Abstract
Psoriasis is a chronic inflammatory disease associated with several comorbidities. A few decades ago, it was considered an exclusive skin disease but today it is considered a multisystem disease. It is believed that 73% of psoriasis patients have at least one comorbidity. Studies have demonstrated the association of psoriasis with inflammatory bowel disease, uveitis, psychiatric disorders, metabolic syndrome and its components and cardiovascular diseases. The systemic inflammatory state seems to be the common denominator for all these comorbidities. This work aims at presenting a review of the current literature on some new comorbidities that are associated with psoriasis as osteoporosis, obstructive sleep apnea and chronic obstructive pulmonary disease. While there is still controversy, many studies already point to a possible bone involvement in patients with psoriasis, especially in the male group, generally less affected by osteoporosis. Psoriasis and chronic obstructive pulmonary disease present some risk factors in common as obesity, smoking and physical inactivity. Besides, both diseases are associated with the metabolic syndrome. These factors could be potential confounders in the association of the two diseases. Further prospective studies with control of those potential confounders should be developed in an attempt to establish causality. Existing data in the literature suggest that there is an association between obstructive sleep apnea and psoriasis, but studies performed until now have involved few patients and had a short follow-up period. It is, therefore, premature to assert that there is indeed a correlation between these two diseases.
Keywords: Bone diseases, metabolic; Osteoporosis; Psoriasis; Pulmonary disease, chronic obstructive; Sleep apnea syndromes; Sleep apnea, Obstructive
INTRODUCTION
Psoriasis is a chronic inflammatory disease that affects the skin and joints. It is immunologically mediated and potentially associated with several comorbidities, affecting about 2% of the population in Europe and the United States.1 Decades ago, it was considered an exclusive skin disease, and the treatment primary goal was freeing the patient of skin lesions.2 However, knowledge about this skin disease has evolved considerably in recent decades, and psoriasis is currently recognized as a multisystem disease.3,4
Several comorbidities are associated with psoriasis.4-7 It is believed that 73% of patients with psoriasis present at least one comorbidity.8 Inflammatory bowel disease, uveitis and psychiatric disturbances have been known for some time. Subsequently, the research focused on study of the association between metabolic syndrome and its components, such as obesity, hypertension, dyslipidemia, diabetes mellitus and cardiovascular diseases in the evolution of psoriasis.5,9 The systemic inflammatory state is the probable link between all these comorbidities.7,9 Several inflammatory cytokines have been studied as possible coadjuvants of this process, but the results are not yet fully clear. Apparently the skin lesions produce a diverse range of inflammation products, which, depending on the extent of skin involvement, may be released into systemic circulation, thus influencing the appearance or worsening of other immune-mediated diseases.10 Proper treatment of psoriasis reduces the serum levels of some cytokines such as TNF-alpha (tumor necrosis factor-alfa) and IL-1 (interleukin), which are recognized risk factors for cardiovascular diseases.11 In addition, inflammatory nature of diseases such as metabolic syndrome leads to systemic release of inflammatory mediators that, in turn, contribute to the appearance or worsening of psoriasis.
Recently, new comorbidities associated with psoriasis are being studied.1,3 This paper aims to present a review of current literature on some new comorbidities associated with psoriasis, such as osteoporosis, obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD).
METHODS
In September 2014 we performed a literature review in Pubmed/Medline database, searching for articles published in the last ten years (2004-2014) on three new comorbidities that are possibly associated with psoriasis: osteoporosis/ osteopenia, chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA).
For the search we use the terms "Osteoporosis and Psoriasis", "Osteopenia and Psoriasis", "Bone Density and Psoriasis", "Chronic Obstructive Pulmonary Disease and Psoriasis" and "Sleep apnea syndromes or Sleep Apnea and psoriasis". In all the researches, the terms were searched as subject (Mesh), as well as words found in all fields in an attempt to reach the largest possible number of references on the topics of interest.
