Abstract
Most studies of sleep and health outcomes rely on self-reported sleep duration, although correlation with objective measures is poor. In this study, we defined sociodemographic and sleep characteristics associated with misreporting and assessed whether accounting for these factors better explains variation in objective sleep duration among 2,086 participants in the Hispanic Community Health Study/Study of Latinos who completed more than 5 nights of wrist actigraphy and reported habitual bed/wake times from 2010 to 2013. Using linear regression, we examined self-report as a predictor of actigraphy-assessed sleep duration. Mean amount of time spent asleep was 7.85 (standard deviation, 1.12) hours by self-report and 6.74 (standard deviation, 1.02) hours by actigraphy; correlation between them was 0.43. For each additional hour of self-reported sleep, actigraphy time spent asleep increased by 20 minutes (95% confidence interval: 19, 22). Correlations between self-reported and actigraphy-assessed time spent asleep were lower with male sex, younger age, sleep efficiency <85%, and night-to-night variability in sleep duration ≥1.5 hours. Adding sociodemographic and sleep factors to self-reports increased the proportion of variance explained in actigraphy-assessed sleep slightly (18%–32%). In this large validation study including Hispanics/Latinos, we demonstrated a moderate correlation between self-reported and actigraphy-assessed time spent asleep. The performance of self-reports varied by demographic and sleep measures but not by Hispanic subgroup.
Keywords: actigraphy, Hispanic Americans, Latinos, measurement error, sleep, sleep duration, validation studies
Accumulating evidence links extremes of sleep duration with chronic diseases, including hypertension, obesity, diabetes, and cancer (1–3). Simultaneously, racial/ethnic disparities in sleep duration are gaining attention (4–6). Though a majority of studies use questionnaires to assess sleep duration, little research examines the validity of self-reporting against objective measurements such as wrist actigraphy, the objective method of choice for measuring sleep over multiple days. Even fewer studies have included ethnically diverse participants (though those that have done so have detected racial/ethnic differences in the accuracy of self-reports) (7) or have had sufficient sample sizes to explore sources of systematic bias (7–9). Yet, examining the correlation of self-reported sleep duration with objective measurements in varied populations may be essential to the interpretation of epidemiologic studies. First, prior research shows moderate correlations (Pearson's ρ = 0.31–0.47) between self-reported sleep duration and actigraphy-measured sleep duration and suggests the presence of both random error and systematic bias in self-reported sleep duration (7–9). Second, findings for subjective and objective measures of sleep duration do not always agree. For example, the Coronary Artery Risk Development in Young Adults (CARDIA) Study detected sex differences in the association of sleep duration with body mass index (BMI) using self-reported sleep duration (10) but not actigraphy-measured sleep duration (11).
To our knowledge, no prior study has examined the validity of self-reported sleep duration among diverse Hispanics/Latinos, the largest racial/ethnic minority group in the United States and a population heavily burdened by obesity, diabetes, and other diseases linked to sleep duration. With 2,086 participants, the current study is the largest study in any population (to our knowledge) to validate self-reported sleep duration against wrist actigraphy. This large sample enabled us to examine how well self-reports and actigraphy correlated within subgroups defined by sleep and sociodemographic characteristics, including Hispanic/Latino background, and which characteristics contributed to the difference between self-reported and objective sleep measures. This research contributes to the interpretation of associations with self-reported sleep duration in epidemiologic studies in general, and it may have particular relevance to Hispanic/Latino health disparities. Differences in self-reported sleep habits between Hispanic subgroups have been identified (12), and associations of self-reported sleep with chronic diseases may vary by Hispanic/Latino subgroup (6, 13–16). Understanding whether the validity of self-reported sleep duration varies by Hispanic/Latino background or other characteristics is critical to interpreting these results.
METHODS
Study population
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) enrolled 16,415 self-identified Hispanics/Latinos aged 18–74 years (March 2008–June 2011). Participants were recruited from randomly selected households in 4 US communities (the Bronx, a borough of New York, New York; Chicago, Illinois; Miami, Florida; and San Diego, California). The study was approved by review boards at each participating institution, and written informed consent was obtained from participants. Details of the study design and procedures have been published elsewhere (17, 18).
HCHS/SOL Sueño, an ancillary sleep study, enrolled 2,252 HCHS/SOL participants (October 2010–December 2013) aged <65 years without severe sleep disorders (narcolepsy, apnea-hypopnea index (AHI) ≥50 events/hour, or use of positive airway pressure). The present analysis included 2,086 eligible individuals with actigraphy and self-reported sleep measurements.
Self-reported sleep duration
Self-reported sleep duration was assessed at the Sueño visit of HCHS/SOL through participant report of habitual bed/wake times on weekends/weekdays, from which we calculated a weighted average of sleep duration (2/7 × weekends +5/7 × weekdays), as done in other sleep studies (19). Self-reported sleep duration was examined both continuously and categorized into tertiles and a priori groupings (<7, 7–<9, or ≥9 hours/day) based on published literature.
Actigraphy measurements
At the Sueño visit, participants were instructed to wear an Actiwatch Spectrum actigraph (Philips Respironics, Murrysville, Pennsylvania) on the nondominant wrist for 7 days and to complete sleep diaries upon awakening each day. Sleep/wake status was determine in 30-second epochs based on a standardized method (20) using the Actiware 5.59 algorithm, which has been validated against polysomnography on an epoch-by-epoch basis (21, 22).
The mean number of valid nights of actigraphy was 7 (standard deviation (SD), 1; range, 5–17 days). We calculated a weighted average (2/7 × weekends + 5/7 × weekdays) of amount of time spent in bed (time between getting into and out of bed) and amount of time spent asleep (time within the in-bed interval scored as sleep). Time spent asleep was treated continuously, in tertiles, and in a priori groupings: <7 hours, 7–<9 hours, and ≥9 hours. In secondary analyses, time spent in bed was considered as the actigraphic variable of interest. Sleep efficiency was computed on each night as the proportion of time spent asleep between the first and last epochs of sleep and then averaged across all nights, dichotomized at 85% (<85%/≥85%). Variability in nightly sleep was defined as the standard deviation of time spent asleep across all recorded days. Participants in the top quartile (SD >1.5 hours) were considered to have “high” variability in time asleep.
Covariate assessment
As part of the HCHS/SOL baseline examination, participants underwent home sleep apnea monitoring using the ARES Unicorder 5.2 (B-Alert; Advanced Brain Monitoring, Inc., Carlsbad, California) for computation of the AHI, as has been previously described (16). Severity of sleep apnea was classified as none (AHI <5 events/hour), mild (AHI 5–15 events/hour), or moderate (AHI 15–50 events/hour). The HCHS/SOL baseline examination included interviewer-administered questionnaires in the participant's preferred language (Spanish or English) and other measurements described previously (17, 18). Information was obtained on demographic factors (Hispanic ethnic background (Cuban, Dominican, Mexican, Puerto Rican, Central American, or South American), nativity (mainland United States or elsewhere), education (less than high school, high school diploma, or more than high school), annual household income (<$30,000, ≥$30,000, or not reported), cigarette and alcohol use (current or noncurrent), and physical activity (Global Physical Activity Questionnaire: self-reported days/week of recreational, transportation, or work activity; corresponding metabolic equivalent units were categorized as high, moderate, or low (23)).
