Abstract
An in vivo experiment was conducted to study the effects of probiotic Bacillus coagulans spores, with and without prebiotic, inulin, on gastrointestinal (GI) microbiota of healthy rats and its potentiality to survive in the GI tract. Forty-eight male Wistar rats were randomly divided into four groups (n=12) and fed as follows: standard diet (control), standard diet supplied with 5% w/w long chain inulin (prebiotic), standard diet with 109/day spores of B. coagulans by orogastric gavage (probiotic), and standard diet with 5% w/w long chain inulin and 109 spores/day of B. coagulans by orogastric gavage (synbiotic). Rats were fed the diets for 30 days. At day 10, 20 and 30 of experiment, 24 h post administration, four rats from each group were randomly selected and after faecal collection were sacrificed. Small intestine, cecum, and colon were excised from each rat and used for microbial analysis. Administration of synbiotic and probiotic diets led to a significant (P<0.05) increment in lactic acid bacteria (LAB), total aerobic and total anaerobic population compared the prebiotic and control diets. A significant decrease in Enterobacteriaceae counts of various segments of GI tract (except small intestine) in synbiotic, probiotic and prebiotic fed groups were also seen. The obvious decline in spores count through passing GI tract and high surviving spore counts in faecal samples showed that spores are not a normal resident of GI microbiota and affect intestinal microbiota by temporary proliferation. In conclusion, the present study clearly showed probiotic B. coagulans was efficient in beneficially modulating GI microbiota and considering transitional characteristics of B. coagulans, daily consumption of probiotic products is necessary for any long-term effect.
Key Words: Bacillus coagulans, Intestinal microbiota, Prebiotic, Probiotic, Synbiotic
Introduction
In recent times, the use of probiotics in various food products has been increased in addition to suggesting their use as a food supplement for therapeutical purposes. Probiotic therapy is very attractive because it is an effective and noninvasive low cost approach which attempts to recreate natural flora rather than its disruption (Hammerman et al., 2006 ▶; Sorokulova et al., 2008 ▶).
The bacterial flora of the GI tract play major roles in human physiology by modulating metabolic and immunological processes to prevent overgrowth of opportunistic microorganisms (Plummer et al., 2005 ▶). Many disorders of the gut are believed to be correlated with disturbance in this distribution of normal bacterial species choosing the growth of pathogenic strains (Kalman et al., 2009 ▶).
The balancing action of probiotics upon the intestinal microbiota involves an increase in bacterial components, especially Lactobacilli and Bifidobacteria that may be beneficial to the host and a reduction in the potentially harmful microorganisms such as coliforms and clostridia, thus reducing risk of diarrhea, inflammatory and allergic diseases (Marzotto et al., 2006 ▶).
Micro-organisms used as a probiotic for human are mainly Gram-positive bacteria belonging to the Lactobacillus and Bacillus spp. Bacillus probiotics differ in many characteristics from those based on Lactobacillus spp. While Lactobacillus represents a normal resident GI microflora of humans, Bacillus belongs only to the transitory GI bacteria (Sorokulova et al., 2008 ▶).
The members of genus Bacillus are endospore forming bacteria that make it extremely heat-stable and resistant to adverse gastrointestinal tract conditions and, when germinate in GI tract, cause positive effects for the host (Hoa et al., 2001 ▶; Losada and Olleros, 2001 ▶; Casula and Cutting, 2002 ▶). Recently probiotic strain of Bacillus coagulans that can withstand the low pH of stomach acid and is activated in the intestines to modulate the gut microbiota has been granted self-affirmed GRAS status by the FDA (Cutting, 2011 ▶).
Prebiotics are food ingredients that cannot be digested by the human digestive system but are metabolized by discrete enteric microbes, thus stimulating proliferation of selected GI bacteria species thought to be beneficial for human health. Fructooligosaccharides (FOS), which are extracted from chicory root in the form of inulin is a recommended prebiotic compound (Mikkelsen et al., 2003 ▶; Ogawa et al., 2005 ▶).
A synbiotic, on the contrary, represents a defined supplement comprised of a mixture of probiotics and prebiotics purposed to enhance the survival and colonization of the supplemented species in the GI tract (Rauch and Lynch, 2012 ▶).
The role of B. coagulans in changing GI flora of broilers has been studied by Lin et al. (2011) ▶. They reported a significant increase in cecal Lactobacillus population and decrease in E. coli counts in treatments fed diets supplied with B. coagulans (Lin et al., 2011 ▶). Also, studies suggest that B. coagulans decreases the symptoms of abdominal pain and bloating in subjects with inflammatory bowel disease. Another study showed that B. coagulans-based product was effective in improving the quality of life and reducing gastrointestinal symptoms in adults with post prandial intestinal gas related symptom in sixty-one adult healthy subjects (Kalman et al., 2009 ▶). Based on the results obtained in the above mentioned studies, the objective of this study was to determine the effect of oral administration of B. coagulans spores and inulin as single supplements as well as a dietary combination on the microbial population of different parts of GI of rats.
