Abstract
The National Institute of Child Health and Human Development's Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be (nuMoM2b) Heart Health Study (HHS) was designed to investigate the relationships between adverse pregnancy outcomes and modifiable risk factors for cardiovascular disease. The ongoing nuMoM2b-HHS, which started in 2013, is a prospective follow-up of the nuMoM2b cohort, which included 10,038 women recruited between 2010 and 2013 from 8 centers across the United States who were initially observed over the course of their first pregnancies. In this report, we detail the design and study procedures of the nuMoM2b-HHS. Women in the pregnancy cohort who consented to be contacted for participation in future studies were approached at 6-month intervals to ascertain health information and to maintain ongoing contact. Two to 5 years after completion of the pregnancy documented in the nuMoM2b, women in the nuMoM2b-HHS were invited to an in-person study visit. During this visit, they completed psychosocial and medical history questionnaires and had clinical measurements and biological specimens obtained. A subcohort of participants who had objective assessments of sleep-disordered breathing during pregnancy were asked to repeat this investigation. This unique prospective observational study includes a large, geographically and ethnically diverse cohort, rich depth of phenotypic information about adverse pregnancy outcomes, and clinical data and biospecimens from early in the index pregnancy onward. Data obtained from this cohort will provide mechanistic and clinical insights into how data on a first pregnancy can provide information about the potential development of subsequent risk factors for cardiovascular disease.
Keywords: cardiovascular disease, cohort study, heart health, methods, pregnancy, women's health
Cardiovascular disease (CVD) is the leading cause of death among women in the United States and other developed countries (1). In addition, the US age-adjusted mortality rates for coronary heart disease and stroke are 24% and 30% higher, respectively, for non-Hispanic black women than for non-Hispanic white women (2). Evidence suggests that dyslipidemia, inflammation, and central adiposity are strongly related to the risk of heart disease among women (3–5). Identification of pathophysiology unique to women has considerable potential to inform CVD prevention strategies (6).
Hypertensive disorders (including preeclampsia and gestational hypertension) are common pregnancy complications with potentially major long-term implications (7). Evidence from epidemiologic studies supports an association between the development of preeclampsia and higher risks of hypertension, CVD, and renal diseases later in life (8–10). There is accumulating evidence that women who experience other adverse pregnancy outcomes (APOs), specifically preterm birth, neonates born small for their gestational ages, and gestational diabetes, also have a higher CVD risk later in life (11, 12). These findings have led to the characterization of pregnancy as a woman's first cardiovascular “stress test” (13, 14).
Systemic abnormalities that occur during pregnancies complicated by preeclampsia, which typically resolve after delivery, include vascular dysfunction, coagulopathy, and liver and renal diseases (15). A “silent” period in which neither clinical hypertension nor CVD is evident can last many years. However, in several studies, researchers found ongoing evidence of endothelial dysfunction, dyslipidemia, metabolic syndrome, cardiac dysfunction, renal abnormalities, and insulin resistance in the absence of clinical disease (7, 16–27). Better understanding of the causal pathways and identification of markers of risk will enable investigators to study the evolution of cardiovascular abnormalities after pregnancy, as well as the effects of modifying factors before the onset of clinical disease (13).
The mechanistic pathways that lead to APOs and CVD share several common features. Systemic endothelial dysfunction is a hallmark of preeclampsia (28–30). Endothelial dysfunction also characterizes the pathway to the development of atheromatous plaque and involves the contributions of dyslipidemia, inflammation, insulin resistance, and prothrombotic processes (31–34). Among women with preeclampsia, vascular changes in uterine spiral arteries resemble the early stages of atherosclerosis (35).
Prior epidemiologic studies in which the link between APOs and CVD were evaluated have generally involved large US or international registry databases with limited clinical details or prospective cohorts that lacked sufficient sample sizes to evaluate APOs such as preeclampsia (27, 36–38). Additionally, very few included biologic markers measured during pregnancy. The largest of these cohorts were assembled outside of the United States. The National Institute of Child Health and Human Development's Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be (nuMoM2b) included a large, diverse, US-based cohort from which biological specimens and data have been longitudinally collected and in which pregnancy outcomes have been systematically characterized.
Sleep-disordered breathing (SDB) refers to a group of disorders characterized by abnormal respiratory patterns (e.g., apneas, hypopneas, and periodic breathing) or the quantity of ventilation during sleep (39, 40). In addition to functional impairments, SDB has been linked to other adverse health outcomes in nonpregnant populations, principally CVD and metabolic disease (41–48). In several large epidemiologic studies, it has been estimated that SDB outside of pregnancy confers a 1.4- to 4.0-fold higher risk of diabetes, hypertension, CVD, and CVD-related mortality (41–51).
