc tests (Newman–Keuls) using Statistica software [36]. As in our previous reports, data points within each group that were outliers (scores exceeding +/−2 standard deviations from other data points in their group) were removed from the statistical analyses. In total, about 9% of animals (25 of 271) were removed with the average z-score for all outliers being 3.26. Details concerning experimental factors and designs appear separately for each experiment below.
2.4. Experiment 1
The multiple-exposure CPFE procedure has been previously described by our lab [12]. The purpose of Experiment 1 was to determine how long juvenile rats could retain a context memory and whether the duration of retention differed from adolescent rats that show comparable levels of initial acquisition. Because the CPFE is fully developed by PND 24 and doesn't develop further at PND 31 [36], Experiment 1 also sought to examine possible ontogenetic differences in retention of the context memory between PND 24 and PND 31.
2.4.1. Method
Subjects were from 25 litters with 79 pups (41 males, 38 females) preexposed on PND 24 and 74 pups (35 males, 39 females) preexposed on PND 31. The design was a 2 (Sex: male vs. female) × 2 (Age: PND 24 vs. 31) × 2 (Preexposure group: Pre vs. Alt-Pre) × 4 (Retention interval: 1, 8, 15, and 22 days) between-groups factorial design, in which the retention interval refers to the period between preexposure and training (see below). The pups were assigned to these groups as follows: PND24-Pre-1 day (7 males, 6 females), PND24-Alt-Pre-1 day (6 males, 6 females), PND24-Pre-8 days (3 males, 4 females), PND24-Alt-Pre- 8 days (4 males, 2 females), PND24-Pre-15 days (7 males, 5 females), PND24-Alt-Pre-15 days (5 males, 5 females), PND24-Pre-22 days (5 males, 6 females), PND24-Alt-Pre-22 days (4 males, 4 females), PND 31-Pre-1 day (4 males, 5 females), PND31-Alt-Pre-1 day (4 males, 4 females), PND31-Pre-8 days (4 males, 4 females), PND31-Alt-Pre-8 days (4 males, 5 females), PND31-Pre-15 days (5 males, 5 females), PND31-Alt-Pre-15 days (4 males, 5 females), PND31-Pre-22 days (5 males, 5 females), and PND31-Alt-Pre-22 days (5 males, 6 females).
The CPFE procedure took place in three phases: preexposure, training and testing with the preexposure-to-training interval varying across groups but with the training and testing sessions always occurring 24 h apart. On the preexposure day (PND 24 or PND 31), pups were preexposed using a multiple preexposure procedure [12,27] to either the training context (Pre Group) or the alternate context (Alt-Pre Group). The Pre Group pups were taken from their home cages, weighed, placed into opaque transport boxes and wheeled to a waiting area outside of the conditioning room while the chambers were cleaned using a 5% ammonium hydroxide solution. The Alt-Pre Group pups experienced the same procedure but were taken to the alternate context on the preexposure day for a total time approximately equal to that of the Pre Group. Pups were taken from their opaque transport boxes and placed inside the chambers for 5 min then removed and placed back in the chamber 5 times for 1 min at approximately 1 minute intervals.
There were 4 retention intervals between preexposure and training: 1 day (replicating the conventional CPFE), 8 days, 15 days and 22 days. After the designated retention interval, all pups (Pre or Alt-Pre) were trained in the Plexiglas conditioning context in which the Pre Group had previously been preexposed. Training consisted of immediate delivery of a 2 s, 1.5 mA scrambled foot shock [36]. In order to ensure immediate delivery of the shocks, pups were placed in their conditioning chamber one at a time. The placement-to-shock interval was less than 5 s. Following the immediate shock, pups were removed as quickly as possible and returned to their transport box and, after all pups were trained (~5 min) they were returned to their home cages.
Twenty-four hours after training all animals were returned to the conditioning context for a testing session in the same chamber in which they were trained. Transport to and from the test chamber was otherwise exactly as described on the training day, except that 4 rats were placed in the chambers at the same time and their behavior was recorded simultaneously for 5 min.
2.4.2. Results
There was one instance in which two same sex littermates were inadvertently assigned to the same group and so their data were averaged to yield a single data point. In addition, data points within each group that were outliers (scores exceeding +/−2 standard deviations from other data points in their group) were removed [36]. The number of outliers removed from each group were as follows: PND 24-Pre 1 day (1), PND 24-Alt-Pre 1 day (2), PND 24-Pre 8 days (1), PND 24-Alt-Pre 8 days (2), PND 24-Pre 15 days (1), PND 24-Alt-Pre 15 days (1), PND 24-Pre 22 days (0), PND 24-Alt-Pre 22 days (1), PND 31-Pre 1 day (1), PND 31-Alt-Pre 1 day (1), PND 31-Pre 8 days (2), PND 31-Alt-Pre 8 days (1), PND 31-Pre 15 days (0), PND 31-Alt-Pre 15 days (1), PND 31-Pre 22 days (1), and PND 31-Alt-Pre 22 days (1). The number of animals per group, after outliers were removed, was as follows: for PND24 1 day (13 Pre, 12 Alt-Pre), 8 days (7 Pre, 8 Alt-Pre), 15 days (12 Pre, 10 Alt-Pre) and for PND 31 1 day (9 Pre, 8 Alt-Pre), 8 days (8 pre, 9 Alt-Pre), 15 days (10 Pre, 9 Alt-Pre) and 22 days (10 Pre, 11 Alt-Pre).