Materials and Methods
Preparation of spore suspension of probiotic bacteria
Lyophilized probiotic B. coagulans was donated by the Pardis Roshd Mehregan Co., Iran. It was grown aerobically in nutrient yeast extract salt medium (NYSM) agar (Russell et al., 1989 ▶) at 37°C for 24 h. A single colony from the NYSM plate was inoculated into 500 ml of NYSM broth and incubated at 37°C with shaking at 250 rpm for 48 h. The bacterial suspension was pelleted three times by centrifugation at 3000 × g for 20 min, and washed with sterile normal saline. Final pellet was resuspended in 100 ml sterile normal saline. To determine the spore per ml of suspension, the solution was heated at 80°C for 15 min to kill the vegetative cells before appropriate serial dilution and plating in NYSM agar. Finally, the spore suspension was prepared at a concentration of 1 × 109 spore/ml in sterile saline and kept in the refrigerator until use.
Animals and diets
Forty-eight male Wistar rats (200 ± 8.4 g) were provided by the Animal Centre of Razi Research Institute, Shiraz, Iran. Animals were housed in groups of six rats per cage in a temperature controlled environment (22 ± 2°C) with 55 ± 10% relative humidity and controlled lighting (12 h light/dark cycle).
Rats were randomly divided into 4 groups and fed as follows: 1) standard diet (control), 2) standard diet supplemented with 5% w/w long chain inulin (Sensus, Netherlands) (prebiotic), 3) standard diet with 109 spores/day B. coagulans (gavage 1 ml of prepared spore suspension using a blunt ended needle) (probiotic), and 4) standard diet supplied with 5% w/w long chain inulin and 109 spores/day B. coagulans (synbiotic). The standard pellet feedstuff contained 14.5% protein, 4.7% ash, 51.2% starch, 4.3% sugar and 4% fat (3.2 kcal/g). Regarding micronutrients, the feedstuff contained 0.72% calcium, 0.6% phosphorus, 0.23% magnesium and 0.25% chloride among others. To add inulin, the standard pellet feedstuff was first broken to powder and after adding 5% inulin, carboxymethyl cellulose (CMC) 0.1% was used to rebind the ingredients and left at room temperature until dried. The inulin content in the rat diet was calculated based on food intake. The food intake of each rat with mean of 200 g body weight is 10 g/day, it means each rat received 0.5 g inulin/day. Food and tap water were provided ad libitum.
To assimilate the experimental conditions, the control and prebiotic group were gavaged with 1 ml of sterile normal saline once a day.
Experimental design
All animals were acclimatized for 2 weeks before the experimental session. Rats were fed the diets for 30 days and had free access to the experimental diets and tap water. At day 10, 20 and 30 of experiment (24 h post B. coagulans administration) 4 rats from each group were randomly selected and sacrificed after faecal collection. Following this, dissection and ligation of selected organs, small intestine (duodenum, jejunum and ileum), cecum, and colon were excised from each rat. Samples of organs were weighed including luminal contents and used for immediate microbiological analysis. This experiment was accomplished under the approval of the state committee on animal ethics, Shiraz University, Shiraz, Iran. Also, the recommendations of European Council Directive (86/609/EC) of November 24, 1986 were followed, regarding the standards in the protection of animals used for experimental purposes.
Microbiological analysis
Five g of samples (dissected organs or faeces) tissues were transferred into stomacher bags along with 45 ml of sterile buffered peptone water and homogenized in a stomacher for 2 min. A ten-fold serial dilution was performed and appropriate dilutions were pour plated using plate count agar (PCA) (Merck, Germany) and incubated at 37°C for 48 h to obtain the total aerobic and anaerobic bacterial counts. Anaerobic plates were placed in anaerobic jar using an anaerobic gas pack system (Anaerocult A, Merck, Germany). Lactic acid bacteria (LAB) were enumerated using Man Rogosa agar (Merck 5413, Darmstadt, Germany) after incubation in an anaerobic environment at 37°C for 48 h. Enterobacteriaceae were enumerated on MacConkey agar (Merck 5465, Darmstadt, Germany) after incubation at 37°C for 24 h. B. coagulans spore counts were determined on NYSM agar after incubation at 37°C for 24 h.
Statistical analysis
In order to determine the difference among treatments, one way analysis of variance (ANOVA) was used and when differences were detected, a Duncan’s multiple comparison test was used to differentiate the treatment means. The analysis was carried out using SPSS (version 19, SPSS Inc.) at a significance level of 0.05.