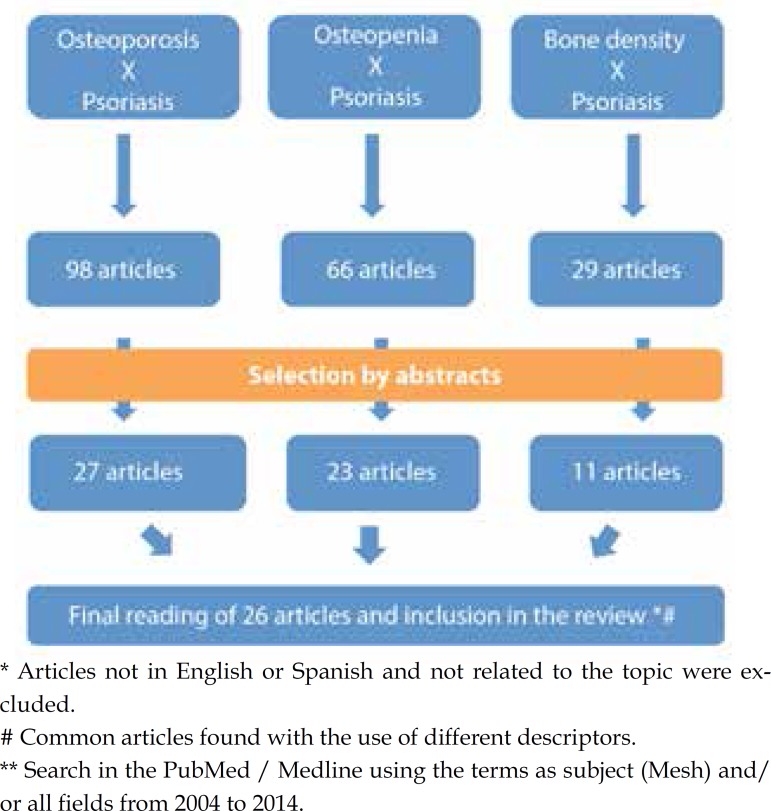
Search for "Osteoporosis and Psoriasis" resulted in 98 references; search for "Osteopenia and Psoriasis" in 66 references; and for "Bone Density and Psoriasis" generated 20 references. We proceeded to the reading of the abstracts and selected and 27, 13 and 11 articles, respectively. Articles not in English or Spanish were excluded. Some of these articles appeared in more than one research since the terms are related. After exclusion of repeated articles, we proceeded with the reading of 26 references on the subject (Figure 1).
Figure 1.
Methodology for the selection of articles related to the research osteoporosis/ osteopenia/ bone density and psoriasis
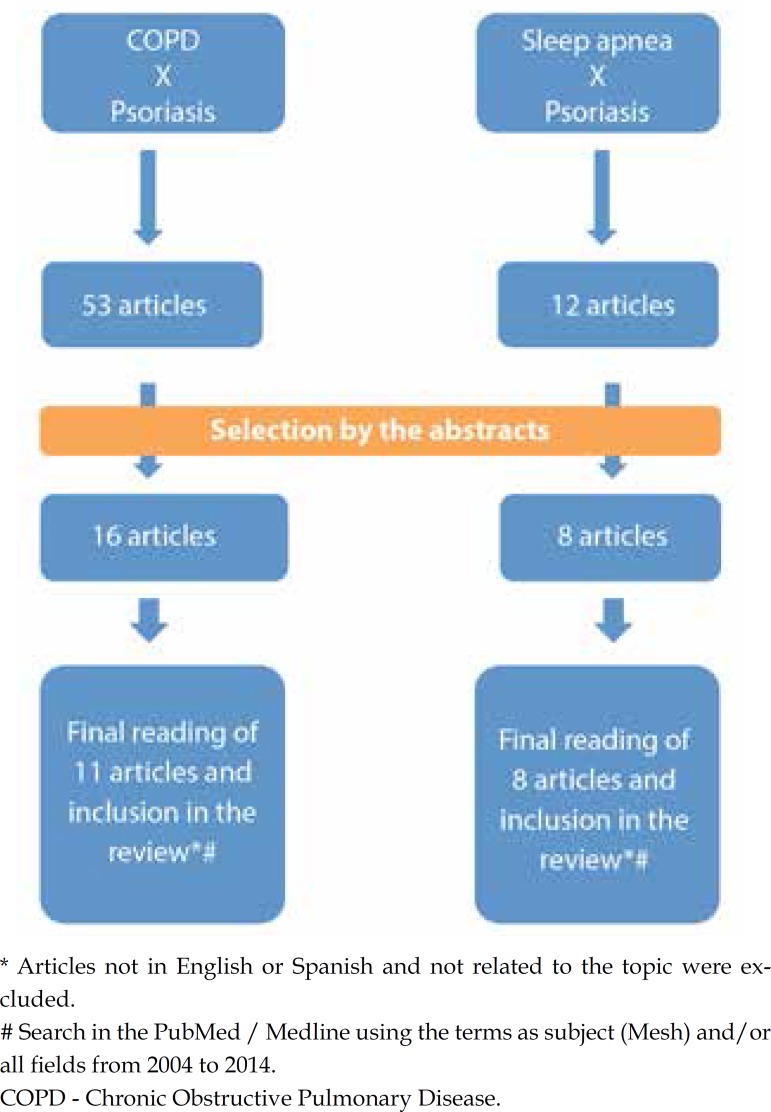
Search for "Chronic Obstructive Pulmonary Disease and Psoriasis" and "Sleep Apnea Syndromes or Sleep Apnea and Psoriasis" resulted in 53 and 12 references, respectively. From there we proceeded with the reading of the abstracts and selected 16 and 8 articles, respectively. Articles not in English or Spanish were excluded. In total we proceeded with the reading of 11 articles related to COPD and 8 articles about sleep apnea (Figure 2).