Pregnancy has been associated with several alterations in sleep patterns and a high incidence of sleep disturbances (52–55). Emerging evidence has linked SDB during pregnancy to APOs (56–59). Postpartum weight retention might contribute to the persistence of incident SDB in the postpartum period and beyond. There have been no large prospective studies with objective measures that were focused on SDB in early pregnancy, its trajectory during pregnancy and postpartum, or its role in mediating CVD risk in women. Improved understanding of the relationships among pregnancy, SDB, and CVD risk in women represents an opportunity for prevention.
nuMoM2b researchers recruited a large and diverse cohort of 10,038 nulliparous women in their first trimesters of pregnancy at 8 US sites between 2010 and 2013 in order to study the mechanisms for and prediction of APOs (60). Women underwent serial assessments over the course of their pregnancies. In nuMoM2b, APOs were defined as any hypertensive disease of pregnancy, preterm birth, and fetal growth restriction. Data were collected at 3 antenatal study visits (6 weeks 0 days to 13 weeks 6 days, 16 weeks 0 days to 21 weeks 6 days, and 22 weeks 0 days to 29 weeks 6 days of gestation) and at delivery. Data were collected via structured interviews, self-administered questionnaires, clinical measurements, ultrasounds, and medical records review. In addition, biospecimens were collected throughout pregnancy and at delivery. The mean maternal age at enrollment for the cohort was 26.9 (standard deviation, 5.7) years. Of the women in the cohort, 59.7% were non-Hispanic white, 14.2% were non-Hispanic black, 16.9% were Hispanic, 4.0% were Asian, and 5.1% were of other races (60). Of the original cohort, 8,830 women had data available for index pregnancy outcomes and agreed to future contact. A subcohort of 3,712 women participated in a SDB substudy that included self-administered overnight sleep breathing assessments (61).
This established nuMoM2b cohort provides a unique opportunity to examine the association between APOs and women's cardiovascular health later in life through prospective follow-up and examination of CVD risk factors and outcomes. The overarching goal of the ongoing nuMoM2b Heart Health Study (HHS), which began in 2013, is to better define the relationships between outcomes of pregnancy and longer-term cardiovascular health of the mother, with emphasis on prediction and mechanisms, in an effort to develop future CVD screening and preventive strategies that involve modifiable CVD risk factors. The inclusion of a longitudinal evaluation of SDB during pregnancy and postpartum is another novel aspect.
Data and biospecimens collected in this follow-up study are linked to those collected during pregnancy in the nuMoM2 and will further enable a mechanistic investigation into these complex pathways between APOs and CVD risk factors. Some of the key aims and hypotheses are listed in Table 1.
Table 1.
Key Study Aims and Hypotheses, Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be Heart Health Study, 2010–2013
| Aim | Related Hypotheses |
|---|---|
| Define the incidence of hypertension and the CVD risk profile of women 2–5 years after a first pregnancy complicated by preeclampsia or other APOs (stillbirth, babies born small for gestational age, preterm birth, preeclampsia, pregnancy-associated hypertension, and gestational diabetes) compared with women who had no APOs in the first pregnancies. |
|
| Identify a profile in early pregnancy that portends subsequent CVD risk at 2–5 years postpartum. |
|
| Determine whether prenatal and postpartum SBD is associated with increased CVD risk and identify specific patterns of prenatal and postpartum SDB that increase CVD risk. |
|
| Identify modifiable factors that influence or mediate the associations between APOs and CVD risk during pregnancy and 2–5 years postpartum. |
|
Abbreviations: APO, adverse pregnancy outcome; BMI, body mass index; CVD, cardiovascular disease; SDB, sleep-disordered breathing.
METHODS
Participants and inclusion and exclusion criteria
All women who completed the nuMoM2b at a participating nuMoM2b-HHS site are eligible to participate if they had obstetrical delivery information available, agreed to be contacted for future studies during the nuMoM2b and did not subsequently withdraw consent, and are 18 years of age or older (Figure 1). Women are eligible for telephone or e-mail contact and interviews at 6-month intervals if they provide verbal consent when contacted. Online surveys (with online consent) that allow for self-interview are also made available. Women are considered enrolled in the nuMoM2b-HHS once they provide this initial verbal or online consent.
Figure 1.
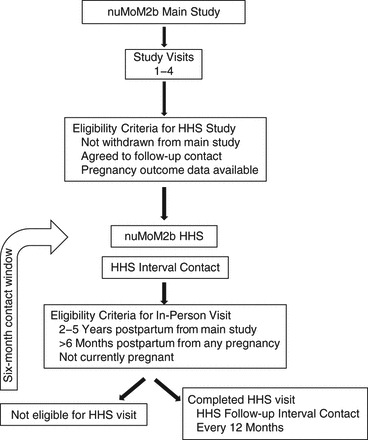
Flow chart for the Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be (nuMoM2b) Study (2010–2013) and the ongoing nuMoM2b-Heart Health Study (HHS).
Women are eligible for an in-person nuMoM2b-HHS visit if they participate in at least 1 telephone/online interview, 2–5 years have elapsed since the nuMoM2b pregnancy ended, they are at least 6 months postpartum from any subsequent pregnancy, they are not currently pregnant, and they are able to provide informed consent. The target enrollment for the in-person nuMoM2b-HHS visit is 5,000 women.