Results
LAB counts
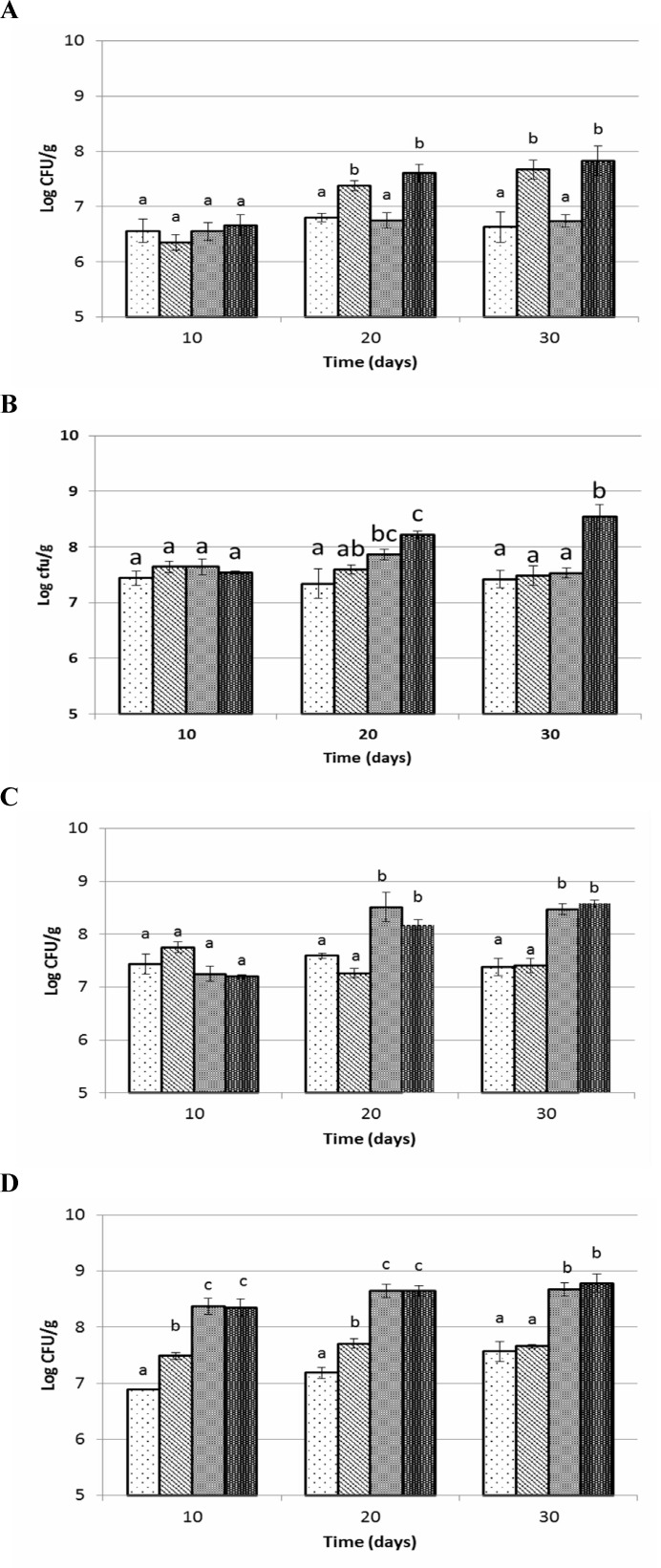
The populations of LAB in the faecal samples and various segments of GI tract of rats were not affected by the experimental diets after 10 days of feeding (Figs. 1A-D). A significant effect was observed, 20 and 30 days post feeding trials. The number of LAB was significantly higher throughout small intestine of synbiotic and prebiotic fed rats as compared with probiotic fed and control diet at 20 (P=0.009) and 30 days (P=0.002) of feeding trials. Results showed that population of LAB in cecum, colon and faecal samples of rats fed with synbiotic and probiotic diets were significantly (P<0.05) higher than other experimental groups. Population of LAB in colon and faecal samples were 8.4-8.8 log CFU/g in synbiotic and probiotic group following 30 days, these values were 7.3-7.6 log CFU/g in prebiotic and control groups.
Fig. 1.
Lactic acid bacteria counts of small intestine (A), cecum (B), colon (C) and faeces (D) of Wistar rats fed with synbiotic, probiotic , prebiotic and control during 30 days trial. Values not sharing the same superscript are significantly different (P<0.05). Bars represent standard error values
Enterobacteriaceae counts
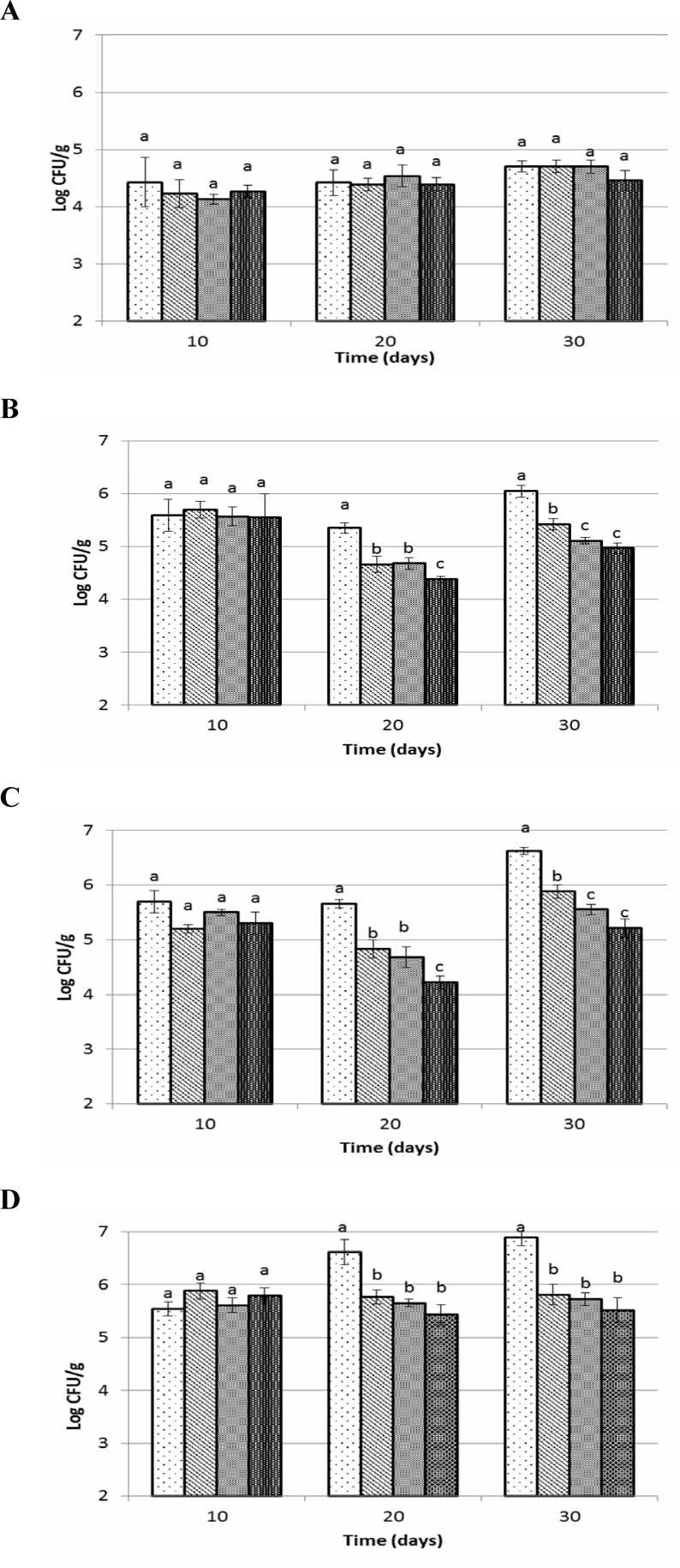
The enterobacterial populations were not significantly different during first 10 days but then decreased in the supplemented groups (P<0.05) (Figs. 2A-D). At day 20 and 30 the numbers of Enterobacteriaceae in various segments of the GI tract (except small intestine) of synbiotic, probiotic and prebiotic