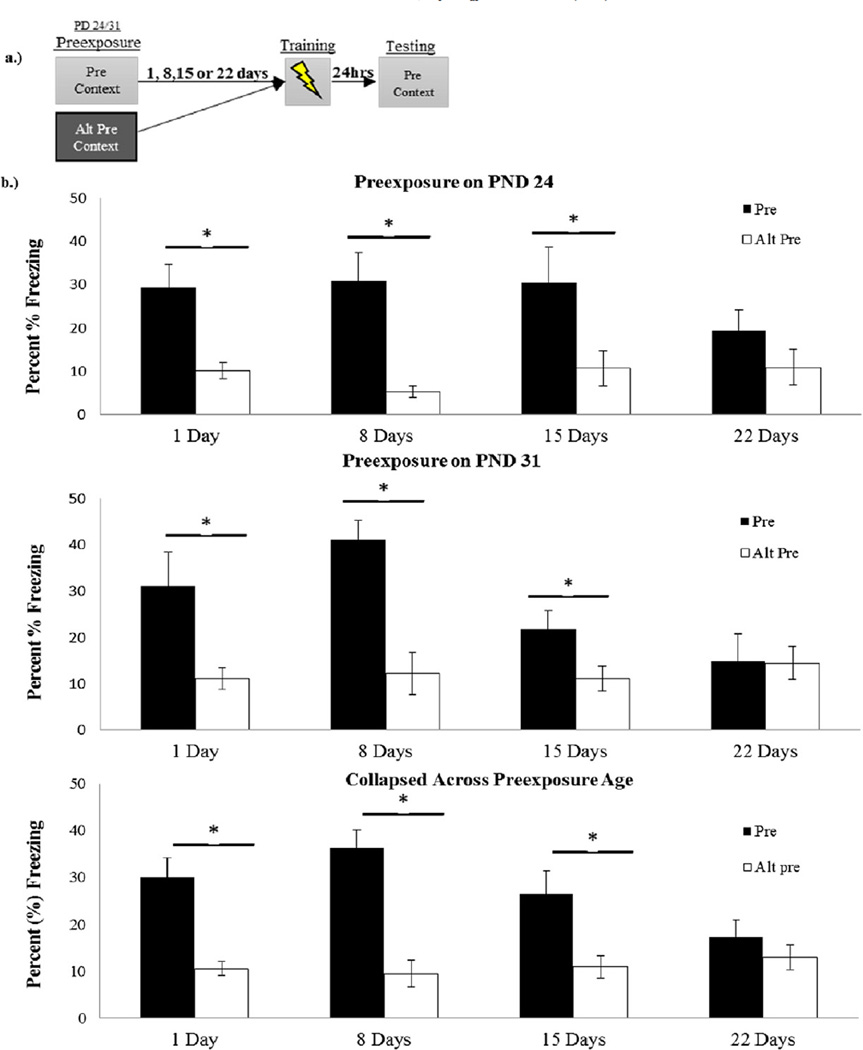
The results of Experiment 1 appear in Fig. 1. ANOVA indicated no main effect or interaction involving Sex, so the data were collapsed across this variable and analyzed via a 2 (age) × 2 (Preexposure Condition) × 4 (Retention Interval) ANOVA (Fig. 1). At both ages, the CPFE was evident at the 1-, 8-, and 15-day retention intervals but not the 22-day retention interval. This is indicated by higher freezing scores in the Pre Group relative to the Alt-Pre Group at all retention intervals except for the 22-day interval. A 2 × 2 × 4 ANOVA, indicated no main effect or interaction involving age (F < 1). There was a highly significant main effect of Preexposure Condition (F(1,139) = 40.80, p < .001) reflecting an increase in freezing in the Pre Group versus the Alt-Pre Group as well as a significant Preexposure Condition × Retention Interval interaction (F(3,139) = 3.172, p = .026). Post hoc Newman–Keuls test confirmed that Preexposure group differences were significant at the 1 day, 8 day, and 15 day retention intervals (all ps < .01). However, this difference was not evident at the 22 day retention interval (p > .39). Thus, retention of the conditioning context appears to be retained for up to two weeks but not three weeks regardless of when animals were preexposed (PND 24 vs. PND 31).
Fig. 1.