Women who successfully completed at least 1 of the sleep breathing assessments in the SDB substudy during their nuMoM2b pregnancy are screened for eligibility to participate in nuMoM2b-HHS SDB assessment after completing their in-person nuMoM2b-HHS visit. Participants cannot be using positive airway pressure therapy or other approved treatments for sleep apnea or oxygen supplementation to treat a medical condition and cannot have used continuous oral steroid therapy for the previous 14 days or more to treat asthma. The enrollment goal for the nuMoM2b-HHS SDB assessment is 1,332 women.
Study procedures
Elements of the interval contacts
Study personnel attempt to contact all potentially eligible nuMoM2b participants in the first 6 months of recruitment. A variety of communication methods are utilized, including mail, telephone, e-mail, social media, and a study website. Women who agree to participate will answer study questions and then will continue to be contacted at 6-month intervals. Women who are eligible are invited to participate in the in-person CVD assessment study and SDB substudy. In addition, contact information is updated to facilitate future contacts. After the in-person study CVD visit, the contact interval is extended to every 12 months.
During the interval contacts, women are asked about access to health care, weight, the child from the index nuMoM2b pregnancy, breastfeeding status and duration, and subsequent pregnancies and adverse outcomes. Participants self-report information about any diagnoses of and medication use for hypertension, heart problems, stroke, pulmonary embolism, deep vein thrombosis, peripheral vascular disease, diabetes, or kidney disease. Appendix 1 lists the data collected during the interval contacts.
In-person nuMoM2b-HHS visit
One in-person nuMoM2b-HHS visit is conducted 2–5 years after the nuMoM2b pregnancy ended, and it generally lasts 1–1.5 hours. A urine pregnancy test is performed to confirm participant eligibility. Baseline data are collected on demographic characteristics, access to health care, current medication and supplement use, contraceptive history, updated family medical history, exposure to secondhand smoke, physical activity level, and use of alcohol, tobacco, and illegal drugs. Participants complete validated self-administered questionnaires detailing food intake frequency, sleep (sleep duration and sleep apnea symptoms), eating habits, usual physical activities, urinary incontinence, perceived stress, symptoms of depression, and gynecological health.
Detailed CVD and related medical histories are obtained at the study visit. Women are asked questions to ascertain whether they have been diagnosed with hypertension, heart problems (e.g., myocardial infarction, coronary artery disease, heart failure, stroke), transient ischemic attack, venous thromboembolism (deep venous thrombosis or pulmonary embolus), diabetes, or kidney disease and whether they have undergone cardiovascular procedures (e.g., echocardiogram, coronary angioplasty) or peripheral vascular disease procedures (for diagnosis or treatment). To the extent possible, we use questions that have been previously demonstrated to be predictive of a clinical diagnosis or that were standardized in large cohort studies (9, 62). The dates and places of diagnosis, relevant procedures, and hospitalizations are collected so that medical releases can be obtained to authorize medical record review to confirm the self-reported diagnoses. Biometric data and specimens are collected from the women as described below. Tables 2 and 3 display the procedures administered and measurements, questionnaires, and biospecimens obtained at the in-person nuMoM2b-HHS visit.
Table 2.
Question Domains, Samples, and Clinical Evaluations Performed in the Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be Study and the Heart Health Study, 2010–2013
| Question Domains, Samples, and Clinical Evaluations |
Pregnancy Trimester (nuMoM2b)a |
Postpartum (nuMoM2b-HHS) |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Delivery | Interval Contact | 2–5 Year In-Person NuMoM2b-HHS Visit | |
| Demographic characteristics | Yes | Yes | ||||
| Demographic characteristics of participant/changes | Yes | Yes | Yes | Yes | ||
| Background of participant's parents | Yes | |||||
| Demographic characteristics of the father of baby and background of father of baby's parents (if appropriate) | Yes | |||||
| Standard instruments | Yes | Yes | Yes | Yes | ||
| Medical history | Yes | Yes | ||||
| Participant's history | Yes | Yes | Yes | Yes | Yes | |
| Family history | Yes | Yes | Yes | Yes | Yes | |
| Medications and supplements | Yes | Yes | Yes | Yes | Yes | |
| Substance use (alcohol, tobacco, or illegal drugs) | Yes | Yes | Yes | Yes | ||
| Subsequent pregnancies | Yes | |||||
| Other psychological factors | ||||||
| Reactions to raceb | Yes | |||||
| Pregnancy intendedness | Yes | |||||
| Difficulties in pregnancy | Yes | Yes | ||||
| Relationship with father of baby | Yes | Yes | Yes | |||
| Symptoms or diagnoses between the third study visit and the admission for delivery | Yes | |||||
| Participant assessment of delivery route/reasonsc | Yes | |||||
| Blood pressured | Yes | Yes | Yes | Yes | ||
| Weight | Yes | Yes | Yes | Yes | ||
| Height and waist, hip, and neck circumferences | Yes | Yes | ||||
| Ultrasound for crown-rump length | Yes | |||||
| Ultrasound for fetal biometry | Yes | Yes | ||||
| Ultrasound for cervical length | Yes | Yes | ||||
| Ultrasound for uterine artery Doppler measurement | Yes | Yes | ||||
| Biospecimens | ||||||
| Urine | Yes | Yes | Yes | Yes | ||
| Plasma and serum | Yes | Yes | Yes | Yes | Yes | |
| Whole bloode | Yes | Yes | ||||
| Cervicovaginal fluid | Yes | Yes | Yes | Yes | ||
| Cord blood or neonatal saliva for DNA | Yes | |||||
| Cord blood for plasma | Yes | |||||
| Placenta, fetal membranes, or umbilical cord segment | Yes | |||||
Abbreviations: HHS, Heart Health Study; nuMoM2b, Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be.