fed groups were significantly lower compared to control. Highest level of decrease in enterobacterial population referred to synbiotic fed group then probiotic and prebiotic fed groups, respectively. Enterobacterial counts in cecum, colon and faecal sample of control group increased about 1 log CFU/g after 30 days. Results showed that feeding trials inhibited enterobacterial growth. After twenty days of feeding trials, enterobacterial count in cecum and colon of synbiotic fed groups were significantly (P<0.05) lower than prebiotic and probiotic fed groups.
Fig. 2.
Entrobacterial counts of small intestine (A), cecum (B), colon (C) and faeces (D) of Wistar rats fed with synbiotic, probiotic, prebiotic and control during 30 days trial. Values not sharing the same superscript are significantly different (P<0.05). Bars represent standard error values
Total aerobic bacteria
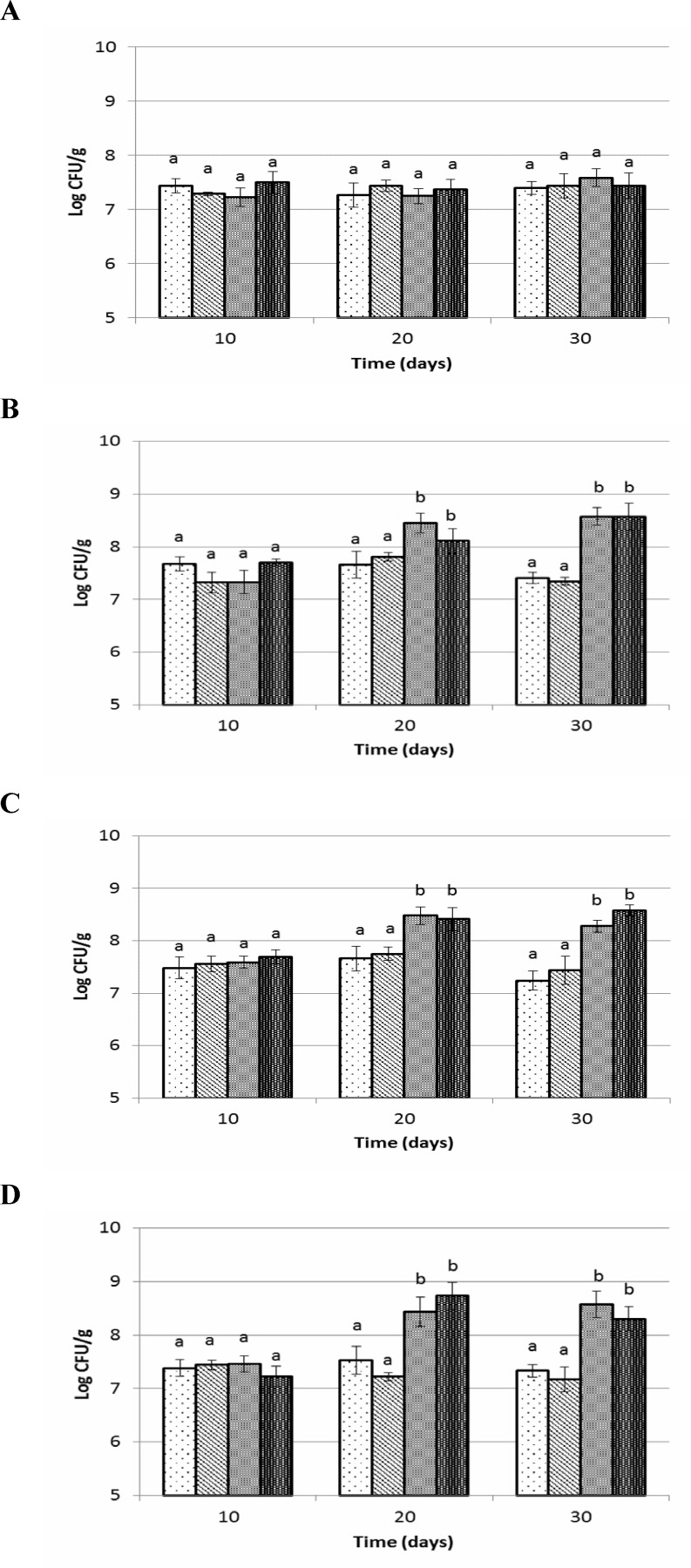
Results for number of total aerobic bacteria are shown in Figs. 3A-D. In groups fed with synbiotic and probiotic diet, significantly higher numbers of aerobic bacteria were observed in the caecum, colon and faecal samples (P<0.05) as compared with prebiotic fed group and control in the days 20 and 30. Feeding with synbiotic and probiotic diets led to 1 log CFU/g increase in total aerobic bacteria level of GI tract. Total aerobic bacteria was not changed in prebiotic fed group compared to control during experiment. There were no significant changes (P>0.05) seen in the number of aerobic bacteria in small intestine during feeding trials.