As part of the Sueño visit, interviewers administered questionnaires about sociodemographic factors, health, and sleep behaviors. Sleep-related symptoms were assessed using the Sleep Heart Health Study Sleep Habits Questionnaire (24), the Epworth Sleepiness Scale (25), and the Insomnia Severity Index (ISI) (26). Daytime sleepiness score on the Epworth Sleepiness Scale was dichotomized as <10 versus ≥10, and insomnia was divided into 4 categories: none (ISI score 0–7), subthreshold (ISI score 8–14), moderate (ISI score 15–21), and severe (ISI score 22–28). Depressive symptoms were evaluated using the 10-item Center for Epidemiological Studies Depression Scale, with scores dichotomized as <10 versus ≥10 (27, 28). Use of sleep medication was categorized as less than once/week versus once/week or more. Participants were asked about employment and work schedules. Nighttime, irregular, on-call, or rotating shifts that included late nights or early mornings were considered shift work. Intake of caffeinated beverages (including coffee, tea, soda, and energy drinks) was categorized as <3 cups/day versus ≥3 cups/day. Age was categorized into 18–44 years and 45–64 years. BMI was calculated as measured weight (in kilograms) divided by measured height (in meters) squared and was categorized into underweight (<18.5)/normal weight (18.5–<25), overweight (25–<30), and obese (class I: 30–<35; class II: 35–<40; or class III: 40).
Statistical analysis
We computed descriptive statistics for the HCHS/SOL cohort and the Sueño subsample by category of self-reported sleep duration. Next, we calculated Pearson correlation coefficients for the correlation of self-reported sleep duration with actigraphy-assessed time spent in bed and time spent asleep, overall and in subgroups defined by participant characteristics. We estimated unadjusted β coefficients and 95% confidence intervals for self-reported sleep duration as a predictor of actigraphy-assessed time in bed and time asleep using linear regression. Regression intercepts represented bias, the disparity between subjects’ reported sleep duration and their actigraphy-measured values. If subjective and objective reports of sleep matched perfectly, there would be no bias and the intercept would be 0 minutes. We report the intercept at the average of 480 minutes (or 8 hours) of self-reported sleep.
To examine predictors of actigraphy-assessed time asleep and time in bed, we used multivariable linear regression with actigraphy outcomes predicted by self-reported sleep duration and participant characteristics: age, sex, BMI, insomnia, sleep apnea, sleepiness, efficiency, variability, sleep medication use, employment, education, Hispanic ethnicity, nativity, language, depression, caffeine intake, smoking, alcohol drinking, and physical activity. We also considered interaction terms for the interaction of each characteristic with self-reported sleep duration. To identify predictors of actigraphy measurements using self-reports and participant characteristics, we used stepwise regression with 10-fold cross-validation. Candidate variables introduced into this procedure included the above characteristics and their interactions with self-reported sleep duration. Interaction terms were entered into the model only with the corresponding main association.
Self-reported sleep duration is often treated categorically because of a U-shaped relationship with many disease outcomes (1). Thus, we assessed the accuracy of ranking by calculating κ scores and confidence intervals based on tertiles of self-reported and actigraphy-measured sleep duration. We also report the area under the receiver operating characteristic curve for logistic models using tertiles of self-reported sleep duration to predict measured actigraphy-assessed short sleep duration (excluding “long sleep,” i.e., persons in the top tertile of sleep time) and actigraphy-assessed long sleep duration (excluding “short sleep,” i.e., persons in the bottom tertile of sleep time).
In sensitivity analyses, we excluded participants in the top quartile of variability to assess the influence of variation in night-to-night sleep duration on the correlation between self-reported and actigraphy-assessed sleep duration. Further, we repeated all analyses using weekday data only. We also compared the fits of models with and without nonlinear relationships, adding quadratic and cubic terms for self-reported sleep to models, and testing linear and cubic splines. A 2-sided P value less than 0.05 was used to indicate statistical significance.
RESULTS
Table 1 shows characteristics of the Sueño sample by category of self-reported sleep duration. Overall, Sueño sample characteristics were similar to those of the HCHS/SOL parent cohort with respect to key demographic/health variables such as BMI, education, income, and employment (see Web Table 1, available at http://aje.oxfordjournals.org/). Mean age was 47.1 (SD, 11.5) years, and mean time between the HCHS/SOL baseline and Sueño examinations was 24 (SD, 5) months (range, 4–30 months). Mean self-reported sleep duration was 7.86 (SD, 1.28) hours; actigraphy-assessed time spent asleep was more than 1 hour shorter (6.74 (SD, 1.02) hours). Participants reporting short sleep were slightly older, had slightly higher scores for depression, sleepiness, insomnia, sleep apnea, and adiposity, and were more likely to consume 3 or more caffeinated beverages per day. Those reporting long sleep were younger and more likely to have incomes under $30,000 per year, to be unemployed, and to engage in no or low physical activity.
Table 1.
Characteristicsa of the Study Sample by Self-Reported Daily Sleep Duration, HCHS/SOL Sueño (n = 2,086), 2010–2013
| Self-Reported Sleep Duration at Sueño Visit, hours/day |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <7 (Short) (n = 460) |
7–9 (Intermediate) (n = 1,292) |
≥9 (Long) (n = 334) |
|||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Age, years | 48.1 (10.6) | 46.9 (11.5) | 46.5 (12.7) | ||||||
| Body mass indexb | 31.1 (6.8) | 29.8 (6.0) | 29.7 (6.6) | ||||||
| CES-D-10 score | 8.2 (6.3) | 7.2 (6.1) | 7.5 (5.8) | ||||||
| Female sex | 283 | 62 | 849 | 66 | 219 | 66 | |||
| Educational attainmentc | |||||||||
| Less than high school/GED | 137 | 30 | 387 | 30 | 140 | 42 | |||
| High school/GED | 115 | 25 | 349 | 27 | 75 | 22 | |||
| More than high school/GED | 208 | 45 | 553 | 43 | 119 | 36 | |||
| Annual household incomec | |||||||||
| <$30,000 | 309 | 67 | 846 | 65 | 245 | 73 | |||
| ≥$30,000 | 132 | 29 | 393 | 30 | 68 | 20 | |||
| Not reported | 19 | 4 | 53 | 4 | 21 | 6 | |||
| Employment status | |||||||||
| Employed, non–shift worker | 227 | 49 | 614 | 48 | 102 | 31 | |||