Figure 2.
Methodology for the selection of articles related to COPD and psoriasis and sleep apnea and psoriasis
In addition to these, we selected some important items for introducing the topic under study.
NEW COMORBIDITIES
Osteoporosis/ Osteopenia
Osteoporosis is defined by reduced bone mass and changes in bone microarchitecture, resulting in decreased bone strength with increased risk of fracture. The World Health Organization (WHO) defines osteoporosis as bone mineral density, in postmenopausal women, ≤2.5 standard deviations of the mean of healthy young individuals of the same sex (T-score < -2.5) and low bone mass when the T-score is between -2.5 and -1 standard deviations.12,13 It occurs more frequently in women than in men, particularly after menopause, and is more common with increasing age.14 When evaluating women in premenopausal period or men between 20 and 50-year old, the Z-score should be analyzed. Z-score compares the patient's bone mass with the mean of individuals at the same age. Result is not of osteopenia or osteoporosis, but within or below the mean for the age. Z-score ≤ -2.0 is defined as "below the expected range for age" and Z-score > -2.0 should be classified as "within the expected range for age". At this age, the term osteoporosis can be used for patients with low bone mass associated to risk factors for fracture or osteoporosis secondary causes, e.g., hyperparathyroidism or taking glucocorticoids.15
Bone mass peak reached in the third decade of life is an important determinant of bone mass in subsequent years and is influenced by several factors. Nutritional factors, including intake of calcium and vitamin D, hormonal profile and physical activity influences the bone mass peak. Other interfering factors include smoking, alcohol abuse, chronic inflammatory diseases, malabsorption states, endocrine disorders, hypogonadism, severe liver disease, rheumatoid arthritis, ankylosing spondylitis, hematologic malignancies, sarcoidosis, amyloidosis, chronic obstructive pulmonary disease, multiple sclerosis and other diseases.14,16
Current advances in the pathogenesis of osteoporosis have observed the involvement of cytokines such as IFN-γ (interferon-gamma), IL-6 and TNF-α. These cytokines also participate in the pathogenesis of psoriasis and psoriatic arthritis, which suggests that patients could be more susceptible to the development of osteoporosis than normal subjects despite studies associating psoriasis with reduced bone mineral density are still limited and controversial.16-20
Osteoclasts are formed from osteoclast precursor cells derived from hematopoietic cells. They express C-FMS (receptor for M-CSF) and RANK and bind to stromal cells/ osteoblasts that express M-CSF, bound to membrane and soluble, RANKL bound to membrane and osteoprotegerin (OPG) under the influence of stimuli resulting in bone resorption (parathyroid hormone, 1.25-vitamin D, TNF, IL-6, IL-11 or prostaglandins). If the osteoblast cells produce more RANKL than osteoprotegerin, osteoclast formation and activation occurs, increasing bone resorption. During inflammatory conditions, T cells are activated and produce soluble RANKL bound to membrane, which can stimulate bone resorption mediated by osteoclasts. IL-1 and TNF cytokines may enhance the effects of RANKL and M-CSF, resulting in bone resorption by the direct stimulation of osteoclast precursors and mature osteoclasts. Osteoprotegerin is an inhibitor of osteoclastogenesis. It is a soluble receptor for RANKL that binds to it and prevents the interaction with its receptor RANK and subsequent stimulus for bone resorption.21-23 It has been proposed that the relative expression of RANKL and its natural antagonist OPG control osteoclastogenesis.24,25
The fundamental role of TNF-α and IL-6 in bone remodeling has been demonstrated in other situations such as in children with idiopathic osteoporosis that exhibit high levels of IL-6 besides, the treatment with anti-TNF-α drugs present a beneficial effect on bone metabolism of patients with rheumatoid arthritis.17 New proinflammatory cytokines, such as IL-20, are being studied. This is a cytokine from the IL-10 family that participates in the pathogenesis of psoriasis and is capable of increasing the expression of RANK and RANKL, inducing the increase of osteoclastogenesis.26