a Study visits were during the following gestational age intervals: first trimester, 6 weeks 0 days to 13 weeks 6 days; second trimester, 16 weeks 0 days to 21 weeks 6 days; and third trimester, 22 weeks 0 days to 29 weeks 6 days.
b Perceptions and reactions regarding racism experienced.
c Participants were asked why they thought they were admitted for their delivery to determine whether their feelings matched those in the chart.
d During the nuMoM2b-HHS visit (2–5 years after the index pregnancy), blood pressure was measured 3 times using an automated device. During the nuMoM2b pregnancy visits, blood pressure was measured using a manual device. It was generally measured once; however, if a systolic reading greater than 140 mm Hg or a diastolic reading greater than 90 mm Hg was obtained, the participant rested for 10 minutes and the test was repeated. The second blood pressure measurement was then reported.
e Whole blood for DNA (nuMoM2b visit) or biochemical assays (nuMoM2b-HHS visit).
Table 3.
Questionnaires Completed During the Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be Study and the Heart Health Study, 2010–2013
| Characteristic or Symptom Assessed | Instrument Name (Abbreviationa) | Pregnancy Trimester (nuMoM2b) |
Postpartum (nuMoM2b-HHS) |
||||
|---|---|---|---|---|---|---|---|
| First | Second | Third | Delivery | Interval Contact | 2–5 Years | ||
| Medical and social history | Yes | Yes | Yes | Yes | Yes | Yes | |
| Interval pregnancy history | Yes | ||||||
| Lactation | Yes | Yes | |||||
| Family history | Yes | Yes | |||||
| Physical activity | Behavioral Risk Factor Surveillance System (BRFSS) | Yes | Yes | Yes | Yes | ||
| Physical activity | Modifiable Activity Questionnaire (MAQ) | Yes | |||||
| Nutrition | Modified Block-Bodnar food frequency questionnaire | Yes | Yes | ||||
| Eating habits | Three Factor Eating Questionnaire (TFEQ) | Yes | |||||
| Depression | Edinburg Postnatal Depression Scale (EPDS) | Yes | Yes | Yes | |||
| State-trait anxiety | State-Trait Anxiety (STAI-T) | Yes | |||||
| Social support | Multidimensional Scale of Perceived Social Support (MSPSS) | Yes | |||||
| Stress | Perceived Stress Scale-10 question (PSS-10) | Yes | Yes | Yes | |||
| Health literacy | Rapid Estimate of Adult Literacy in Medicine (REALM) | Yes | |||||
| Experiences of discrimination | Experiences of Discrimination (EOD) | Yes | |||||
| Resilience | Connor-Davidson Resilience Scale (CD-RISC) | Yes | |||||
| Sleep scales | |||||||
| Sleep apnea | Berlin Questionnaire for Sleep Apnea | Yes | Yes | Yes | |||
| Insomnia | Women's Health Initiative Insomnia Rating Scale | Yes | Yes | Subsetb | |||
| Sleepiness | Epworth Sleepiness Scale | Yes | Yes | Subsetb | |||
| Restless leg syndrome | Restless Legs Syndrome Diagnostic Criteria | Yes | Yes | Subsetb | |||
| Sleep disturbance and impairment | Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance (SD) and Sleep-Related Impairment (SRI) item banks | Subsetb | |||||
a Abbreviations are not available for all instruments.
b Collected from subset of participants who completed a sleep assessment.
Clinical measurements
Standardized blood pressure measurements are performed at the study visit with the participant seated. Blood pressure is measured in the right arm. The appropriate cuff size is selected based on measured arm circumference at the midpoint of the upper arm. An automated device (OMRON HEM-907XL, Omron Healthcare Incorporated, Lake Forest, Illinois) is used to measure blood pressure unless the arm circumference is greater than 50 cm, in which case a thigh cuff and aneroid manometer is required. After a 5-minute rest period during which the participant sits with her feet on floor, her back supported by chair, and her arm resting on hard surface at heart level, blood pressure is measured 3 times. Between measurements is a 30-second rest period, during which the participant raises her arm with her hand unclenched for at least 5 seconds. All 3 measurements are recorded, and the averages of the last 2 systolic and diastolic pressures are recorded and provided to the participant.
Body composition is assessed as body mass index (weight in kilograms divided by height in meters squared) from measures of height obtained using a stadiometer and weight obtained using regularly calibrated balance beam scales. Waist circumference over the iliac crest and at the natural waist, hip circumference, and neck circumference are measured to the nearest 0.1 centimeter using a measuring tape that does not stretch. Each measurement is taken twice, and if the difference between the 2 is greater than 0.5 cm, a third measurement is recorded.