| Shift worker | 79 | 17 | 157 | 12 | 44 | 13 | |||
| Unemployed or retired | 154 | 33 | 521 | 40 | 188 | 56 | |||
| Hispanic ethnic backgroundc | |||||||||
| Cuban | 86 | 19 | 233 | 18 | 57 | 17 | |||
| Dominican | 66 | 14 | 160 | 12 | 35 | 10 | |||
| Mexican | 102 | 22 | 366 | 28 | 93 | 28 | |||
| Puerto Rican | 97 | 21 | 241 | 19 | 90 | 27 | |||
| Central American | 67 | 15 | 172 | 13 | 45 | 13 | |||
| South American | 42 | 9 | 120 | 9 | 14 | 4 | |||
| Nativity | |||||||||
| Mainland US | 68 | 20 | 202 | 16 | 73 | 16 | |||
| Outside mainland US | |||||||||
| Had lived in US for ≥10 years | 198 | 59 | 742 | 58 | 257 | 56 | |||
| Had lived in US for <10 years | 67 | 20 | 343 | 27 | 129 | 28 | |||
| English language preference | 100 | 22 | 242 | 19 | 82 | 25 | |||
| Current alcohol drinker | 212 | 46 | 579 | 45 | 147 | 44 | |||
| Current cigarette smoker | 102 | 22 | 229 | 18 | 71 | 21 | |||
| Physical activity levelc | |||||||||
| High | 45 | 10 | 119 | 9 | 20 | 6 | |||
| Moderate | 201 | 44 | 575 | 45 | 144 | 43 | |||
| Low | 214 | 47 | 595 | 46 | 169 | 51 | |||
| Insomnia severity | |||||||||
| None | 254 | 55 | 814 | 63 | 190 | 57 | |||
| Subthreshold | 103 | 22 | 288 | 22 | 80 | 24 | |||
| Moderate | 77 | 17 | 143 | 11 | 45 | 14 | |||
| Severe | 26 | 6 | 45 | 3 | 18 | 5 | |||
| Caffeinated beveraged intake ≥3 servings/day | 192 | 42 | 441 | 34 | 118 | 35 | |||
| Use of sleep medication at least once/week | 66 | 14 | 170 | 13 | 57 | 17 | |||
| Sleep efficiency <85% | 156 | 34 | 420 | 33 | 155 | 46 | |||
| SD of actigraphy-assessed time spent asleep ≥1.5 hours/day | 112 | 34 | 285 | 22 | 124 | 27 | |||
| Apnea-hypopnea index scoree | 2.4 (0.4–6.9) | 1.5 (0.4–5.1) | 1.8 (0.4–6.3) | ||||||
| Epworth Sleepiness Scale score | 6.9 (5.0) | 5.4 (4.3) | 5.0 (4.0) | ||||||
| Self-reported sleep duration, minutes/dayf | 368 (41) | 478 (36) | 587 (33) | ||||||
| Actigraphy-assessed time spent asleep, minutes/dayg | 366 (58) | 408 (54) | 442 (62) | ||||||
| Actigraphy-assessed time spent in bed, minutes/dayh | 425 (65) | 474 (58) | 521 (65) | ||||||
| Difference between self-reported and actigraphy-assessed time spent asleep, minutes/day | 2 (62) | 70 (59) | 145 (65) | ||||||
| Difference between self-reported and actigraphy-assessed time spent in bed, minutes/day | −57 (70) | 4 (60) | 66 (66) | ||||||
| Difference between actigraphy-assessed time in bed and actigraphy-assessed time asleep, minutes/day | 59 (29) | 66 (30) | 79 (30) | ||||||
Abbreviations: CES-D-10, 10-item Center for Epidemiologic Studies Depression Scale; GED, General Educational Development; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; SD, standard deviation; US, United States.
a All characteristics were measured at the Sueño visit of the HCHS/SOL unless otherwise indicated.
b Weight (kg)/height (m)2.
c Measured at the HCHS/SOL baseline visit.
d Caffeinated beverages included coffee, tea, soda, and energy drinks.
e Values are presented as median (interquartile range).
f Sleep duration was the weighted average of weekend and weekday self-reported habitual bed time minus wake time measured at the Sueño visit.
g Actigraph-measured time spent asleep was the weighted average amount of time spent asleep during the main rest period, excluding periods of wakefulness, over weekends and weekdays.
h Actigraph-measured time spent in bed was the weighted average duration of the main rest period, including periods of wakefulness, over weekends and weekdays.
Table 2 shows descriptive statistics for self-reported sleep duration, actigraphy-assessed time spent asleep, and their difference, correlation, and bivariate associations. Overall, the correlation of self-reported sleep duration with time spent asleep was 0.43 (95% confidence interval (CI): 0.39, 0.46), with higher correlations for weekdays (Pearson's ρ = 0.44) versus weekends (Pearson's ρ = 0.27).
Table 2.
Prediction of Sleep-Related Actigraphy Measurements by Self-Reports, HCHS/SOL Sueño (n = 2,086), 2010–2013
| Sleep Variable | Mean Time (SD), minutes/day |
Correlation |
480-Minute Intercept,a minutes |
Self-Reportingb as a Predictor of Actigraphy Time Spent in Bed or Asleep |
||||
|---|---|---|---|---|---|---|---|---|
| Self-Reports | Actigraphy | Difference | ρ | 95% CI | β | 95% CI | ||
| Time spent in bed | ||||||||
| Overallc | 472 (77) | 471 (67) | 1 (74) | 0.48 | 0.45, 0.52 | 475 | 0.42 | 0.39, 0.46 |
| Weekdays | 459 (85) | 466 (73) | −8 (80) | 0.49 | 0.46, 0.53 | 475 | 0.42 | 0.39, 0.45 |
| Weekends | 503 (99) | 482 (92) | 21 (114) | 0.29 | 0.25, 0.33 | 476 | 0.27 | 0.23, 0.31 |
| Time spent asleep | ||||||||
| Overalld | 472 (77) | 404 (61) | 67 (75) | 0.43 | 0.39, 0.46 | 407 | 0.34 | 0.31, 0.37 |
| Weekdays | 459 (85) | 400 (65) | 59 (81) | 0.44 | 0.40, 0.47 | 407 | 0.34 | 0.31, 0.37 |
| Weekends | 503 (99) | 415 (84) | 88 (111) | 0.27 | 0.23, 0.31 | 410 | 0.23 | 0.19, 0.26 |
Abbreviations: CI, confidence interval; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; SD, standard deviation.
a The regression intercept represents bias, the disparity between subjects’ reported sleep duration and their actigraphy-measured values. If subjective and objective reports of sleep matched perfectly, there would be no bias and the intercept would be 0 minutes. We report the intercept at the average of 480 minutes (or 8 hours) of self-reported sleep.
b Overall self-reported sleep duration was the weighted average of weekend and weekday self-reported habitual time in bed minus wake time measured at the Sueño visit of the HCHS/SOL.
c Overall actigraph-measured time spent in bed was the weighted average duration of the main rest period, including periods of wakefulness, over weekends and weekdays.
d Overall actigraph-measured time spent asleep was the weighted average amount of time spent asleep during the main rest period, excluding periods of wakefulness, over weekends and weekdays.
Before multivariable adjustment, actigraphy-assessed time spent asleep increased by 20 minutes (95% CI: 19, 22) for each additional hour of self-reported sleep duration. When results were stratified by sociodemographic, sleep, and health characteristics (Table 3), the association of self-reported sleep duration with measured time asleep differed significantly by sex (P = 0.02), age (P = 0.04), sleep efficiency (P = 0.0003), nightly sleep variability (P < 0.0001), and education (P = 0.01), with weaker associations among males, younger participants, those with sleep efficiency <85%, and those with nightly variability ≥1.5 hours or greater education. We found no evidence that the association varied by Hispanic/Latino background (P for interaction > 0.05).
Table 3.