Fig. 3.
Total aerobic counts of small intestine (A), cecum (B), colon (C) and faeces (D) of Wistar rats fed with synbiotic, probiotic, prebiotic and control during 30 days trial. Values not sharing the same superscript are significantly different (P<0.05). Bars represent standard error values
Total anaerobic bacteria count
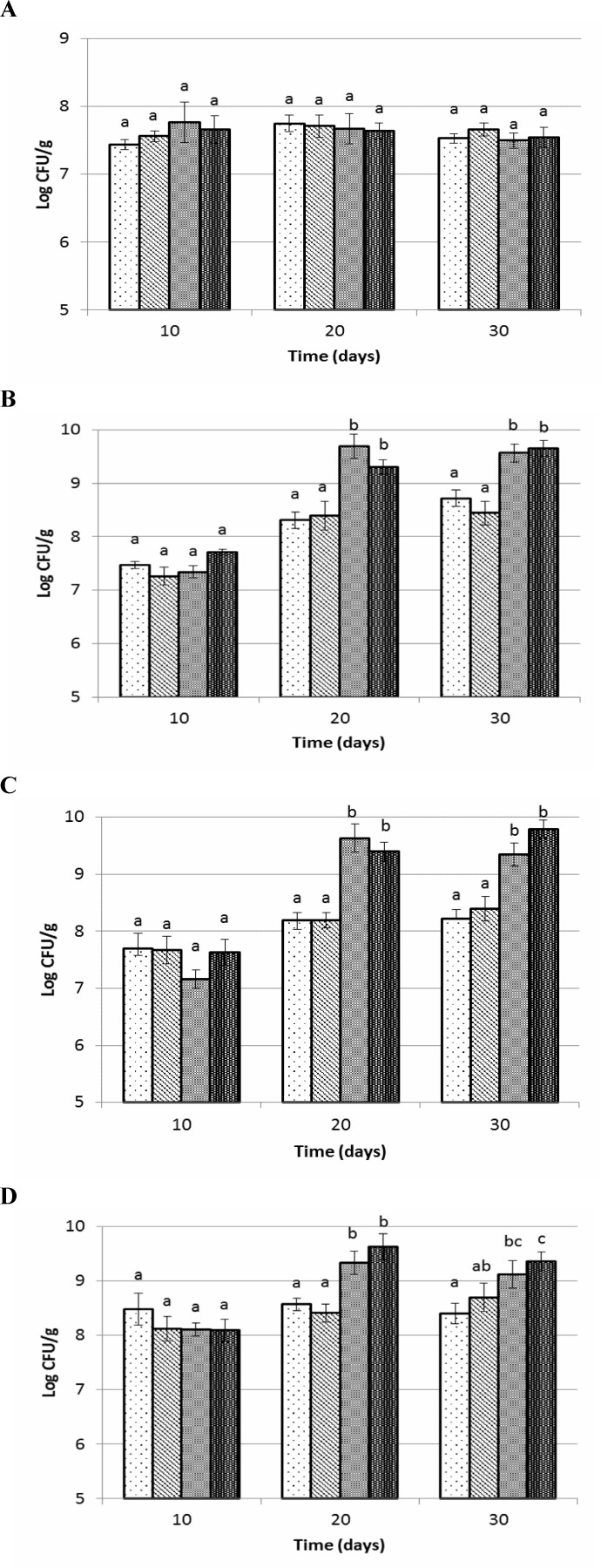
The populations of total anaerobic bacteria in small intestine of rats were not affected by the experimental diets (P>0.05) (Figs. 4A-D). Results obtained showed that total anaerobic bacteria in caecum, colon and faecal samples of B. coagulans fed groups were significantly higher compared to prebiotic fed and control. The level of total anaerobic bacteria on days 20 and 30 in synbiotic and probiotic fed groups were 1 log CFU/g higher than prebiotic fed and control groups. An additive effect of inulin with B. coagulans on total anaerobic bacterial growth was seen in synbiotic fed group compared to probiotic group, which was not significant (P>0.05).
Fig. 4.