In addition to osteoprotegerin and RANKL, there are other serum markers that indicate bone formation and resorption, the latter can be estimated by determining the carboxyl-terminal telopeptide of type I collagen (CTx), which is generated after the collagen breakdown, main bone protein, and can predict changes in bone mineral density and fracture risk.27
Several mechanisms are involved in the association of psoriasis with osteoporosis, such as elevation of inflammatory cytokines, drug use in the treatment of psoriasis and joint immobilization due to dysfunction and joint pain secondary to psoriatic arthritis.6
In the psoriatic arthritis (PsA), bone involvement is complex because it involves both bone loss and formation mechanisms.28-30 It is believed that in PsA occurs not only local but also systemic bone loss. Some authors have demonstrated this association of PsA with osteopenia/ osteoporosis. Teichmann (2009) showed that 57.3% of studied patients with PsA presented reduced bone mineral density (BMD) of the lumbar spine.31 A study with 2212 patients with PsA found that patients with bone erosion have lumbar spine BMD lower than patients without bone erosion, even after adjusting for confounding factors.32 A cross-sectional study of 116 patients with PsA found osteopenia in 1/3 of women and men while osteoporosis was much more prevalent in men (10.2%) than in women (1.75%). It is noteworthy that the mean age of men in this study was 49 years old, age in which is not expected to find such a frequency of osteoporosis in men.33 Xue (2012) found significantly higher RANKL concentration in psoriasis patients than in control patients, as well as a lower concentration of OPG/RANKL in this group, showing a higher osteoclastogenesis in patients with PsA.29 However, some studies found no difference in BMD of the spine and femur in patients with PsA and control patients, while Osmancevic (2008) found higher BMD at the spine and hip of women with psoriasis after menopause.34-36 This author attributed the higher BMD to the higher weight and body mass index (BMI) of this group, to physical activity and to UVB exposure. Borman (2008) also found no significant difference in BMD of psoriasis patients with and without arthritis, although patients with psoriatic arthritis with longer duration of joint disease have high risk of osteoporosis.18
Association of psoriasis without joint involvement with osteoporosis is even more controversial. A recent study that included both patients with psoriasis and PsA, found no higher prevalence of osteoporosis than in the general population as well as no association between bone resorption and severity of skin involvement evaluated by PASI (Psoriasis Area Severity Index). The study observed that the probability of a patient with psoriasis develop osteopenia/ osteoporosis correlates directly with the number of years with the disease.6 Pereira (2011) showed that postmenopausal women with psoriasis and PsA didn't present lower bone mass, however, this group seems to have a greater chance of osteoporotic fractures.37 Moreover, Keller (2013), included in its population-based study 17,507 osteoporosis cases and 52,521 control cases without osteoporosis, and found that subjects with osteoporosis had a higher prevalence of psoriasis previously diagnosed (OR 1.65; CI 1.42 -1.94).19 Another major case-control study with 7936 psoriasis cases and 14835 control cases, showed an association of osteoporosis in men with psoriasis, which cannot be demonstrated in women after multivariate analysis and confounding factors control.15 Study conducted by the authors of this article also showed that men have lower bone mass and bone mineral density than control patients (unpublished data). Attia (2011) showed a higher prevalence of osteoporosis only in the group with PsA despite high levels of OPG have been noted both in patients with psoriasis and control patients.21
While there is still controversy in this association, many studies have indicated a possible bone involvement in patients with psoriasis, especially among men, usually less affected by osteoporosis/ osteopenia. Thus, this factor should be measured carefully during psoriasis patient care and laboratory evaluation of bone metabolism (vitamin D dosage, calcium, phosphorus, parathyroid hormone) and bone densitometry should be considered individually, especially in older patients with more time with the disease and other risk factors for bone loss.