Panel a.) Schematic of CPFE timeline with preexposure occurring on PD 24 or 31. Panel b.) Context freezing 24-h after immediate-shock training in animals preexposed to the training (Pre) or alternate context (Alt-Pre) on PND 24 (top) or PND 31 (middle) either 1, 8, 15 or 22 days before training. Retention of context memory collapsed across preexposure age (bottom). Asterisks represent results of Newman–Keuls post-hoc analyses of Retention × Preexposure Condition ANOVA and p < .05.
The CPFE was replicated [20,36] in the one day retention group but this is the first study to demonstrate retention of the preexposure memory for 8 and 15 days. At 22 days, this memory was not retained sufficiently to support the CPFE.
2.5. Experiment 2
In Experiment 1, there were no differences in retention or the context memory when it was acquired on PND 24 vs. PND 31. It is possible that retention of the context memory for longer periods emerges after PND 31 [35]. To test this hypothesis, Experiment 2 assessed retention of context memory in adult rats at 24 h versus the 22-day interval that yielded forgetting in juvenile rats. The CPFE procedure used in this experiment was similar to that used in Experiment 1 and in other studies of adult rats from this laboratory [19].
2.5.1. Methods
Subjects were from 16 litters with 47 rats (23 males, 24 females) preexposed on PND 52. The rats were assigned to a 2 (Sex: male vs. female) × 2 (Preexposure group: Pre vs. Alt-Pre) × 2 (Retention interval: 1 vs. 22 days) between-group factorial design as follows: Pre-1 day (8 males, 5 females), Alt Pre-1 day (4 males, 8 females), Pre-22 days (4 males, 7 females) and Alt Pre-22 days (7 males, 4 females).
The apparatus and CPFE procedure in Experiment 2were identical to that of Experiment 1 except that rats were briefly handled for two days prior to the experiment [19]. The design was the same except that only one age group was examined and there were only two retention intervals between preexposure and training: 1 day (replicating the traditional CPFE) and the longest retention interval used—22 days. Animals in the Pre Group were exposed to the training context during the Preexposure phase of the experiment and were then subjected to either a 1 day or a 22 day retention interval before immediate shock training in that context, while animals in the Alt Pre Group were preexposed to the alternate context and then subjected to the same retention intervals before training in the same context as the Pre Group. Twenty-four hours after training all animals underwent a testing session in the same chamber in which they were trained.
2.5.2. Results
The number of outliers (scores exceeding+/−2 standard deviations from other animals in their group) removed from each group was as follows: 1 day (0 Pre Group, 1 Alt Pre Group) and 22 days (1 Pre Group, 2 Alt Pre Group). The final group numbers were as follows: 1 day (13 Pre, 12 Alt-Pre) and 22 days (11 Pre, 11 Alt-Pre).
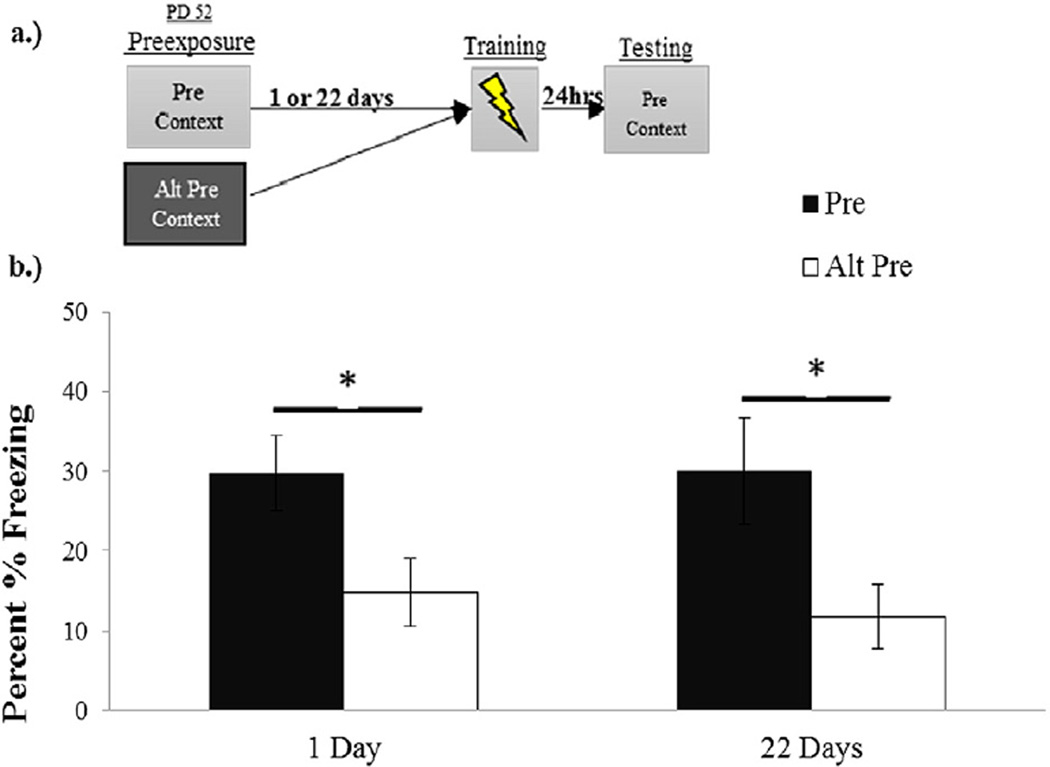
The results of Experiment 2 appear in Fig. 2. ANOVA indicated no main effect or interaction involving Sex (all ps > .1), so the data were pooled across this variable and analyzed via a 2 (Preexposure Condition) × 2 (Retention Interval) ANOVA. The CPFE was evident at both the 1- and 22-day retention interval. This is indicated by higher freezing scores in the Pre Group relative to Alt-Pre Group at all retention intervals. There was a highly significant main effect of Preexposure Condition (F(1,43)=11.21, p = .002) reflecting an increase in freezing in the Pre Group versus the Alt Pre Group but no significant main or interaction effects involving retention interval (Fs < 1).