Actigraphy-Assessed Amount of Time Spent Asleep Each Day as Predicted by Self-Reported Sleep Duration, According to Participant Characteristics, HCHS/SOL Sueño (n = 2,086), 2010–2013
| Participant Characteristic | No. of Persons | Mean Time Spent Asleep (SD), minutes/day |
Correlation |
Self-Reportinga as a Predictor of Actigraphy Time Spent Asleep |
Interaction P Value (F Test)b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Self-Reports | Actigraphyc | Difference | ρ | 95% CI | 480-Minute Intercept,d minutes | β | 95% CI | |||
| Sex | ||||||||||
| Male | 735 | 468 (77) | 391 (64) | 77 (81) | 0.35 | 0.28, 0.41 | 395 | 0.29 | 0.23, 0.34 | 0.02 |
| Female | 1,351 | 473 (76) | 412 (59) | 62 (71) | 0.47 | 0.43, 0.51 | 414 | 0.36 | 0.33, 0.40 | |
| Age, years | ||||||||||
| 18–44 | 724 | 481 (75) | 403 (59) | 78 (76) | 0.38 | 0.31, 0.44 | 403 | 0.30 | 0.25, 0.35 | 0.04 |
| 45–64 | 1,362 | 467 (73) | 405 (62) | 61 (74) | 0.45 | 0.41, 0.49 | 410 | 0.37 | 0.33, 0.40 | |
| Body mass indexe category | ||||||||||
| Normal (18.5–<25) or underweight (<18.5) | 405 | 477 (76) | 408 (66) | 69 (78) | 0.40 | 0.31, 0.48 | 409 | 0.35 | 0.27, 0.42 | 0.63 |
| Overweight (25–<30) | 791 | 477 (72) | 409 (60) | 68 (72) | 0.42 | 0.36, 0.48 | 410 | 0.36 | 0.30, 0.41 | |
| Obese (≥30) | ||||||||||
| Obese I (30–<35) | 506 | 462 (80) | 401 (58) | 61 (77) | 0.42 | 0.34, 0.49 | 408 | 0.30 | 0.24, 0.36 | |
| Obese II (35–<40) | 249 | 470 (78) | 398 (61) | 72 (71) | 0.49 | 0.39, 0.58 | 402 | 0.38 | 0.30, 0.47 | |
| Obese III (≥40) | 135 | 465 (89) | 394 (63) | 71 (84) | 0.43 | 0.28, 0.56 | 399 | 0.30 | 0.20, 0.41 | |
| Insomnia Severity Index category | ||||||||||
| Severe | 89 | 460 (100) | 418 (77) | 42 (87) | 0.54 | 0.38, 0.67 | 426 | 0.42 | 0.28, 0.55 | 0.16 |
| Moderate | 265 | 456 (87) | 403 (68) | 55 (88) | 0.37 | 0.26, 0.47 | 409 | 0.29 | 0.21, 0.38 | |
| Subthreshold | 471 | 474 (76) | 410 (62) | 64 (72) | 0.48 | 0.40, 0.54 | 412 | 0.39 | 0.32, 0.45 | |
| None | 1,258 | 474 (72) | 402 (58) | 72 (71) | 0.41 | 0.37, 0.46 | 404 | 0.33 | 0.29, 0.37 | |
| Apnea-hypopnea index score | ||||||||||
| ≤5 (no sleep apnea) | 1,516 | 474 (75) | 407 (61) | 66 (75) | 0.40 | 0.36, 0.44 | 409 | 0.32 | 0.29, 0.36 | 0.30 |
| >5–<15 (mild sleep apnea) | 388 | 462 (79) | 395 (60) | 66 (73) | 0.48 | 0.40, 0.55 | 402 | 0.36 | 0.30, 0.43 | |
| ≥15 (moderate/severe sleep apnea) | 182 | 474 (84) | 400 (68) | 75 (78) | 0.49 | 0.37, 0.59 | 402 | 0.39 | 0.29, 0.50 | |
| Epworth Sleepiness Scale score | ||||||||||
| ≥10 | 386 | 453 (78) | 383 (65) | 70 (82) | 0.35 | 0.26, 0.44 | 391 | 0.29 | 0.22, 0.37 | 0.27 |
| <10 | 1,700 | 476 (76) | 409 (59) | 66 (73) | 0.43 | 0.39, 0.47 | 411 | 0.34 | 0.30, 0.37 | |
| Day-to-day variability, hours/day | ||||||||||
| <1.5 | 1,565 | 470 (73) | 408 (59) | 62 (68) | 0.48 | 0.44, 0.52 | 412 | 0.39 | 0.35, 0.42 | <0.0001 |
| ≥1.5 | 521 | 476 (86) | 395 (67) | 81 (91) | 0.32 | 0.24, 0.39 | 396 | 0.25 | 0.18, 0.31 | |
| Sleep efficiency | ||||||||||
| <85% | 731 | 478 (85) | 383 (64) | 95 (84) | 0.39 | 0.33, 0.45 | 383 | 0.29 | 0.24, 0.34 | 0.0002 |
| ≥85% | 1,355 | 468 (71) | 416 (57) | 52 (65) | 0.51 | 0.47, 0.55 | 421 | 0.40 | 0.37, 0.44 | |
| Employment status | ||||||||||
| Employed, non–shift worker | 943 | 463 (67) | 401 (54) | 62 (65) | 0.44 | 0.38, 0.49 | 407 | 0.36 | 0.31, 0.40 | 0.25 |
| Shift worker | 280 | 456 (83) | 387 (66) | 72 (86) | 0.35 | 0.24, 0.44 | 393 | 0.28 | 0.19, 0.37 | |
| Unemployed or retired | 863 | 485 (83) | 414 (65) | 71 (81) | 0.42 | 0.36, 0.47 | 412 | 0.33 | 0.28, 0.38 | |
| Educational attainment | ||||||||||
| Less than high school/GED | 664 | 478 (82) | 410 (65) | 68 (75) | 0.50 | 0.44, 0.56 | 410 | 0.40 | 0.35, 0.45 | 0.01 |
| High school/GED | 539 | 471 (76) | 404 (58) | 67 (75) | 0.40 | 0.32, 0.47 | 407 | 0.31 | 0.25, 0.37 | |
| More than high school/GED | 880 | 467 (73) | 401 (60) | 67 (75) | 0.37 | 0.31, 0.43 | 404 | 0.30 | 0.25, 0.35 | |
| Hispanic ethnic background | ||||||||||
| Cuban | 376 | 468 (78) | 403 (64) | 65 (76) | 0.44 | 0.35, 0.51 | 407 | 0.36 | 0.28, 0.43 | 0.25 |
| Dominican | 261 | 464 (78) | 405 (58) | 59 (78) | 0.37 | 0.26, 0.47 | 409 | 0.27 | 0.19, 0.36 | |
| Mexican | 561 | 480 (69) | 414 (57) | 66 (66) | 0.46 | 0.39, 0.52 | 414 | 0.38 | 0.32, 0.44 | |
| Puerto Rican | 428 | 476 (87) | 401 (71) | 75 (85) | 0.44 | 0.36, 0.51 | 402 | 0.36 | 0.29, 0.43 | |
| Central American | 284 | 469 (74) | 400 (52) | 69 (72) | 0.38 | 0.28, 0.48 | 403 | 0.27 | 0.19, 0.35 | |
| South American | 176 | 459 (67) | 393 (60) | 65 (72) | 0.36 | 0.23, 0.49 | 400 | 0.32 | 0.20, 0.45 | |
| Nativity | ||||||||||
| Mainland US | 343 | 476 (83) | 398 (67) | 79 (87) | 0.34 | 0.25, 0.43 | 399 | 0.28 | 0.20, 0.36 | 0.12 |
| Outside mainland US | ||||||||||
| Had lived in US for ≥10 years | 539 | 473 (76) | 408 (61) | 65 (73) | 0.44 | 0.37, 0.51 | 406 | 0.35 | 0.29, 0.41 | |
| Had lived in US for <10 years | 1,197 | 466 (73) | 401 (58) | 65 (70) | 0.45 | 0.40, 0.49 | 410 | 0.36 | 0.32, 0.40 | |