Chronic obstructive pulmonary disease
COPD affects approximately 10% of the population and encompasses chronic obstructive bronchitis and emphysema. It is characterized by a persistent and progressive limitation of airflow.38 The main factor associated with COPD is smoking, followed by lung inflammation, which is responsible for small airways thickening and alveolar destruction. There is evidence that COPD seems to be a more complex disorder than only airways obstruction.39 The inflammation appears to be the link between COPD and various other diseases such as metabolic syndrome and psoriasis.40
An abnormal chronic inflammatory response associated with alteration of the immune system can aggravate or initiate a series of inflammatory diseases. In psoriasis it is observed a Th-1 and Th-17 cells disruption with elevation of IL-1, IL-6, IL-8 and TNF-α, as well as elevation of systemic inflammation markers such as C-reactive protein (CRP). In COPD both these cytokines and CRP are high and associated with disease severity. Furthermore, high levels of neutrophils, TNF -α, IL-6 and IL-8 were found in sputum and bronchoalveolar lavage fluid of patients with COPD.40 IL-17, already known to be associated with psoriasis by Th-17 response, was recently associated with pulmonary diseases, including COPD. In these patients, there was an increase in expression of this cytokine on bronchial submucosa and neutrophils IL-17+ are present in the sputum of COPD patients.41 These two diseases also share inflammatory markers such as the chemokine receptor CXCR2, which is essential in the recruitment of neutrophils and angiogenesis at sites of acute and chronic inflammation as well as increased expression of IL-8 and its receptors.42,43
Another common pathophysiological feature is the fraction of exhaled nitric oxide (FeNO). It is known that FeNO levels in COPD are associated with smoking and disease severity being increased in exacerbations of the disease. Psoriasis seems to be related to higher levels of FeNo and potentially greater risk of COPD. Identification of patients with high FeNo may sign lung involvement.38
Population-based study of 2096 psoriasis cases and 8384 control cases found that the risk of developing COPD by patients with psoriasis was 2.35 times higher than in control patients after 18 months of follow-up. The study also pointed out that patients >50 years and men were more susceptible to COPD. According to the authors, confounding factors were controlled in this study.40 Another major case-control study including 12502 psoriasis cases and 24287 control cases found that the prevalence of COPD was significantly higher in patients with psoriasis than in control patients, 5.7% versus 3.6%, respectively (p<0.001). This difference remained even after controlling confounding factors.39 Data from a cohort study conducted in the USA (National Health and Wellness Survey) found a higher prevalence of a number of comorbidities in patients suffering from psoriasis, among them COPD (OR 1.68; CI 1.03-2.78).5 Al-Mutai (2010) found higher prevalence of COPD in patients with psoriasis, however the difference was not statistically significant compared to control patients.9 In a study including only patients with psoriatic arthritis, COPD is the fifth most frequently found comorbidity, behind only to hypertension, obesity, diabetes, and kidney disease.1
Although both diseases present multifactorial etiology, the risk of patients with psoriasis develop COPD should be highlighted. Preventive and therapeutic actions, in control of risk factors such as smoking cessation or in treatment of the inflammatory process, must be observed in an attempt to prevent the emergence of COPD in patients with psoriasis.40
It is noteworthy that psoriasis and COPD present some risk factors in common such as obesity, smoking and physical inactivity, and both diseases are associated with metabolic syndrome. These factors could be confounding factors in the association between these two diseases.40 The vast majority of studies are not prospective, which does not allow the establishment of causal relation. New prospective studies with control of these possible confounding factors should be developed in an attempt to establish the causal link.
Obstructive Sleep Apnea
OSA is a common form of sleep disorder that affects 2-4% of the general population and is characterized by repetitive episodes of partial or complete obstruction of the upper airway during sleep, resulting in recurrent awakening and intermittent hypoxia, besides daytime symptoms due to excessive sleepiness.44,45 Scientific evidence has shown that patients with OSA are at increased risk of various comorbidities such as cardiovascular risk, stroke, insulin resistance, type 2 diabetes, obesity and metabolic syndrome.46-49
OSA patients have inflammation of the upper airways, as well as increased oxidative stress and systemic inflammation indicated by elevated levels of TNF- α, IL-6 and C-reactive protein.50
It is noteworthy that obesity has a high prevalence both in OSA and in psoriasis, thus it is natural to anticipate that some association may exist between the last two.51
Buslau et al published a study in 1999 aiming to determine the prevalence of OSA in patients with psoriasis. The authors evaluated 25 adults with psoriasis and 19 control patients with bronchitis matched for age and sex. The study observed that not only the prevalence of OSA is elevated in patients with psoriasis as these patients have higher apnea index.52 In 2011, Ya-Wen Wang et al evaluated 2258 patients diagnosed with OSA for 3 years, and concluded that patients with this comorbidity has a risk two times higher of developing psoriasis and psoriatic arthritis than the general population.53
In another study published in 2013, the authors evaluated 33 patients with psoriasis and found that the frequency of OSA was much higher among the studied patients than in the general population. They also concluded that OSA may represent a risk factor for the onset of psoriasis. However, this study did not present control group, and also did not make statistical adjustments for the coexistence of obesity and metabolic syndrome in patients.54
Literature data suggest that there is an association between OSA and psoriasis, but studies conducted so far have involved few patients and performed a short follow-up. It is premature, therefore, to say that in fact there is a correlation between these two diseases. Development of prospective studies involving a large number of participants would be necessary, as well as statistical adjustments for potential confounders such as lifestyle habits, comorbidities and treatments performed.55-57
CONCLUSION
Comorbidities are very common in patients with psoriasis and their presence has important practical implications for the clinical management of patients. These should always be oriented to adopt a healthier lifestyle, including diet and exercise, and avoiding smoking and alcohol consumption. The dermatologist's role is crucial in this process because he is often the first doctor to be sought by these patients. Identification of comorbidities and collaboration of physicians from other specialties are essential for the proper treatment of patients. We highlight the difficulty of proving the association of comorbidities with psoriasis. Many studies use databases not designed for this purpose and due to the low prevalence of some diseases there is a need for large populations to prove an association.