Total anaerobic counts of small intestine (A), cecum (B), colon (C) and faeces (D) of Wistar rats fed with synbiotic, probiotic , prebiotic and control during 30 days trial. Values not sharing the same superscript are significantly different (P<0.05). Bars represent standard error values
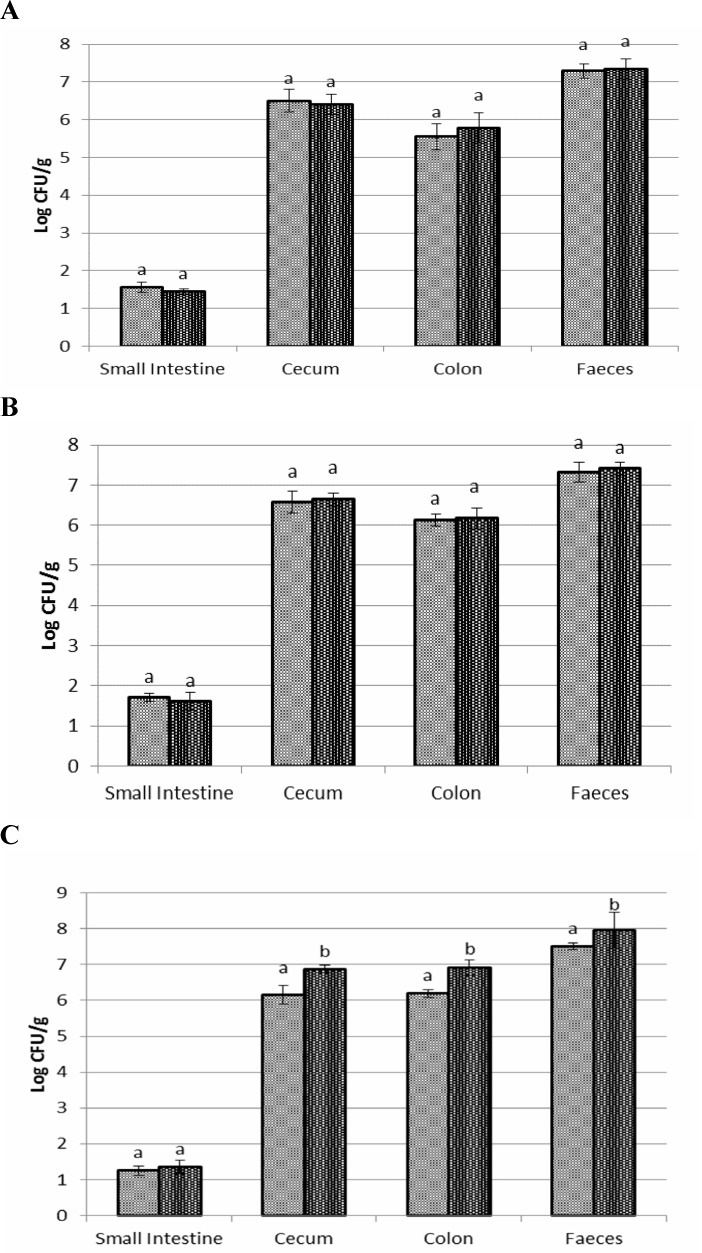
Bacillus coagulans spores count
Results for the number and distribution of B. coagulans spores are shown in Figs. 5A-C. A significant increase in the mean level of B. coagulans spores was observed following passing through from small intestine to distal parts of GI tract. The highest and lowest levels of spores were found in faecal samples and small intestine, respectively, 24 h post dosing. The number of spores was significantly higher in synbiotic fed rats compared to probiotic fed in faecal sample and various segments of gastrointestinal tract except small intestine.
Fig. 5.
Bacillus coagulans spore counts after ten (A), twenty (B), thirty (C) days of different organs of Wistar rats fed with synbiotic , and probiotic, during 30 days trial. Values not sharing the same superscript are significantly different (P<0.05). Bars represent standard error values
Discussion
The ability of probiotic strains to survive, transit and to colonize the GI tract is considered as an important factor for providing potential health benefits. Thus, the use of Bacillus products raised a number of questions, including their mode of action. In the present study, response of rat microbiota at different times during oral intake of B. coagulans spores was investigated. There has been limited information on probiotic activity of B. coagulans spores on GI tract flora. Studies on the effects of B. coagulans on animals have been limited to those who investigated the effects of administration of B. coagulans spores on the chicken growth performance and composition of intestinal microflora. Cavazzoni et al. (1998) ▶ reported significant improvement in chicken performance treated with Bacillus as compared with chicken receiving no additive with highest mean body weights and daily weight gains. Another study by Lin et al. (2011) ▶ examined the effect of B. coagulans spores on the intestinal microbiota of broiler chicken.
The present study is the first study where the effect of B. coagulans spores alone and in combination with inulin was studied on GI flora of rat as an animal model.