| Language preference | ||||||||||
| English | 424 | 475 (85) | 399 (68) | 76 (87) | 0.37 | 0.28, 0.45 | 400 | 0.3 | 0.23, 0.37 | 0.12 |
| Spanish | 1,662 | 471 (74) | 406 (59) | 65 (72) | 0.45 | 0.41, 0.48 | 409 | 0.36 | 0.32, 0.39 | |
| Depressive symptoms | ||||||||||
| CES-D-10 score ≥10 | 643 | 473 (75) | 403 (58) | 70 (72) | 0.42 | 0.36, 0.48 | 411 | 0.35 | 0.29, 0.41 | 0.56 |
| CES-D-10 score <10 | 1,443 | 468 (81) | 407 (68) | 61 (81) | 0.43 | 0.39, 0.47 | 406 | 0.33 | 0.30, 0.37 | |
| Use of sleep medication, times/week | ||||||||||
| ≥1 | 293 | 471 (75) | 402 (60) | 69 (73) | 0.38 | 0.28, 0.47 | 419 | 0.29 | 0.21, 0.38 | 0.19 |
| <1 | 1,793 | 473 (85) | 417 (66) | 55 (86) | 0.44 | 0.40, 0.47 | 405 | 0.35 | 0.32, 0.38 | |
| Current smoker | ||||||||||
| Yes | 402 | 467 (84) | 394 (72) | 74 (88) | 0.36 | 0.27, 0.44 | 398 | 0.31 | 0.23, 0.39 | 0.34 |
| No | 1,683 | 473 (75) | 407 (58) | 66 (71) | 0.45 | 0.41, 0.48 | 410 | 0.35 | 0.31, 0.38 | |
Abbreviations: CES-D-10, 10-item Center for Epidemiologic Studies Depression Scale; CI, confidence interval; GED, General Educational Development; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; SD, standard deviation; US, United States.
a Actigraph-measured time spent asleep was the weighted average over weekends and weekdays of the time spent asleep during the main rest period, excluding periods of wakefulness.
b Interaction P value for the product of the participant characteristic and self-reported habitual sleep duration.
c Self-reported sleep duration was the weighted average of weekend and weekday self-reported habitual amount of time spent in bed minus wake time measured at the Sueño visit of the HCHS/SOL.
d The regression intercept represents bias, the disparity between subjects’ reported sleep duration and their actigraphy-measured values. If subjective and objective reports of sleep matched perfectly, there would be no bias and the intercept would be 0 minutes. We report the intercept at the average of 480 minutes (or 8 hours) of self-reported sleep.
e Weight (kg)/height (m)2.
Self-report measurements did not uniformly overestimate actigraphy-assessed time spent asleep; self-reports underestimated actigraphy-assessed time asleep for 17% of participants. On average, self-reports of ≤6 hours were underestimates of actigraphy-assessed time spent asleep, while self-reports of >6 hours were overestimates (Figure 1). However, sensitivity analyses provided no evidence of nonlinear associations of self-reported sleep duration with actigraphy measurements (likelihood ratio test: P > 0.05).
Figure 1.
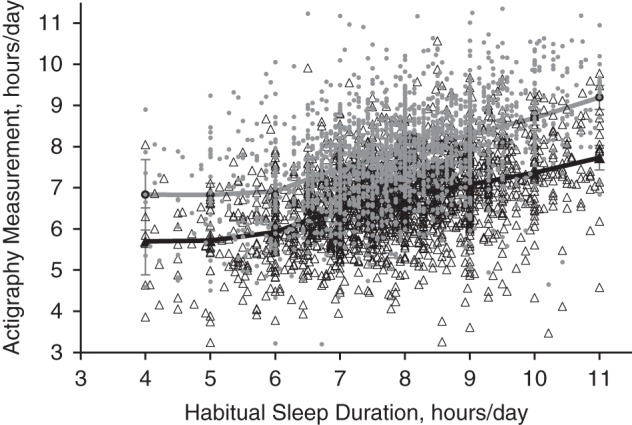
Actigraphy-assessed amounts of time spent asleep and time spent in bed per day, by self-reported sleep duration, in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sueño ancillary study (n = 2,086), 2010–2013. Self-reported sleep duration is plotted on the x-axis. White triangles represent individual data points for the observed values of actigraphy-assessed time spent asleep, while gray dots represent individual data points for the observed values of actigraphy-assessed time spent in bed (y-axis). The black line represents mean values for actigraphy-assessed time spent asleep within 1-hour segments of self-reported habitual sleep duration, while the gray line represents mean values for actigraphy-assessed time spent in bed within 1-hour segments of self-reported habitual sleep duration. Bars, 95% confidence intervals.
As shown in Web Table 2, we then tested multivariable-adjusted regression models for actigraphy-assessed time asleep and time in bed. Self-reported sleep duration made the largest contribution to the overall model R2 (partial R2 =0.18), with minimal additional variance being explained by sociodemographic, health, or sleep characteristics (R2 rising to 0.32). Bias (the intercept) at a self-reported sleep duration of 8 hours was almost 1 hour. Holding self-reported sleep duration and other characteristics constant, males, participants with daytime sleepiness, persons with sleep efficiency <85%, persons with BMI ≥35, shift workers, and current smokers and drinkers spent 6–36 fewer minutes asleep per day. Persons who had insomnia, used sleep medication at least once per week, or were unemployed spent 7–22 more minutes asleep per day.
Table 4 shows results of a stepwise selection procedure using internal cross-validation to identify predictors of actigraphy-assessed time spent asleep given self-reported habitual sleep duration. From the list of candidate variables shown in Table 3 and Web Table 2, sex, daytime sleepiness, sleep efficiency, insomnia severity, and employment were selected as predictors of time asleep. The interaction of sex and sleep efficiency with self-reported sleep duration were also entered into the model. Results indicated that little information was added to the models by sociodemographic, health, and sleep characteristics, precluding the development of calibration models.
Table 4.