Fig. 2.
Panel a.) Schematic of CPFE timeline with preexposure occurring on PD 52. Panel b.) Context freezing 24-h after immediate-shock training in animals preexposed to the training (Pre) or alternate context (Alt-Pre) on PND 52 either 1 or 22 days before training in the context.
The CPFE in the one day retention group replicates previous studies of adult rats [19,27] and 22-day retention confirms, with our training procedures and parameters, a previous finding by Rudy and Wright-Hardesty [35]. This finding indicates that long-term retention of context learning by weanling–juvenile rats in Experiment 1 has not reached durations that are observed in adult rats.
2.6. Experiment 3
We have previously shown that pups are incapable of performing the CPFE at PND 17 [20,36]. Other labs [14] suggest that pups preexposed on PND 17 acquire the context memory but are incapable of expressing that memory until at least 1 week later (training on PND 24 and testing on PND 25). The purpose of Experiment 3 was to see if this same trend in acquisition/expression can be replicated and, if so, how retention at longer intervals would compare with juvenile–adolescent rats. In addition, because we employed the multiple exposure CPFE protocol [12,27], this is the first study to determine if the additional exposures to the training context could cause the CPFE to be present on PND 17.
2.6.1. Methods
Subjects were from 13 litters with 71 rats (37 males, 34 females) preexposed on PND 17. The rats were assigned to a 2 (Sex: male vs. female) × 2 (Preexposure group: Pre vs. Alt-Pre) × 3 (Retention interval: 1, 8 or 15 days) between-groups factorial design as follows: Pre-1 day (5 males, 5 females), Alt Pre-1 day (7males, 3 females), Pre-8 day (6males, 7 females), Alt Pre-8 day (6 males, 6 females), Pre-15 days (6 males, 7 females) and Alt Pre-15 days (7 males, 6 females).
The apparatus, CPFE procedure, and data analysis in Experiment 3 were identical to that of Experiment 1 and our previous studies of PND 17 rats [36]. The design was the same except that only one age group was examined and there were only three retention intervals between preexposure and training: 1, 8, and 15 days. Animals in the Pre Group were preexposed to the training context during the Preexposure phase of the experiment and were then subjected to either a 1, 8, or a 15 day retention interval before immediate shock training in that context, while rats in the Alt Pre Group were preexposed to the alternate context and then subjected to the same retention intervals before training in the same context as the Pre Group. Twenty-four hours after training all animals were returned to the same chamber in which they were trained for a 5-min testing session.
2.6.2. Results
The number of outliers (scores exceeding +/−2 standard deviations from other animals in their group) removed from each group were as follows: 1 day (1 Pre Group, 1 Alt Pre Group), 8 days (1 Pre Group, 1 Alt Pre Group) and 15 days (1 Pre Group, 1 Alt Pre Group). The final group numbers after outliers were removed were as follows: 1 day (11 Pre, 11 Alt- Pre), 8 days (13 Pre, 13 Alt-Pre), and 15 days (13 Pre, 13 Alt-Pre).