Footnotes
Conflicts of Interest: None
Financial Support: None
Study performed at Santa Casa de Belo Horizonte - Belo Horizonte (MG), Brazil.
REFERENCES
- 1.Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J, Sampalis JS. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol. 2011;30:877–885. doi: 10.1007/s10067-011-1692-7. [DOI] [PubMed] [Google Scholar]
- 2.Furst DE, Mandell B, Calabrese LH, Cather JC, Clauw DJ, Deodhar A, A, et al. Proceedings of the 5th annual perspectives in rheumatic diseases. Semin Arthritis Rheum. 2013;43:416–419. doi: 10.1016/j.semarthrit.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Lotti T, Hercogova J, Prignano F. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29:10–15. doi: 10.1016/j.sder.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lotti T, Hercogova J, Prignano F. The concept of psoriatic disease: can cutaneous psoriasis any longer be separated by the systemic comorbidities? Dermatol Ther. 2010;23:119–122. doi: 10.1111/j.1529-8019.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Mills D, Bala M. Psoriasis: cardiovascular risk factors and other disease comorbidities. J Drugs Dermatol. 2008;7:373–377. [PubMed] [Google Scholar]
- 6.D'Epiro S, Marocco C, Salvi M, Mattozzi C, Luci C, Macaluso L, et al. Psoriasis and bone mineral density: implications for long-term patients. J Dermatol. 2014;41:783–787. doi: 10.1111/1346-8138.12546. [DOI] [PubMed] [Google Scholar]
- 7.Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. J Invest Dermatol. 2009;129:1601–1603. doi: 10.1038/jid.2009.55. [DOI] [PubMed] [Google Scholar]
- 8.Sanz LP. Psoriasis, a systemic disease? Actas. Dermosifiliogr. 2007;98:396–402. [PubMed] [Google Scholar]
- 9.Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol. 2010;37:146–155. doi: 10.1111/j.1346-8138.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Fan X, Zhang K, Yin G, Liu Y. Influence of psoriatic peripheral blood CD4+ T and CD8+ T lymphocytes on C-myc, Bcl-xL and Ki67 gene expression in keratinocytes. Eur. J Dermatol. 2007;17:392–396. doi: 10.1684/ejd.2007.0236. [DOI] [PubMed] [Google Scholar]
- 11.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie WD, Schousboe JT. A review of osteoporosis diagnosis and treatment options in new and a recently updated guidelines on case finding around the world. Curr Osteoporos Rep. 2011;9:129–140. doi: 10.1007/s11914-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Melton 3rd LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 14.Dreiher J, Weitzman D, Cohen AD. Psoriasis and osteoporosis: a sex-specific association? J Invest Dermatol. 2009;129:1643–1649. doi: 10.1038/jid.2008.432. [DOI] [PubMed] [Google Scholar]
- 15.Uptodate.com Becker CB, Cohen A. Evaluation and treatment of premenopausal osteoporosis. Uptodate Waltham; Massachusetts: 2015. [2015 April 15]. Internet. Available from: http://www.uptodate.com. [Google Scholar]
- 16.Millard TP, Antoniades L, Evans AV, Smith HR, Spector TD, Barker JN. Bone Mineral density of patients with chronic plaque psoriasis. Clin Exp Dermatol. 2001;26:446–448. doi: 10.1046/j.1365-2230.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- 17.Kastelan D, Kastelan M, Massari LP, Korsic M. Possible association of psoriasis and reduced bone mineral density due to increased TNF-alpha and IL-6 concentrations. Med Hypotheses. 2006;67:1403–1405. doi: 10.1016/j.mehy.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 18.Borman F, Babaoğlu S, Gur G, Bingol S, Bodur H. Bone mineral density and bone turnover in patients with psoriatic arthritis. Clin Rheumatol. 2008;27:443–447. doi: 10.1007/s10067-007-0725-8. [DOI] [PubMed] [Google Scholar]