Questionnaires
A unique aspect of this study is the linkage of characteristics assessed during the index pregnancy, including self-reported health-related attributes and behaviors, to the emergence of CVD risk in the years after the pregnancy. Table 3 displays the instruments used in both the nuMoM2b and the nuMoM2b-HHS. At the nuMoM2b visit, physical activity was assessed using standardized leisure activity questions adapted from the Behavioral Risk Factor Surveillance System (63). In addition, an adapted version of the Modifiable Activity Questionnaire (64–66) was completed to assess leisure and occupational activities performed over the previous 12 months, as well as sedentary time. Nutrition was measured using the modified Block 2005 Food Frequency Questionnaire, which has been validated in many populations (67–71). In addition, eating habits were assessed using the disinhibition subscale of the Three Factor Eating Questionnaire (72–75). Symptoms of depression were measured using the validated Edinburgh Postnatal Depression Scale (76–83). The 10-question version of the Perceived Stress Scale was administered (84–88). All nuMoM2b-HHS participants complete the Berlin Questionnaire for Sleep Apnea (89), and the subset undergoing a home sleep test complete additional questionnaires (Women's Health Initiative Insomnia Rating Scale (90); Epworth Sleepiness Scale (91); restless legs syndrome diagnostic criteria (92); the Patient-Reported Outcomes Measurement Information System sleep disturbance and sleep-related impairment item banks) (93).
Biospecimens
Aliquots of urine, serum, whole blood, and plasma, as well as a sample for buffy coat, are stored locally at each site at −70°C degrees. Data are entered into a secure web-based data capture system. Multiple data quality checks are performed to ensure high-fidelity information in the database. The sites ship frozen specimens in batches to the study biorepository for long-term storage at −70°C. Samples will be assayed in batches for the analytes described in the Outcomes section.
Overnight SDB assessment
Participants in the nuMoM2b-HHS who complete in-person visits and who are eligible for and consent to the nuMoM2b-HHS SDB substudy are instructed on how to complete a self-administered overnight SDB assessment using the Embletta Gold portable sleep system (Natus Medical Incorporated, Pleasanton, California). All studies are scored at a central sleep reading center using the protocol in the nuMoM2b SDB study (61). The SDB notification protocol for this study was based on guidelines used in cohorts comprising nonpregnant women (94, 95).
Chart review of subsequent pregnancies
Using structured questionnaires adapted from surveys used by the Stillbirth Collaborative Research Network (96), participants are asked about any pregnancies subsequent to the index nuMoM2b pregnancy. Participants who self-report 1 or more APOs (as defined in nuMoM2b), stillbirth, or multiple gestation are asked for authorization to release medical records for the identified pregnancy (97). These records are obtained by research staff and abstracted for characterization and verification of pregnancy complications and outcomes using methods similar to those in the nuMoM2b parent study (60).
Outcomes
The primary outcome of the nuMoM2b-HHS is incident hypertension that developed after the nuMoM2b index pregnancy, as determined by blood pressure measurement and medication use at the 2–5 year postpartum study visit. The diagnostic threshold for hypertension will be based on current guidelines (98, 99). Prehypertension and systolic and diastolic blood pressures modeled as continuous variables are secondary outcomes.
Other cardiovascular risk factors include total cholesterol, high-density lipoprotein cholesterol, triglycerides, and calculated low-density lipoprotein cholesterol at the time of the study visit, as well as the trajectory of lipid levels in the time period between early in the index pregnancy and 2–5 years after delivery. Fasting blood glucose and insulin levels (for calculation of the homeostasis model assessment of insulin resistance) (100) will be measured, as will levels of glycated hemoglobin. Additional outcome measures include urine albumin/creatinine ratio, which is an indicator of subclinical renal disease and vascular dysfunction, and concentration of natriuretic peptide B, which is a measure of subclinical ventricular dysfunction (101). High-sensitivity C-reactive protein, a measure of inflammation, will also be assessed. The trajectory since early pregnancy may be assessed for glycated hemoglobin, high-sensitivity C-reactive protein, microalbuminuria, and potentially other markers.
The combination of medical histories and clinical and laboratory measurements also will enable evaluation of composite outcomes such as metabolic syndrome and CVD risk prediction scores. Because CVD events will be rare in this young female population, published CVD risk scores will provide a link to estimates of CVD risk as a function of CVD risk factors. These include the Framingham score (102), the Reynolds score (http://www.reynoldsriskscore.org), and the 2013 American College of Cardiology/American Heart Association atherosclerotic cardiovascular disease risk equations (103). A limitation of these scores is that none have been validated in a younger population. However, there is evidence that they may still be prognostic of future risk (104). To address this limitation, we also will evaluate risk scores calculated using the method of the Pathobiological Determinants of Atherosclerosis in Youth Study (105), which predicts coronary artery calcification rather than CVD events and is designed for a young-adult population (15–34 years of age).
We will also obtain information on some outcomes via self-reports from participants. Patients will be considered hypertensive if they reported having high blood pressure diagnosed by a doctor or other health professional on 2 or more health care visits or currently taking antihypertensive medication (106). Whether a patient has been diagnosed with diabetes by a doctor or other health care professional and whether the condition has been treated with medication also will be ascertained. Major CVD events will be ascertained by self-report and will facilitate further future evaluation (e.g., including medical record review). Diabetes and renal disease are independent outcomes of interest and also fall in the causal pathway towards development of CVD.