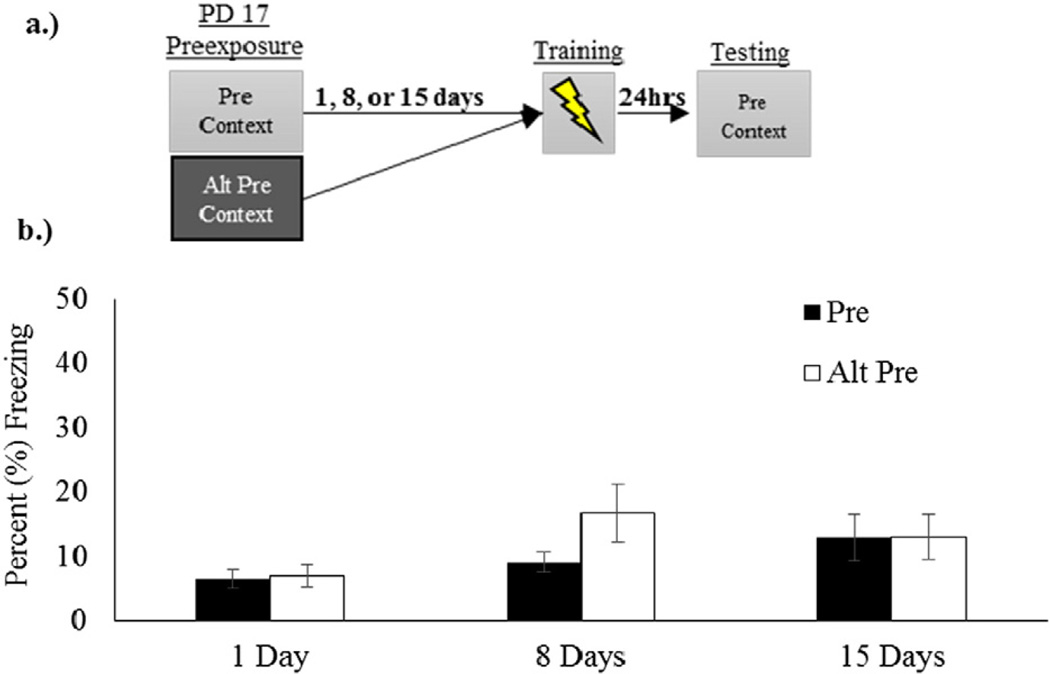
The results of Experiment 3 appear in Fig. 3. There was no main effect of sex (F(1,59) = .229, p = 0.63) but there was a significant sex by Preexposure Condition interaction (F(1,59) = 4.494, p = 0.04) and sex by retention interaction (F(2,59) = 6.281, p = 0.003) both driven by a higher level of freezing in the male group at the 15 day retention interval. Newman–Keuls post-hoc analyses revealed that at 15 days, males (M=19.27, SE=4.01) froze significantly more than both females (M= 6.75, SE=1.37) at this retention interval and males at the 1 day retention interval (M = 5.02, SE=1.06). Although males at the 15 day retention froze more overall than these other groups, independent sample t-test (t(11) = −.433, p = .675) revealed no difference between the male Pre (M = 21.20, SE = 5.94) and male Alt-Pre (M = 17.61, SE = 5.79) groups, illustrating a lack of the preexposure effect. Because these sex effects did not alter the conclusions we are reporting, our subsequent ANOVA collapsed across this variable in a 2 (Preexposure Condition) × 3 3 (Retention Interval) ANOVA. The CPFE was not evident at any retention interval. Both Pre and Alt-Pre Groups showed low and similar levels of freezing at all retention intervals. ANOVA revealed no main effect of Retention condition (F(2,65) = 2.473, p = .092) or Preexposure group (F(1,65)=1.138, p=.290) nor was there a Retention × Preexposure interaction (F(2,62) = .965, p = .386).
Fig. 3.
Panel a.) Schematic of CPFE timeline with preexposure occurring on PD 17. Panel b.) Failure to obtain the CPFE in rats preexposed on PND 17 and trained 1, 8 or 15 days after preexposure.
Contrary to a previous report [14], there was no evidence that the context memory was encoded on PND 17 and then associated with shock after 8 or 15 days. The failure to observe the CPFE at the 1-day retention interval extends findings from our previous reports [20,36] to the multiple-preexposure procedure used in this study.
3. Discussion
The ontogeny of long-term retention of context memory was explored using the context preexposure facilitation effect (CPFE). Experiment 1 found that juvenile rats preexposed on PND 24 or PND 31 remember the preexposure context for up to 15 days but not for 22 days. In Experiment 2, adult rats preexposed on PND 52 were able to retain memory for the preexposure context after 22 days as evidenced by a strong CPFE at that retention interval. Comparable levels of initial CPFE expression at the 1-day retention interval suggests that forgetting in the younger age groups does not reflect weaker acquisition of the context memory. Other interpretations are possible because this report does not include a direct measure of strength of context learning on the preexposure day. However, other studies suggest that this learning is robust in juvenile rats [33]. Thus, there appear to be ontogenetic differences in long-term memory of context learning between juvenile and adult rats. Experiment 3 determined that pre-weanlings were incapable of displaying the CPFE after any retention intervalwhenpreexposedonPND17.Thismay reflect the absence of conjunctive encoding the context memory at this age [33] or an inability to retain the memory for 24 h [20] or for 8 or 15 days (present findings), or to associate the context memory with shock at any of these retention intervals. The combined results of Experiments 1, 2 and 3 showed that the ability to acquire the CPFE (based on 24-h context memory) develops between PND 17 and PND 24 whereas the ability to retain the contextual memory beyond 15 days further develops between PND 31 and PND 52.