B. coagulans spores, when administered at a level of 109 spores/day for 30 days, caused an increase in LAB number. Results of LAB count showed that B. coagulans spores were activated in cecum and promote the LAB growth in distal segments of gastrointestinal tract. The numbers of LAB were significantly higher throughout small intestine of synbiotic and prebiotic fed rats as compared with probiotic fed and control diet (P<0.05). This could be due to the fact that B. coagulans spores in small intestine were still not activated and proliferated and only inulin promoted the LAB growth. It is in agreement with data shown by Casula and Cutting (2002) ▶ who indicated that spore germination was not detected in the duodenum but was readily detectable in the ileum. The small intestine contains regions of different physiochemical conditions, and the high content of stomach acids and bile salts in duodenum may support spore germination (Casula and Cutting, 2002 ▶). Higher LAB count in synbiotic fed rats indicates additive effect between inulin and B. coagulans. This idea was supported by Gallaher and Khil (1999) ▶ who showed that administration of prebiotic oligofructose with probiotic Bifidobacteria to rats resulted in additive effects compared to their individual administration.
In the present study, enterobacterial count of control group increased over time (P<0.05), while their number decreased in other treatment groups. Strongest effect of different diets in decreasing enterobacterial population referred to synbiotic fed group then probiotic and prebiotic fed group, respectively. Enterobacteriaceae family contains many potentially pathogenic/harmful bacteria. Thus, reduction or control of proliferation of these bacteria plays a role in maintaining health and well-being and in reducing the risk of some diseases including antibiotic associated diarrhea, acute gastroenteritis, inflammatory bowel disease, food allergy, and colon cancer (DeRoos and Katan, 2000 ▶).
The results obtained in this study are in agreement with a previous study by Lin et al. (2011) ▶ who found an increase in LAB counts and a decrease in E. coli counts in the duodenum and cecum of broilers fed with B. coagulans spores.
Supplementation with B. coagulans spores significantly increased total anaerobic bacteria in faecal sample and various segments of GI tract (except small intestine). The reason for the increase in the total bacterial counts at the follow up period is not clear, it may be due to facultative anaerobic characteristic of B. coagulans (De Vecchi and Drago, 2006 ▶), which is proved by Ripamonti et al. (2009) ▶ who showed the ability of B. coagulans to grow in anaerobic conditions. Thus anaerobic conditions of small intestine and presence of suitable nutritional environment (e.g., fructose, L-alanine) stimulated spores germination (Casula and Cutting, 2002 ▶).
It is possible that higher number of total aerobic bacteria in B. coagulans spores fed groups (synbiotic and probiotic) compared with prebiotic fed and control was correlated to increase in Lactobacillus. This result is similar to results shown by Matsumoto et al. (2010) ▶ who reported an increase in total aerobic bacteria following Lactobacillus casei consumption. These data are in agreement with earlier studies that showed probiotics supplementation increases faecal total Lactobacilli, anaerobic counts and Bifidobacteria concentrations significantly (Kuisma et al., 2003 ▶). Perhaps the characteristics of B. coagulans probiotic in changing GI microbiota prevent GI disorders, considering previous studies indicated that the total faecal obligate anaerobe, Bacteroidaceae, Bifidobacterium, and Lactobacillus counts in patients with acute diarrhea were lower than in healthy adults (Hong et al., 2005 ▶). Moreover, another study specified that, in patients with irritable bowel syndrome characterized by diarrhea (IBS-D), the Bifidobacterium spp. counts were lower than in a healthy control group (Malinen et al., 2005 ▶).
One of the important findings of this study was the high concentration of B. coagulans spores (9 log spores/g) in faecal samples that was equal to inoculum dose. This data shows B. coagulans spores are stable to bile and acidic condition of GI tract. Previous studies showed the ability of Bacillus species to survive in host GI tract (Hoa et al., 2001 ▶; Duc et al., 2004; Ripamonti et al., 2009 ▶). Another study indicated B. coagulans GanedenBC30 highly survived (70%) in a dynamic, validated, in vitro model of the stomach and small intestine, although germination of the spores was minimal (<10%) under the conditions tested (Maathuis et al., 2010 ▶).
The obvious decline in spores count through passing rat GI tract while affecting GI microbiota supports the fact that Bacillus spp. could have an endosymbiotic relationship with the host, being able to temporarily survive and proliferate within the GI tract (Hong et al., 2005 ▶). An in vivo study reported B. coagulans being lost one week after administration (Cavazzoni et al., 1998 ▶).
The results of this study showed that use of inulin caused significant lowering effect on enterobacterial population. Inulin, which was used in combination with B. coagulans as synbiotic, showed an increase, but not a significant effect, in promoting both LAB and B. coagulans populations. Several studies showed significant differences in the microbiota of rats fed with inulin. Most studies reporting inulin-derived benefits have assayed relatively high inulin doses, especially in animals. However, the present study showed a low inulin intake could also exert some benefits by exploring differential effects on intestinal microbiota (Azorin-Ortuno et al., 2009 ▶).
In conclusion, using an animal model, the present study clearly showed that B. coagulans was efficient in beneficially modulating GI microbiota and considering transitional characteristic of probiotic B. coagulans that are not able to colonize the intestine and are quickly eliminated in faeces, daily consumption of probiotic products is necessary for any long-term effect.
Acknowledgment
The work was financially supported by School of Veterinary Medicine, Shiraz University.