The primary outcome of interest for the SDB substudy is the apnea-hypopnea index, which is defined as the total number of apneas and hypopneas per hour of estimated sleep (including central and obstructive apneas) and the number of hypopneas associated with oxygen desaturation of 3% or more. Specifically, the primary outcome is an apnea-hypopnea index of 5 or higher (mild sleep apnea). The scoring procedures followed in the nuMoM2b SDB substudy will be utilized (61).
Statistical considerations
To relate the occurrence of APOs during the nuMoM2b index pregnancy to cardiovascular risk factors 2–5 years postpartum, we will exclude women who had chronic hypertension (approximately 1.8%) and pregestational diabetes (approximately 1.9%) in the index pregnancy (107). After the combined exclusion of 3.7% of the expected 5,000 women with an in-person study visit, 4,815 will remain for analysis. Web Table 1 (available at http://aje.oxfordjournals.org/) shows the minimum detectable hypertension risk ratios for specific APOs compared with a control group at 2–5 years postpartum without adjustment for covariates. Assuming a 2% incidence of hypertension at follow-up for the non-APO controls (108), unadjusted risk ratios from 2.7 to 2.1 can be detected for specific APOs, with prevalence ranging from 4% to 10%. This range is consistent with what we expect for the main APOs of interest: preeclampsia, 7% (109, 110); preterm birth, 8%; spontaneous preterm birth, 5%, and fetal growth restriction (5th, 10th percentile). Web Table 2 shows the detectable mean differences in various continuous measures of CVD risk at 2–5 years postpartum. Small differences in means will be detectable with the anticipated sample size.
There will be some ability to examine the impact of subsequent pregnancies (main associations and interactions) on the continuous outcomes. However, these associations might be complex, so an alternative analysis restricted to the subset of women without a second pregnancy will be performed. Based on birth spacing in the United States (111), we estimate that approximately 19.5% of participants who undergo an in-person visit will have had a completed second pregnancy more than 6 months before their in-person visit (Web Table 1).
Data collection and management
The Data Coordinating and Analysis Center's central database for the nuMoM2b-HHS contains all data records maintained for nuMoM2b participants who consented to participate in the nuMoM2b-HHS. The records are linked using the nuMoM2b identification number. This will allow for potential future studies (with appropriate consent by the participant and institutional review board approval).
The majority of the nuMoM2b-HHS data are collected by clinical site staff and entered via a secure website into electronic files located at the Data Coordinating and Analysis Center. Some questionnaires may be completed directly by participants via the website. Data entry is under the control of data entry programs within a data management system developed by the Data Coordinating and Analysis Center. Each individual accessing the website is provided a unique username and password. The website is secure with secure sockets layer (SSL) encryption.
Home sleep test data are uploaded from collection equipment to a dedicated computer at each clinical site and reviewed for completeness. Home sleep test data are transmitted electronically to a secure file transfer protocol (FTP) server at the Sleep Reading Center. The protocols and data monitoring procedures are the same as the ones used in the nuMoM2b SDB substudy (61).
Study personnel training
Study personnel at each clinical site underwent standardized training on study procedures. The training was provided by the Data Coordinating and Analysis Center, as well as by key clinical staff at local sites. Training in the setup and operation of the Embletta Gold home sleep test devices was done by study personnel, as described in the original nuMoM2b SDB substudy (61).
DISCUSSION
Although most women return to apparent normal health after a pregnancy complicated by preeclampsia or other APOs, current medical practice is not tailored to assess their potentially increased risk for subsequent hypertension, CVD, and renal disease (8, 9). Though both the American Heart Association and the American College of Obstetricians and Gynecologists recommend closer follow-up and evaluation of women who have hypertensive disorders in pregnancy (6, 112), there is little evidence on which to base prevention strategies and recommendations. The nuMoM2b-HHS will elucidate aspects of CVD risk associated with APOs and address gaps in knowledge in the years after a first pregnancy.
The nuMoM2b-HHS is a unique prospective observational study involving a large, geographically and ethnically diverse cohort, rich depth of phenotypic information regarding APOs, and clinical data and biospecimens that were collected starting early in the index pregnancy. Analyses of incident CVD risk factors and other outcomes collected during the early postpartum years will enable better risk stratification and are expected to help guide strategies for maternal follow-up and medical management. The designs of the study and of the data and specimen collection will support research to address these gaps and are expected to ultimately guide interventional studies and prevention programs in clinical or public health settings.
The nuMoM2b-HHS also serves as a platform for ancillary studies. The biospecimens collected provide great potential for a variety of mechanistic studies. In addition, certain self-reported clinical outcomes can be further evaluated (e.g., through medical record review) in future studies.
In addition to in-depth information about a woman's first pregnancy, some information about all subsequent pregnancies, including any APOs that were experienced, will be ascertained. In the short-term, this collection will facilitate evaluation of factors associated with recurrence of APOs (either the same or a different APO). In the longer term, if the nuMoM2b-HHS is continued beyond the initial study period, these data may become even more valuable because they could provide a complete picture of all of a woman's pregnancies, which would allow us to evaluate associations with CVD and related outcomes.