Beta Coefficients and R2 Values for Predictors of Actigraphy-Assessed Daily Amounts of Time Spent Asleep and in Bed, HCHS/SOL Sueño (n = 2,086), 2010–2013a,b,c
| Participant Characteristic | Partial R2 | Time Spent Asleep, minutes/day |
Partial R2 | Time Spent in Bed, minutes/day |
||
|---|---|---|---|---|---|---|
| β | SE | β | SE | |||
| Intercept | 436.93 | 3.93 | 468.01 | 3.09 | ||
| Self-reported sleep duration, centered at 480 minutes/day | 0.18 | 0.40 | 0.04 | 0.23 | 0.35 | 0.03 |
| Sex | ||||||
| Male | 0.02 | −11.21 | 3.48 | |||
| Male × self-reported sleep duration | 0 | −0.12 | 0.04 | |||
| Insomnia Severity Index | ||||||
| Subthreshold | 0 | −13.99 | 3.15 | 0 | 17.85 | 4.79 |
| Moderate | 0 | 1.21 | 3.74 | 0 | 17.61 | 5.86 |
| Severe | 0 | −2.96 | 4.24 | 0.01 | 46.65 | 9.90 |
| Epworth Sleepiness Scale score ≥10 | 0.02 | −22.00 | 4.37 | 0.01 | −24.83 | 4.90 |
| Sleep efficiency | ||||||
| <85% | 0.08 | −40.40 | 3.61 | |||
| <85% × self-reported sleep duration | 0 | −0.12 | 0.05 | |||
| Employment status | ||||||
| Shift worker | 0 | −11.11 | 4.98 | 0.01 | −12.13 | 5.58 |
| Unemployed | 0 | 8.38 | 3.82 | 0.01 | 11.34 | 4.26 |
| Full-model R2 | 0.31 | 0.28 | ||||
| Root mean squared error | 50.69 | 57.01 | ||||
Abbreviations: HCHS/SOL, Hispanic Community Health Study/Study of Latinos; SE, standard error.
a Each column (time asleep and time in bed) represents a separate statistical model with adjustment for all of the variables in the column. The β coefficients were estimated through stepwise selection with the full list of candidate variables plus the interaction of each variable with self-reported sleep duration.
b Reference categories for selected variables were: female sex; Epworth Sleepiness Scale score <10; no clinical insomnia; and employed non–shift worker.
c All terms were statistically significant at the P < 0.05 level.
Table 5 shows κ scores indicating the level of agreement among subjects ranked in the low, medium, or high tertile of actigraphy-assessed time asleep given the participant's tertile of self-reported sleep duration, overall and by subgroup. Agreement between actigraphy and self-reported sleep duration was poor (κ values ranged from 0.17 to 0.33).
Table 5.
Scores for Agreement Between Tertilesa of Actigraphy-Assessed Time Spent Asleep and Self-Reported Sleep Duration, According to Selected Participant Characteristics, HCHS/SOL Sueño (n = 2,086), 2010–2013b
| Participant Characteristic | Total No. of Persons | Time Spent Asleep |
Time Spent in Bed |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed Agreement |
κ | 95% CI | Observed Agreement |
κ | 95% CI | ||||
| No. of Persons | % | No. of Persons | % | ||||||
| Overall | 2,086 | 0.23 | 0.20, 0.27 | 0.27 | 0.24, 0.31 | ||||
| Sex | |||||||||
| Male | 735 | 129 | 54 | 0.19 | 0.14, 0.24 | 381 | 52 | 0.28 | 0.22, 0.33 |
| Female | 1,351 | 682 | 50 | 0.26 | 0.22, 0.30 | 694 | 51 | 0.27 | 0.23, 0.31 |
| Age, years | |||||||||
| 18–44 | 724 | 337 | 47 | 0.2 | 0.15, 0.25 | 347 | 48 | 0.22 | 0.16, 0.27 |
| 45–64 | 1,362 | 685 | 50 | 0.25 | 0.21, 0.29 | 728 | 53 | 0.3 | 0.26, 0.34 |
| Body mass indexccategory | |||||||||
| Obese (≥30) | 890 | 454 | 51 | 0.26 | 0.21, 0.31 | 462 | 52 | 0.28 | 0.23, 0.33 |
| Nonobese (<30) | 1,196 | 568 | 47 | 0.21 | 0.17, 0.25 | 613 | 51 | 0.27 | 0.23, 0.31 |
| Insomnia severity | |||||||||
| Severe | 89 | 49 | 55 | 0.33 | 0.18, 0.47 | 51 | 57 | 0.36 | 0.22, 0.51 |
| Nonsevere | 1,997 | 973 | 49 | 0.23 | 0.20, 0.26 | 1,024 | 51 | 0.27 | 0.24, 0.30 |
| Day-to-day variability, hours | |||||||||
| ≥1.5 | 521 | 232 | 45 | 0.17 | 0.11, 0.23 | 234 | 45 | 0.18 | 0.11, 0.24 |
| <1.5 | 1,565 | 790 | 50 | 0.26 | 0.22, 0.29 | 841 | 54 | 0.31 | 0.30, 0.34 |
| Sleep efficiency | |||||||||
| <85% | 731 | 328 | 45 | 0.18 | 0.13, 0.23 | 368 | 50 | 0.25 | 0.20, 0.30 |
| ≥85% | 1,355 | 694 | 51 | 0.27 | 0.23, 0.31 | 707 | 52 | 0.28 | 0.24, 0.32 |
| Educational attainment | |||||||||
| Less than high school diploma/GED | 664 | 348 | 52 | 0.28 | 0.23, 0.34 | 355 | 53 | 0.3 | 0.24, 0.36 |
| High school diploma/GED or more | 1,422 | 674 | 47 | 0.21 | 0.17, 0.25 | 720 | 51 | 0.26 | 0.22, 0.30 |
Abbreviations: CI, confidence interval; GED, General Educational Development; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; SD, standard deviation.
a Mean values for low, intermediate, and high tertiles were as follows: for self-reported habitual sleep duration, 6.47 (SD, 0.73), 7.86 (SD, 0.29), and 9.24 (SD, 0.66) hours/day; for actigraph-assessed amount of time spent asleep, 5.64 (SD, 0.64), 6.78 (SD, 0.23), and 7.81 (SD, 0.55) hours/day; and for actigraph-assessed amount of time spent in bed, 6.65 (SD, 0.65), 7.85 (SD, 0.25), and 9.05 (SD, 0.64) hours/day.
b Self-reported sleep duration was calculated as the weighted average of weekday and weekend habitual amounts of time spent in bed minus wake time; actigraphy-measured amounts of time spent in bed and in sleep were also weighted averages of weekend and weekday measurements.
c Weight (kg)/height (m)2.
Web Figure 1 shows the receiver operating characteristic curves predicting tertile of actigraphy-assessed sleep duration from tertile of self-reported sleep duration, with and without adjustment for covariates. The probability that a randomly selected individual would be correctly classified as having an actigraphy-assessed sleep time in the lowest tertile (short sleep) based on tertile of self-reported sleep duration was 0.62. The corresponding probability for actigraphy-assessed sleep time in the highest tertile (long sleep) was 0.64. Both probabilities increased slightly, to 0.68, with addition of the covariates shown in Table 3.