Although literature exploring adult rodent retention of long-term contextual memory has begun to emerge [5,16,17,35], and there is a large literature on infantile amnesia in rodent models [2,4,8,10,21], studies of the ontogeny of long-term memory of context representations is limited to mice [1,2]. In Experiment 2, adult rats showed robust freezing to the context after a 22 day retention between preexposure and training. This is consistent with reports of context retention for 28 days by Rudy and Wright-Hardesty [34], who used the same multiple preexposure CPFE procedure used here to examine the retention of preexposure memory in rats over multiple time intervals. The finding that PND 24 and PND 31 rats acquire and retain context memories, as shown by the traditional CPFE after retention intervals of 1, 8 and 15 days but not for 22 days (Experiment 1), is consistent with previous studies of infantile amnesia in the rat [9]. This previous study employed a passive avoidance paradigm but the contextual component of this task makes the findings relevant to the present study. Similarly to the juvenile groups in the current study, animals trained on PND 23 showed retention 7 days after training but they did not show retention 21 days after training. In contrast, a recent study of standard context fear conditioning (sCFC) in developing mice showed that adolescents (PND 30) retain sCFC as well as PND 60 mice, i.e., for up to 28 days following training, while PND 15 mice showed little or no retention after only 7 days [1]. Thus, PND 30 mice appear to retain sCFC for a longer period than PND 31 rats were able to retain the incidental learning of context in the present study of the CPFE. In a recent mouse CPFE study that appeared after the present study was completed, Akers et al. [2] showed that PND 17 mice retain context memories completely for 1 day, partially for 7- or 14-days, but not at all at 28 days. Adjusting for differences between rat and mouse in rate of neurobehavioral development (for comparative modeling, see [11]), this outcome agrees fairly well with the findings of the present study.
In Experiment 3, pre-weanling rats showed no evidence of context conditioning at the 1 day retention interval which is consistent with previous reports by our lab [20,36] and with a study by Rudy and Morledge [33], who demonstrated that preweanling rats could detect context cues during training but showed no retention of contextual fear 24 h later. Our experiment utilized a multiple preexposure paradigm which has been shown to increase freezing to the preexposure context in both juveniles and adults [12,27]. It therefore shows that, even with extensive preexposure training, PND 17 rats show no evidence of the CPFE. Experiment 3 also showed that retention of context memory in PND 17 rats was not expressed at later retention intervals (8 or 15 days). This fails to replicate an earlier report that rats preexposed on PND 17 can acquire context– shock associations when immediate-shock training occurs 8 days later [14]. The basis for the discrepancy between those findings and the present ones requires further study.
Context fear conditioning requires the encoding of a context representation and that representation is then associated with the aversive stimulus during training (see [32] for review). The adolescent groups (PND 24 and PND 31) in the present study are essentially showing the immediate shock deficit [13] at the 22 day retention but not at the 15 day or shorter retention intervals. This could occur because the context representation encoded on the preexposure day has either decayed in strength or can no longer be retrieved (via pattern completion) and thus can no longer support conditioning to an immediate shock. The literature on reinstatement of memory in infantile amnesia [22–24,38,39] supports a retrieval failure mechanism. Our attempts to use reminder treatments to reactivate the preexposure memory in juvenile rats have thus far been unsuccessful because the reminder treatments alone can support the CPFE (Robinson-Drummer & Stanton, unpublished observations). However, the role of decay vs. retrieval is another fruitful direction for future research with this CPFE paradigm.
The neurobiological basis of infantile amnesia is not known but this has recently become a topic of renewed interest [2,8,21]. Long-term memory of contextual fear may depend on a process whereby memory is transformed from being dependent on hippocampus to being dependent on other cortical structures like the prefrontal cortex [15]. Callaghan et al. [8] have noted that immaturity of prefrontal cortex may prevent this transfer and impair long-term memory. The role of the prefrontal cortex in fear conditioning does indeed show ontogenetic change between PND 18 and PND 25 [25] but the developmental timing of this role seems to precede the period over which long-term context memories are developing in the present study. There are also molecular mechanisms of long-term memory that change over the course of postnatal development. Callaghan et al. [8] argue that the balance between protein kinases and phosphatases can determine memory retention vs. forgetting. Calcium2+/calmodulin-dependent protein kinase II (CaMKII),which is important for long-term potentiation (LTP) and experience-dependent plasticity [26], maintains long-term memory and emerges as the dominant driver of LTP after PND 27 [41]. Here, too, the developmental profile of this mechanism seems to precede the period after adolescence over which long-term context memory emerges in the present study.