One of the unique features of the nuMoM2b cohort is that data and biospecimens were collected early in the first pregnancy, at 6–13 weeks of gestation. These early pregnancy features that were assessed before clinically evident findings arose later in pregnancy may help distinguish whether APOs are markers of predisposing factors that increase CVD risk or just causal contributors to that risk. In addition, the robust data and samples collected longitudinally during the index nuMoM2b pregnancy and at the time of delivery represent a valuable source of information about the changes that occur during pregnancy and how they might impact long-term health. No preconception measurements were performed in the nuMoM2b, but self-reported prepregnancy weight was recorded. Although a preconception cohort study is currently being initiated in China (113), the challenges in enrolling a preconception cohort are substantial (114). These include a longer period of observation, a major increase in sample size requirements (because only a subset of the women will become pregnant), and a lack of representativeness (because only women who report planning their first pregnancy in the near term will be included). Importantly, pregnancy marks a time of almost universal access to health care in the United States. Thus, pregnancy-related risk that portends future cardiovascular risk offers an opportunity to translate findings into methods to improve the health of women and their offspring.
Discovery of early pregnancy factors that increase long-term CVD risk might have a substantial impact on public health if they facilitate intervention earlier than does traditional CVD risk-factor screening or if they increase adherence to interventions because of messages that are better tailored for women. Just as the nuMoM2b researchers strived to find early biomarkers that would predict and allow patients to avert APOs, the nuMoM2b-HHS aims to discover factors in pregnancy and soon after delivery that facilitate risk stratification or that may serve as targets for intervention to prevent later CVD in women. Translating these findings into screening, preventive, and therapeutic interventions could impact the incidence of CVD in women.
ACKNOWLEDGMENTS
Author affiliations: Department of Obstetrics and Gynecology, School of Medicine, Indiana University, Indianapolis, Indiana (David M. Haas, Shannon E. Barnes); Department of Medicine, University of Wisconsin-Madison, Madison, Wisconsin (Deborah B. Ehrenthal); RTI International, Research Triangle Park, North Carolina (Matthew A. Koch, Corette B. Parker); Department of Obstetrics and Gynecology, University of Pittsburgh, Pittsburgh, Pennsylvania (Janet M. Catov, Francesca Facco, Hyagriv N. Simhan); Department of Obstetrics and Gynecology, Case Western Reserve University, Cleveland, Ohio (Brian M. Mercer); Division of Cardiology, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California (C. Noel Bairey-Merz); Department of Obstetrics and Gynecology, University of Utah and Intermountain Healthcare, Salt Lake City, Utah (Robert M. Silver); Department of Obstetrics and Gynecology, Columbia University, New York, New York (Ronald J. Wapner); Department of Obstetrics and Gynecology, Christiana Care, Wilmington, Delaware (Matthew K. Hoffman); Department of Obstetrics and Gynecology, Northwestern University, Chicago, Illinois (William A. Grobman); Division of Cardiology, Department of Medicine, Northwestern University, Chicago, Illinois (Philip Greenland); Department of Obstetrics and Gynecology, University of California, Irvine, Irvine, California (Deborah A. Wing); Department of Obstetrics and Gynecology, University of Texas Medical Branch, University of Texas, Galveston, Texas (George R. Saade); Department of Obstetrics and Gynecology, University of Pennsylvania, Philadelphia, Pennsylvania (Samuel Parry); Department of Neurology, Northwestern University, Chicago, Illinois (Phyllis C. Zee); Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Uma M. Reddy); and National Heart, Lung, and Blood Institute, Bethesda, Maryland (Victoria L. Pemberton, Dale R. Burwen).
This study is supported by cooperative agreement funding from the National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development: grant U10-HL119991 to RTI International; grant U10-HL119989 to Case Western Reserve University; grant U10-HL120034 to Columbia University; grant U10-HL119990 to Indiana University; grant U10-HL120006 to the University of Pittsburgh; grant U10-HL119992 to Northwestern University; grant U10-HL120019 to the University of California, Irvine; grant U10-HL119993 to University of Pennsylvania; and grant U10-HL120018 to the University of Utah. In addition, support was provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health to Clinical and Translational Science Institutes at Indiana University (grant UL1TR001108) and University of California, Irvine (grant UL1TR000153).