In Sueño, sleep duration was reported as bed time minus wake time. However, not all time in bed is spent asleep; to assess the influence of this on our results, we repeated all analyses using actigraphy-measured time in bed in place of time asleep. As anticipated, mean actigraphy-assessed time in bed was longer than time asleep and was similar to self-reported sleep duration at 7.85 (SD, 1.12) hours/day (Table 1). However, the correlation with self-reported sleep duration was only slightly higher than that for time asleep, at r = 0.48 (95% CI: 0.45, 0.52), and for each additional hour of self-reported sleep duration, time in bed increased by 25 minutes (95% CI: 24, 27) (Table 2). The addition of covariates to self-reported sleep duration improved the overall model R2 only slightly, from 0.23 to 0.30 (Web Table 2). In the stepwise selection procedure, at the same level of self-reported sleep duration, shift workers and participants with daytime sleepiness spent less time in bed, while those who had insomnia or were unemployed spent more time in bed (Table 4).
DISCUSSION
Study findings
In this diverse cohort of US Hispanics/Latinos, we found moderate correlations between self-reported habitual sleep duration and actigraphy-assessed time spent asleep (r = 0.43). To our knowledge, this is the largest study of sleep duration in Hispanics/Latinos to date and the only study we know of to identify predictors of actigraphy measurements from self-reported sleep duration in Hispanics/Latinos. Other studies have also observed moderate correlations between reported and actigraphy-assessed sleep, ranging from 0.31 to 0.47, but with few exceptions (7, 29), those studies have recruited older (8) and non-Hispanic white (9, 30, 31) populations.
One of the few studies examining racial/ethnic differences in the correlation between self-reported and actigraphy-measured sleep duration is CARDIA, which found higher correlations among white participants (r = 0.56, 95% CI: 0.44, 0.68) than among black participants (r = 0.29, 95% CI: 0.10, 0.48), suggesting the presence of important racial differences. In our study, the correlation between self-reports and actigraphy in a Hispanic/Latino population was intermediate to correlations reported for whites and blacks. Furthermore, the correlations did not differ significantly among Hispanic/Latino subgroups. This is important in interpreting the reported differences in the relationship between self-reported sleep duration and chronic disease outcomes. For example, in the National Health and Nutrition Examination Survey, the relationship between hypertension and self-reported short sleep duration was seen only among non-Mexican Hispanics/Latinos (32), while in the Hispanic Health and Nutrition Examination Survey, short sleep duration was associated with larger body size only among Mexican Americans (14).
In interpreting our findings, it is important to note that while some studies (e.g., CARDIA) based sleep self-assessments on a single question about the actual number of hours of sleep obtained on a typical night, habitual bed and wake times are commonly used to measure sleep duration in epidemiologic studies because participants may more easily report these habits than their hours of actual sleep (33, 34). However, not all time in bed is spent asleep. Consistent with this, the unadjusted difference between measured time asleep and self-reported sleep duration in our study exceeded 1 hour, while the difference between objectively measured time in bed and self-reported sleep duration was only 1 minute (though with substantial variation). Yet, correlations of actigraphy-assessed time asleep and time in bed with self-reported sleep duration were close (Pearson's ρ: ρ = 0.43 and ρ = 0.48), indicating that the measurement approach for self-reported sleep duration did not fully explain discrepancies between self-report and actigraphy assessments.
We observed higher correlations for weekdays than for weekends, perhaps due to consistent weekday routines enabling more accurate reporting. However, fewer nights of actigraphy recording were collected on weekends (median of 5 weekday nights of actigraphy vs. 2 weekend nights), which weakened the correlation with self-reported sleep duration.
Self-reported sleep duration made the largest contribution to the overall variance in actigraphy-assessed time spent asleep among all the variables we considered but only accounted for 18% of this variance; the model R2 rose to 32% with the addition of sociodemographic, sleep, and health characteristics. Thus, the addition of covariates to self-reported habitual sleep duration did not result in a model that explained sufficient variation in actigraphy, making it challenging to develop calibration equations.
When investigators conducting prior research in nutrition attempted to improve the accuracy of self-report measures of diet (compared with an objective measure such as a biomarker), often the addition of participant characteristics to the model substantially increased R2 (35, 36). For example, in the Women's Health Initiative, the addition of characteristics such as BMI and age increased the model R2 value for total energy intake from 4% for the food frequency questionnaire alone to 42% (36). One possibility is that the skills needed to report diet (e.g., portion estimation) are more complex than those needed to report habitual bed/wake times, leading to a less dramatic increase in R2 with the addition of participant characteristics to the model for actigraphy-assessed time spent asleep. Difficulty in estimating habitual sleep duration may arise, in part, from night-to-night variability. In keeping with this hypothesis, the correlation between self-reported and actigraphy-assessed sleep duration was lower among persons in the highest quartile of night-to-night variability (Pearson's ρ = 0.32), while participants in the lowest variability quartile (<0.8 hours) had the highest correlation (ρ = 0.54). However, when high-variability participants were excluded from sensitivity analyses, self-reporting was still only able to explain 31% of the variation in time spent asleep. Restricting the data to weekdays (when night-to-night sleep may be more consistent) yielded correlations (ρ = 0.44 vs. ρ = 0.43) and κ values (κ = 0.26 vs. κ = 0.23) similar to those of the weighted average results.
The association of self-reported sleep duration with actigraphy-assessed time spent asleep varied by sociodemographic, health, and sleep characteristics. Other studies have also observed overreporting of sleep duration relative to actigraphy, with greater discrepancies among men and persons with poor sleep quality (7, 29, 30). In our study, the difference between measured time asleep and self-reported sleep duration did not vary by sleep apnea severity or BMI. In contrast, there were differences identified by age, sex, nightly variability, sleep efficiency, sleep medication use, and smoking.
Other studies have noted that persons with higher BMI, sleep apnea, sleepiness, and depression report shorter sleep durations, which reduces the discrepancy between self-reported and actigraphy-assessed sleep duration (7, 31). In keeping with this, we found that after controlling for self-reported sleep duration, males, shift workers, persons with BMI ≥35, low sleep efficiency, and excessive daytime sleepiness, current smokers, and current drinkers slept for fewer minutes each night, while those who were unemployed, had insomnia, or used sleep medication weekly or more often slept a greater number of minutes per night.
Strengths and limitations
To our knowledge, no prior study has validated self-reported sleep duration in such a large sample or among a diverse group of US Hispanics/Latinos. Compared with other validation studies, the active exclusion of participants with severe sleep apnea and the use of a state-of-the-science actigraphy device with a light sensor and off-wrist detection for increased accuracy represent important strengths (7). Additionally, we included more than 5 days of actigraphy and measured key sleep characteristics, including insomnia, sleep apnea severity, and sleep medication use. The fact that our study population was comprised entirely of Hispanics/Latinos addresses an important gap in sleep research, but it could limit generalizability to other ethnic groups if cultural attitudes or home/neighborhood environments modify reporting of sleep duration relative to actigraphy. Notably, while total sleep time measured by actigraphy and polysomnography have good agreement (r = 0.90) (37, 38), actigraphy is not itself a gold standard measure and may misinterpret inactivity during wake time as sleep, or vice versa. However, polysomnography over the course of multiple nights is impractical in large studies because of the participant burden and expense (39). Additionally, we used an average of 7 nights of actigraphy to estimate habitual sleep duration. Though 7 nights is considered to have reasonable reliability, nightly variation in sleep or episodic variation in sleep duration (e.g., “good weeks” and “bad weeks”) undoubtedly weakens the correlation with self-reported sleep duration, which is based on questions asking explicitly about habitual or “usual” sleep patterns (40). Finally, we asked participants to report bed/wake times but not sleep-onset latency, which may limit comparability with studies in which participants estimated the actual hours of sleep.