References
- Azorin-Ortuno M, Urban C, Ceron J, Tecles F, Allende A, Tomas Barberan F, Carlos Espin J. Effect of low inulin doses with different polymerisation degree on lipid metabolism, mineral absorption, and intestinal microbiota in rats with fat-supplemented diet. Food. Chem. 2009;113:1058–1065. [Google Scholar]
- Casula G, Cutting SM. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002;68:2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzoni V, Adami A, Castrovilli C. Performance of broiler chickens supplemented with Bacillus coagulans as probiotic. Brit. Poult. Sci. 1998;39:526–529. doi: 10.1080/00071669888719. [DOI] [PubMed] [Google Scholar]
- Cutting SM. Bacillus probiotics. Food. Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- DeRoos NM, Katan MB. Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: a review of papers published between 1988 and 1998. Am. J. Clin. Nutr. 2000;71:405–411. doi: 10.1093/ajcn/71.2.405. [DOI] [PubMed] [Google Scholar]
- De Vecchi E, Drago L. Lactobacillus sporogenes or Bacillus coagulans: misidentification or mislabeling? Int. J. Probiotics Prebiotics. 2006;1:3–10. [Google Scholar]
- Gallaher DD, Khil J. The effect of synbiotics on colon carcinogenesis in rats. J. Nutr. 1999;129:1483–1487. doi: 10.1093/jn/129.7.1483S. [DOI] [PubMed] [Google Scholar]
- Hammerman C, Bin-Nun A, Kaplan M. Safety of probiotics: comparison of two popular strains. B. M. J. 2006;333:1006–1008. doi: 10.1136/bmj.39010.630799.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa TT, Duc LH, Isticato R, Baccigalupi L, Ricca E, Van PH, Cutting SM. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001;67:3819–3823. doi: 10.1128/AEM.67.9.3819-3823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HA, Duc LH, Cutting SM. The use of bacterial spore formers as probiotics. FEMS. Microbiol. Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Kalman DS, Schwartz HI, Alvarez P, Feldman S, Pezzullo JC, Krieger DR. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009;9:85–92. doi: 10.1186/1471-230X-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment. Pharmacol. Ther. 2003;17:509–515. doi: 10.1046/j.1365-2036.2003.01465.x. [DOI] [PubMed] [Google Scholar]
- Lin SY, Hung ATY, Lu JJ. Effects of supplement with different level of Bacillus coagulans as probiotic on growth performance and intestinal microflora populations of broiler chickens. J. Anim. Vet. Adv. 2011;10:111–114. [Google Scholar]
- Losada MA, Olleros T. Towards a healthier diet for the colon: the influence of fructooligosaccharides and Lactobacilli on intestinal health. Nutr. Res. 2001;22:71–84. [Google Scholar]
- Maathuis AJH, Keller D, Farmer S. Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef. Microbes. 2010;1:31–36. doi: 10.3920/BM2009.0009. [DOI] [PubMed] [Google Scholar]
- Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- Marzotto M, Maffeis C, Paternoster T, Ferrario R, Rizzotti L, Pellegrino M, Dellaglio F, Torriani S. Lactobacillus paracasei A survives gastrointestinal passage and affects the fecal microbiota of healthy infants. Res. Microbiol. 2006;157:857–866. doi: 10.1016/j.resmic.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Takada T, Shimizu K, Moriyama K, Kawakami K, Hirano K, Kajimoto O, Nomoto K. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J. Biosci. Bioeng. 2010;110:547–552. doi: 10.1016/j.jbiosc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Mikkelsen LL, Jakobsen M, Jensen BB. Effects of dietary oligosaccharides on microbial diversity and fructo-oligosaccharide degrading bacteria in faeces of piglets post-weaning. Anim. Feed. Sci. Technol. 2003;109:133–150. [Google Scholar]
- Ogawa T, Asai Y, Yasuda K, Sakamoto H. Oral immunoadjuvant activity of a new synbiotic Lactobacillus casei subsp casei in conjunction with dextran in BALB/c mice. Nutr. Res. 2005;25:295–304. [Google Scholar]
- Plummer SF, Garaiova I, Sarvotham T, Simon L, Cottrell SL, Scouiller SL, Weaver MA, Tang J, Dee P, Hunter J. Effects of probiotics on the composition of the intestinal microbiota following anti-biotic therapy. Int. J. Antimicrob. Agents. 2005;26:67–74. doi: 10.1016/j.ijantimicag.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rauch M, Lynch SV. The potential for probiotic manipulation of the gastrointestinal microbiome. Curr. Opin. Biotechnol. 2012;23:192–201. doi: 10.1016/j.copbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Ripamonti B, Agazzi A, Baldi A, Balzaretti C, Bersani C, Pirani C, Rebucci R, Savoini G, Stella S, Stenico A, Domeneghini C. Administration of Bacillus coagulans in calves: recovery from faecal samples and evaluation of functional aspects of spores. Vet. Res. Commun. 2009 doi: 10.1007/s11259-009-9318-0. doi: 10.1007/s11259-009-9318-0. [DOI] [PubMed] [Google Scholar]
- Russell BL, Jelley SAA, Yousten AA. Selective medium for mosquito pathogenic strains of Bacillus sphaericus 2362. J. Appl. Environ. Microbiol. 1989;55:294–297. doi: 10.1128/aem.55.2.294-297.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokulova I, Pinchuk I, Denayrolles M, Osipova I, Huang J, Cutting M, Urdaci M. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008;53:954–963. doi: 10.1007/s10620-007-9959-1. [DOI] [PubMed] [Google Scholar]
- Yousten AA, Fretz SA, Jelly SA. Selective medium for mosquito pathogenic strains of Bacillus sphaericus. App. Environ. Microbiol. 1985;49:1532–1533. doi: 10.1128/aem.49.6.1532-1533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]