Finally, there is the recent hypothesis that increased hippocampal neurogenesis could underlie infantile amnesia [2,21]. Neurogenesis may facilitate subsequently established memories but may impair previously acquired memories. As neurogenesis in the dentate gyrus proceeds, input–output connections with new granule cells create competition with mature granule cells that, in turn, cause structural changes (e.g., dislocation, elimination or even silencing of synapses) that may preclude the retrieval of previously acquired memories [18]. Thus memories acquired earlier in ontogeny, when neurogenesis is higher, may be less persistent than the same memories acquired in adulthood, when neurogenesis has subsided to low levels typical of adult neurogenesis. Consistent with this model, Akers et al. [2] have recently shown that eliminating developmental hippocampal neurogenesis in infant mice (using GAN-treated TK+ or TMZ injections) made retention more persistent than control mice after training on both sCFC and the CPFE (context-only memory). In addition, increasing hippocampal neurogenesis in adult mice after training by exercise (running wheels), or drugs (memantine or fluoxetine), impaired long-term retention of context fear. In rats, hippocampal neurogenesis declines with age [3,37] with adult levels of neurogenesis not observed until after adolescence—the age ranges explored in the current study. It is therefore plausible that hippocampal neurogenesis could account for the poorer retention of juvenile– adolescent versus adult rats in this study. If so, disrupting neurogenesis during the juvenile–adolescent period might increase long-term memory of context exposure to adult levels (22–28 days).
4. Conclusion
The current study demonstrates that the ability to recall a long-term context memory after a 24-h retention interval develops between PND 17 and PND 24 in rats, where as retention increases to 15-days in PND 24 and PND 31 rats and is observed for 22 days in adult rats (PND 52). Infantile amnesia is observed when preexposure is on PND 24 or PND 31 with a 22 day interval between preexposure and training but not at shorter intervals (24 h, 8 or 15 days). Animals preexposed on PND 17 show no retention at any of the tested intervals while adult rats showed near perfect retention even at the longest, 22-day interval. Although there are developmental changes that occur in key cortical structures [25] as well as in the molecular mechanisms [41] that control long-term memory, many of these mechanisms reach their mature stages too early in ontogeny to account for the memory differences observed between adolescents and adults in the present study. It is plausible that developmental increases in hippocampal neurogenesis [2] underlie the effects observed here and studies exploring this possibility should be pursued in rats.
HIGHLIGHTS.
Context memory persists in the context preexposure facilitation effect (CPFE).
Context memory persists for 22 days in adult but for only 15 days in juvenile rats.
The CPFE may be useful for studying the neural basis of infantile amnesia.
Acknowledgments
Thanks are due to Dr. Sarah Jablonski for advice and technical training (of PRD) in the early stages of this project. This project was supported by a University of Delaware Graduate Scholar Award (to PRD), research funds from the University of Delaware, and NIH grants 1-R21-HD070662 and 1-R01-HD075066 to MES.
References
- 1.Akers KG, Arruda-Carvalho M, Josselyn SA, Frankland PW. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn. Mem. 2012;19(12):598–604. doi: 10.1101/lm.027581.112. http://dx.doi.org/10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- 2.Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang H, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. doi: 10.1126/science.1248903. (Retrieved from http://search.proquest.com/docview/1543435973?accountid=10457) [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das G. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MJ, Riccio DC. Ontogenetic forgetting of stimulus attributes. Learn. Behav. 2005;33(4):444–453. doi: 10.3758/bf03193183. http://dx.doi.org/10.3758/BF03193183. [DOI] [PubMed] [Google Scholar]
- 5.Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory: implication for region change in brain activation patterns associated with recent and remote memory tests. Learn. Mem. 2007;14(3):200–203. doi: 10.1101/lm.499407. http://dx.doi.org/10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KL, Stanton ME. Cross-modal transfer of the conditioned eyeblink response during interstimulus interval discrimination training in young rats. Dev. Psychobiol. 2008;50(7):647–664. doi: 10.1002/dev.20335. http://dx.doi.org/10.1002/dev.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav. Neurosci. 2009;123(5):1148–1152. doi: 10.1037/a0016733. http://dx.doi.org/10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan BL, Li S, Richardson R. The elusive engram: what can infantile amnesia tell us about memory? Trends Neurosci. 2014;37(1):47–53. doi: 10.1016/j.tins.2013.10.007. http://dx.doi.org/10.1016/j.tins.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BA, Campbell EH. Retention and extinction of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 1962;55:1–8. doi: 10.1037/h0049182. (Retrieved from http://search.proquest.com/docview/82936586?accountid=10457) [DOI] [PubMed] [Google Scholar]