Conclusions
In this cohort of US Hispanics/Latinos, the correlation between self-reported sleep duration and actigraphy-assessed sleep duration was moderate. We confirmed the presence of systematic bias in self-reported sleep duration and showed that participant characteristics (including sex, age, sleep efficiency, and night-to-night variability) influence the association of self-reported sleep duration with actigraphy-measured time spent asleep. However, adding these and other characteristics to models provided little additional information to explain the variation in actigraphy-assessed sleep. Future research comparing associations of subjectively and objectively measured sleep duration with health outcomes and/or using actigraphy measurements in a subset of participants to correct measurement error would be informative as to the degree to which reporting bias influences the observed relationship between sleep duration and health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Departments of Nutrition and Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Elizabeth M. Cespedes, Frank B. Hu); Division of Research, Kaiser Permanente Northern California, Oakland, California (Elizabeth M. Cespedes); Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women's Hospital, Boston, Massachusetts (Susan Redline, Rui Wang, Sanjay R. Patel); Channing Division of Network Medicine, Harvard Medical School, Boston, Massachusetts (Bernard Rosner, Rui Wang); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Bernard Rosner, Rui Wang); Department of Medicine, Columbia University Medical Center, New York, New York (Carmela Alcantara); School of Social Work, Columbia University, New York, New York (Carmela Alcantara); Collaborative Studies Coordinating Center, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Jianwen Cai, Daniela Sotres-Alvarez); Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania (Martica H. Hall); Division of Pulmonary and Critical Care Medicine, Department of Medicine, School of Medicine, University of California, San Diego, San Diego, California (Jose S. Loredo); Sleep Medicine Section, Medicine Service, VA San Diego Healthcare System, San Diego, California (Jose S. Loredo); Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York (Yasmin Mossavar-Rahmani, Neomi A. Shah); Department of Neurology, Miller School of Medicine, University of Miami, Miami, Florida (Alberto R. Ramos); Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kathryn J. Reid); Department of Medicine, Montefiore Medical Center, New York, New York (Neomi A. Shah); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Phyllis C. Zee); and Institute for Minority Health Research, University of Illinois at Chicago, Chicago, Illinois (Phyllis C. Zee).
The Sueño ancillary sleep study was supported by a National Heart, Lung, and Blood Institute (NHLBI) grant (HL098297) to Brigham and Women's Hospital. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts between the NHLBI and the University of North Carolina (N01-HC65233), the University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes/centers/offices contributed to the Hispanic Community Health Study/Study of Latinos through a transfer of funds to the NHLBI: the National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health Office of Dietary Supplements. E.M.C. was supported by grant T32 DK007703 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Conflict of interest: none declared.
REFERENCES
- 1.Kurina LM, McClintock MK, Chen JH et al. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol. 2013;236:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diab Endocrinol. 2015;31:52–62. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H, Yin JY, Yang WS et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac J Cancer Prev. 2013;1412:7509–7515. [DOI] [PubMed] [Google Scholar]
- 4.Jackson CL, Redline S, Kawachi I et al. Association between sleep duration and diabetes in black and white adults. Diabetes Care. 2013;3611:3557–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CL, Hu FB, Redline S et al. Racial/ethnic disparities in short sleep duration by occupation: the contribution of immigrant status. Soc Sci Med. 2014;118:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall MH, Matthews KA, Kravitz HM et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN Sleep Study. Sleep. 2009;321:73–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Lauderdale DS, Knutson KL, Yan LL et al. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;196:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SR, Blackwell T, Ancoli-Israel S et al. Sleep characteristics of self-reported long sleepers. Sleep. 2012;355:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girschik J, Fritschi L, Heyworth J et al. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;225:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Onge MP, Perumean-Chaney S, Desmond R et al. Gender differences in the association between sleep duration and body composition: the Cardia Study. Int J Endocrinol. 2010;2010:726071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauderdale DS, Knutson KL, Rathouz PJ et al. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;1707:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SR, Sotres-Alvarez D, Castañeda SF et al. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;3810:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandner MA, Buxton OM, Jackson N et al. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;365:769–779e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson KL. Association between sleep duration and body size differs among three Hispanic groups. Am J Hum Biol. 2011;231:138–141. [DOI] [PubMed] [Google Scholar]
- 15.Loredo JS, Soler X, Bardwell W et al. Sleep health in U.S. Hispanic population. Sleep. 2010;337:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redline S, Sotres-Alvarez D, Loredo J et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;1893:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;208:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavange LM, Kalsbeek WD, Sorlie PD et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;208:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb DJ, Redline S, Nieto FJ et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;298:1009–1014. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR, Weng J, Rueschman M et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;389:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushida CA, Chang A, Gadkary C et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;25:389–396. [DOI] [PubMed] [Google Scholar]
- 22.Marino M, Li Y, Rueschman MN et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;3611:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoos T, Espinoza N, Marshall S et al. Validity of the Global Physical Activity Questionnaire (GPAQ) in adult Latinas. J Phys Act Health. 2012;95:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lind BK, Goodwin JL, Hill JG et al. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: experience of the Sleep Heart Health Study. Sleep Breath. 2003;71:13–24. [DOI] [PubMed] [Google Scholar]
- 25.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;146:540–545. [DOI] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;24:297–307. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;13:385–401. [Google Scholar]
- 28.Andresen EM, Malmgren JA, Carter WB et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;102:77–84. [PubMed] [Google Scholar]
- 29.Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;88:e71292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Den Berg JF, Van Rooij FJ, Vos H et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;173:295–302. [DOI] [PubMed] [Google Scholar]
- 31.Silva GE, Goodwin JL, Sherrill DL et al. Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS). J Clin Sleep Med. 2007;36:622–630. [PMC free article] [PubMed] [Google Scholar]
- 32.Grandner MA, Chakravorty S, Perlis ML et al. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;151:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasler G, Buysse DJ, Klaghofer R et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;274:661–666. [DOI] [PubMed] [Google Scholar]
- 34.Miller CB, Gordon CJ, Toubia L et al. Agreement between simple questions about sleep duration and sleep diaries in a large online survey. Sleep Health. 2015;12:133–137. [DOI] [PubMed] [Google Scholar]
- 35.Mossavar-Rahmani Y, Shaw PA, Wong WW et al. Applying recovery biomarkers to calibrate self-report measures of energy and protein in the Hispanic Community Health Study/Study of Latinos. Am J Epidemiol; 2015;18112:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentice RL, Mossavar-Rahmani Y, Huang Y et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;1745:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;62:113–124. [DOI] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Cole R, Alessi C et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;263:342–392. [DOI] [PubMed] [Google Scholar]
- 39.Curcio G, Ferrara M, Piergianni A et al. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;1155:1178–1188. [DOI] [PubMed] [Google Scholar]
- 40.Van Someren EJ. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 2007;163:269–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