- 19.Keller JJ, Kang JH, Lin HC. Association between osteoporosis and psoriasis: results from the Longitudinal Health Insurance Database in Taiwan. Osteoporos Int. 2013;24:1835–1841. doi: 10.1007/s00198-012-2185-5. [DOI] [PubMed] [Google Scholar]
- 20.Balato N, Balato A, Gallo L, Napolitano M, Patruno C, Ayala F. Psoriasis and osteoporosis: data from a Southern Italian population. Arch Osteoporos. 2012;7:321–323. doi: 10.1007/s11657-012-0112-1. [DOI] [PubMed] [Google Scholar]
- 21.Attia EA, Khafagy A, Abdel-Raheem S, Fathi S, Saad AA. Assessment of osteoporosis in psoriasis with and without arthritis: correlation with disease severity. Int J Dermatol. 2011;50:30–35. doi: 10.1111/j.1365-4632.2010.04600.x. [DOI] [PubMed] [Google Scholar]
- 22.Walsh NC, Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol. 2004;16:419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 23.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier; 2011. [Google Scholar]
- 24.Stolina M, Adamu S, Ominsky M, Dwyer D, Asuncion F, Geng Z, et al. RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. Res. 2005;20:1756–1765. doi: 10.1359/JBMR.050601. [DOI] [PubMed] [Google Scholar]
- 25.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu YH, Chen WY, Chan CH, Wu CH, Sun ZJ, Chang MS. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J Exp Med. 2011;208:1849–1861. doi: 10.1084/jem.20102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indridason OS, Franzson L, Sigurdsson G. Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos Int. 2005;16:417–423. doi: 10.1007/s00198-004-1699-x. [DOI] [PubMed] [Google Scholar]
- 28.Del Puente A, Esposito A, Parisi A, Atteno M, Montalbano S, Vitiello M, et al. Osteoporosis and psoriatic arthritis. J Rheumatol Suppl. 2012;89:36–38. doi: 10.3899/jrheum.120240. [DOI] [PubMed] [Google Scholar]
- 29.Xue Y, Jiang L, Cheng Q, Chen H, Yu Y, Lin Y, et al. Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLoS One. 2012;7: doi: 10.1371/journal.pone.0046740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husni ME, Mease PJ. Managing comorbid disease in patients with psoriatic arthritis. Curr Rheumatol Rep. 2010;12:281–287. doi: 10.1007/s11926-010-0112-3. [DOI] [PubMed] [Google Scholar]
- 31.Teichmann J, Voglau MJ, Lange U. Antibodies to human tissue ransglutaminase and alterations of vitamin D metabolism in ankylosing spondylitis and psoriatic arthritis. Rheumatol Int. 2010;30:1559–1563. doi: 10.1007/s00296-009-1186-y. [DOI] [PubMed] [Google Scholar]
- 32.Anandarajah AP, El-Taha M, Peng C, Reed G, Greenberg JD, Ritchlin CT. Association between focal erosions and generalised bone loss in psoriatic arthritis. Ann Rheum Dis. 2011;70:1345–1347. doi: 10.1136/ard.2010.148452. [DOI] [PubMed] [Google Scholar]
- 33.Hofbauer LC, Schoppet M, Christ M, Teichmann J, Lange U. Tumour necrosis factor-related apoptosis-inducing ligand and osteoprotegerin serum levels in psoriatic arthritis. Rheumatology (Oxford) 2006;45:1218–1222. doi: 10.1093/rheumatology/kel108. [DOI] [PubMed] [Google Scholar]
- 34.Riesco M, Manzano F, Font P, García A, Nolla JM. Osteoporosis in psoriatic arthritis: an assessment of densitometry and fragility fractures. Clin Rheumatol. 2013;32:1799–1804. doi: 10.1007/s10067-013-2322-3. [DOI] [PubMed] [Google Scholar]
- 35.Grazio S, Cvijetić S, Vlak T, Grubišić F, Matijević V, Nemčić T, et al. Osteoporosis in psoriatic arthritis: is there any? Wien Klin Wochenschr. 2011;123:743–750. doi: 10.1007/s00508-011-0095-8. [DOI] [PubMed] [Google Scholar]
- 36.Osmancevic A, Landin-Wilhelmsen K, Larkö O, Mellström D, Wennberg AM, Hulthén L, et al. Risk factors for osteoporosis and bone status in postmenopausal women with psoriasis treated with UVB therapy. Acta Derm Venereol. 2008;88:240–246. doi: 10.2340/00015555-0403. [DOI] [PubMed] [Google Scholar]