- 10.Campbell BA, Spear NE. Ontogeny of memory. Psychol. Rev. 1972;79(3):215–236. doi: 10.1037/h0032690. http://dx.doi.org/10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- 11.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105(1):7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 12.Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav. Brain Res. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. (Retrieved from http://search.proquest.com/docview/1466096649?accountid=10457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanselow MS. Factors governing one-trial contextual conditioning. Anim. Learn. Behav. 1990;18(3):264–270. (Retrieved from http://search.proquest.com/docview/617878982?accountid=10457) [Google Scholar]
- 14.Foster JA, Burman MA. Evidence for hippocampus-dependent contextual learning at postnatal day 17 in the rat. Learn. Mem. 2010;17(5):259–266. doi: 10.1101/lm.1755810. http://dx.doi.org/10.1101/lm.1755810. [DOI] [PubMed] [Google Scholar]
- 15.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 16.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304(5672):881–883. doi: 10.1126/science.1094804. (Retrieved from http://search.proquest.com/docview/620615218?accountid=10457) [DOI] [PubMed] [Google Scholar]
- 17.Frankland PW, Ding H, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn. Mem. 2006;13(4):451–457. doi: 10.1101/lm.183406. http://dx.doi.org/10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankland PW, Köhler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36(9):497–503. doi: 10.1016/j.tins.2013.05.002. http://dx.doi.org/10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton GF, Murawski NJ, St. Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY. Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Res. 2011;1412:88–101. doi: 10.1016/j.brainres.2011.07.027. http://dx.doi.org/10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev. Psychobiol. 2012;54(7):714–722. doi: 10.1002/dev.20621. http://dx.doi.org/10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josselyn SA, Frankland PW. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 2012;19(9):423–433. doi: 10.1101/lm.021311.110. http://dx.doi.org/10.1101/lm.021311.110. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, McNally GP, Richardson R. Recovery of fear memories in rats: role of gamma-amino butyric acid (GABA) in infantile amnesia. Behav. Neurosci. 2006;120(1):40–48. doi: 10.1037/0735-7044.120.1.40. http://dx.doi.org/10.1037/0735-7044.120.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol. Learn. Mem. 2007;88(1):48–57. doi: 10.1016/j.nlm.2007.03.004. http://dx.doi.org/10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Richardson R. Immediate post-reminder injection of gamma-amino butyric acid (GABA) agonist midazolam attenuates reactivation of forgotten fear in the infant rat. Behav. Neurosci. 2007;121(6):1328–1332. doi: 10.1037/0735-7044.121.6.1328. http://dx.doi.org/10.1037/0735-7044.121.6.1328. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav. Neurosci. 2012;126(2):217–225. doi: 10.1037/a0027151. http://dx.doi.org/10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- 26.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 27.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J. Neurosci. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. http://dx.doi.org/10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee RD, Squire LR. On the development of declarative memory. J. Exp. Psychol. Learn. Mem. Cogn. 1993;19(2):397–404. doi: 10.1037//0278-7393.19.2.397. http://dx.doi.org/10.1037/0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- 29.Rovee-Collier C. Dissociations in infant memory: rethinking the development of implicit and explicit memory. Psychol. Rev. 1997;104(3):467–498. doi: 10.1037/0033-295x.104.3.467. http://dx.doi.org/10.1037/0033-295X.104.3.467. [DOI] [PubMed] [Google Scholar]
- 30.Rovee-Collier C, Hayne H, Colombo M. The Development of Implicit and Explicit Memory. 2000 http://dx.doi.org/10.1075/aicr.24. [Google Scholar]
- 31.Rovee-Collier C, Shyi GC. A functional and cognitive analysis of infant long-term retention. In: Howe ML, Brainerd CJ, Reyna VF, editors. Development of Long-term Retention. 1992. pp. 3–55. http://dx.doi.org/10.1007/978-1-4612-2868-4_1. [Google Scholar]
- 32.Rudy JW. Context representations, context functions, and the parahippocampal–hippocampal system. Learn. Mem. 2009;16(10):573–585. doi: 10.1101/lm.1494409. http://dx.doi.org/10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. http://dx.doi.org/10.1037/0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- 34.Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav. Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. http://dx.doi.org/10.1037/0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 35.Rudy JW, Wright-Hardesty K. The temporal dynamics of retention of a context memory: something is missing. Learn. Mem. 2005;12(2):172–177. doi: 10.1101/lm.84005. http://dx.doi.org/10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol. Learn. Mem. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. http://dx.doi.org/10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuro Report. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 38.Spear NE. The Processing of Memories: Forgetting and Retention. New York: Erlbaum; 1978. [Google Scholar]
- 39.Spear NE, Parsons P. Analysis of a reactivation treatment: ontogenetic determinants of alleviated forgetting. In: Medin D, Roberts W, Davis D, editors. Processes in Animal Memory. Hillsdale, NJ: Erlbaum; 1976. pp. 135–166. [Google Scholar]
- 40.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J. Neurosci. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat. Neurosci. 2003;6(1):15–16. doi: 10.1038/nn985. http://dx.doi.org/10.1038/nn985. [DOI] [PubMed] [Google Scholar]