- 37.Pedreira PG, Pinheiro MM, Szejnfeld VL. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res Ther. 2011;13:R16–R16. doi: 10.1186/ar3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malerba M, Radaeli A, Olivini A, Damiani G, Ragnoli B, Montuschi P, et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014;2014:271918–271918. doi: 10.1155/2014/271918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreiher J, Weitzman D, Shapiro J, Davidovici B, Cohen AD. Psoriasis and chronic obstructive pulmonary disease: a case-control study. Br J Dermatol. 2008;159:956–960. doi: 10.1111/j.1365-2133.2008.08749.x. [DOI] [PubMed] [Google Scholar]
- 40.Chiang YY, Lin HW. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59–65. doi: 10.1111/j.1468-3083.2011.04009.x. [DOI] [PubMed] [Google Scholar]
- 41.Tan HL, Rosenthal M. Rosenthal M. IL-17 in lung disease: friend or foe? Thorax. 2013;68:788–790. doi: 10.1136/thoraxjnl-2013-203307. [DOI] [PubMed] [Google Scholar]
- 42.Ha H, Neamati N. Pyrimidine-based compounds modulate CXCR2-mediated signaling and receptor turnover. Mol Pharm. 2014;11:2431–2441. doi: 10.1021/mp500180e. [DOI] [PubMed] [Google Scholar]
- 43.Qazi BS, Tang K, Qazi A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int J Inflam. 2011;2011:908468–908468. doi: 10.4061/2011/908468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–484. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 45.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 46.Bagai K. Obstructive sleep apnea, stroke, and cardiovascular diseases. Neurologist. 2010;16:329–339. doi: 10.1097/NRL.0b013e3181f097cb. [DOI] [PubMed] [Google Scholar]
- 47.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 48.Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 49.Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29:10–15. doi: 10.1016/j.sder.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Maari C, Bolduc C, Nigen S, Marchessault P, Bissonnette R. Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatolog Treat. 2014;25:57–60. doi: 10.3109/09546634.2012.713458. [DOI] [PubMed] [Google Scholar]
- 51.Thorburn PT, Riha RL. Skin disorders and sleep in adults: where is the evidence? Sleep Med Rev. 2010;14:351–358. doi: 10.1016/j.smrv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Buslau M, Benotmane K. Cardiovascular complications of psoriasis: does obstructive sleep apnoea play a role? Acta Derm Venereol. 1999;79:234–234. doi: 10.1080/000155599750011075. [DOI] [PubMed] [Google Scholar]
- 53.Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285–289. doi: 10.1016/j.sleep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 54.Karaca S, Fidan F, Erkan F, Nural S, Pinarci T, Gunay E, et al. Might psoriasis be a risk factor for obstructive sleep apnea syndrome. Sleep Breath. 2013;17:275–280. doi: 10.1007/s11325-012-0686-2. [DOI] [PubMed] [Google Scholar]
- 55.Dalamaga M, Papadavid E, Vlami K. Unmasking the Janus face of the association between psoriasis, metabolic syndrome and obstructive sleep apnea. Sleep Breath. 2013;17:449–450. doi: 10.1007/s11325-012-0749-4. [DOI] [PubMed] [Google Scholar]
- 56.Papadavid E, Vlami K, Dalamaga M, Giatrakou S, Theodoropoulos K, Gyftopoulos S, et al. Sleep apnea as a comorbidity in obese psoriasis patients: a crosssectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol. 2013;27:820–826. doi: 10.1111/j.1468-3083.2012.04580.x. [DOI] [PubMed] [Google Scholar]
- 57.Gowda S, Goldblum OM, McCall WV, Feldman SR. Factors affecting sleep quality in patients with psoriasis. J Am Acad Dermatol. 2010;63:114–123. doi: 10.1016/j.jaad.2009.07.003. [DOI] [PubMed] [Google Scholar]