ClinicalTrials.gov Registration number NCT02231398.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- APO
adverse pregnancy outcome
- CVD
cardiovascular disease
- HHS
Heart Health Study
- nuMoM2b
Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be
- SDB
sleep-disordered breathing
APPENDIX 1
Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be Heart Health Study: data collected at interval contacts
Informed consent (first contact)
Data on Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be pregnancy (first contact) Answers to general questions (each contact) Data on subsequent pregnancies (each contact) Information on history of the following cardiovascular disease–related conditions (selected contacts) Medical records release (selected births and cardiovascular disease–related hospitalizations and procedures)
Vital status of baby
If the baby is living, how long the baby was breastfed, when other foods were introduced, and what the baby weighed
For pregnancies carried 20 weeks or more, maternal weight 6 weeks postpartum
Whether the participant has access to health care
Maternal weight
Estimated due date
Date pregnancy ended
- Whether the mother experienced the following pregnancy complications:
- ○ Preterm birth
- ○ Hypertension or preeclampsia
- ○ Gestational diabetes
- ○ Premature labor or ruptured membranes
- ○ Multiple gestation pregnancy
Outcome (live birth, stillbirth, or loss at <20 weeks)
For pregnancies carried 20 weeks or more, prepregnancy weight and amount of weight gain during pregnancy
For live births, whether the mother is breastfeeding
Hypertension
Heart problems (myocardial infarction, coronary artery disease, heart failure, other)
Stroke, transient ischemic attack, or cerebrovascular accident
Venous thromboembolism (deep venous thrombosis or pulmonary embolism)
Peripheral vascular disease
Diabetes
Kidney disease
Invitation to in-person visit and sleep breathing assessment at appropriate time if eligible
Contact information (each contact)
REFERENCES
- 1. Rosamond W, Flegal K, Friday G et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;1155:e69–e171. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;1314:e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Folsom AR, Kaye SA, Sellers TA et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;2694:483–487. [PubMed] [Google Scholar]
- 4. Gordon T, Castelli WP, Hjortland MC et al. Diabetes, blood lipids, and the role of obesity in coronary heart disease risk for women. The Framingham Study. Ann Intern Med. 1977;874:393–397. [DOI] [PubMed] [Google Scholar]
- 5. Manson JE, Colditz GA, Stampfer MJ et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;32213:882–889. [DOI] [PubMed] [Google Scholar]
- 6. Mosca L, Benjamin EJ, Berra K et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;12311:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnussen EB, Vatten LJ, Smith GD et al. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;1145:961–970. [DOI] [PubMed] [Google Scholar]
- 8. Bellamy L, Casas JP, Hingorani AD et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;3357627:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vikse BE, Irgens LM, Bostad L et al. Adverse perinatal outcome and later kidney biopsy in the mother. J Am Soc Nephrol. 2006;173:837–845. [DOI] [PubMed] [Google Scholar]
- 10. Vikse BE, Irgens LM, Leivestad T et al. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;3598:800–809. [DOI] [PubMed] [Google Scholar]
- 11. Catov JM, Dodge R, Barinas-Mitchell E et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt). 2013;2210:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rich-Edwards JW, Fraser A, Lawlor DA et al. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;361:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rich-Edwards JW, McElrath TF, Karumanchi SA et al. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;563:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;3257356:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sibai BM, Caritis S, Hauth J et al. What we have learned about preeclampsia. Semin Perinatol. 2003;273:239–246. [DOI] [PubMed] [Google Scholar]
- 16. Agatisa PK, Ness RB, Roberts JM et al. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;2864:H1389–H1393. [DOI] [PubMed] [Google Scholar]
- 17. Bar J, Kaplan B, Wittenberg C et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999;145:1129–1132. [DOI] [PubMed] [Google Scholar]
- 18. Berends AL, de Groot CJ, Sijbrands EJ et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;514:1034–1041. [DOI] [PubMed] [Google Scholar]
- 19. Kvehaugen AS, Dechend R, Ramstad HB et al. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. 2011;581:63–69. [DOI] [PubMed] [Google Scholar]
- 20. Saxena AR, Karumanchi SA, Brown NJ et al. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;555:1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yinon Y, Kingdom JC, Odutayo A et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;12218:1846–1853. [DOI] [PubMed] [Google Scholar]
- 22. Ehrenthal DB, Goldstein ND, Wu P et al. Arterial stiffness and wave reflection 1 year after a pregnancy complicated by hypertension. J Clin Hypertens (Greenwich). 2014;1610:695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehrenthal DB, Rogers S, Goldstein ND et al. Cardiovascular risk factors one year after a hypertensive disorder of pregnancy. J Womens Health (Larchmt). 2015;241:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamad RR, Larsson A, Pernow J et al. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertens. 2009;2711:2257–2264. [DOI] [PubMed] [Google Scholar]
- 25. Melchiorre K, Sutherland GR, Liberati M et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;584:709–715. [DOI] [PubMed] [Google Scholar]
- 26. Fraser A, Nelson SM, Macdonald-Wallis C et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;12511:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Catov JM, Lewis CE, Lee M et al. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension. 2013;613:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Germain AM, Romanik MC, Guerra I et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;491:90–95. [DOI] [PubMed] [Google Scholar]
- 29. Levine RJ, Lam C, Qian C et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;35510:992–1005. [DOI] [PubMed] [Google Scholar]
- 30. Noori M, Donald AE, Angelakopoulou A et al. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;1225:478–487. [DOI] [PubMed] [Google Scholar]
- 31. Held C, Hjemdahl P, Rehnqvist N et al. Haemostatic markers, inflammatory parameters and lipids in male and female patients in the Angina Prognosis Study in Stockholm (APSIS). A comparison with healthy controls. J Intern Med. 1997;2411:59–69. [DOI] [PubMed] [Google Scholar]
- 32. Hoffmeister A, Rothenbacher D, Bäzner U et al. Role of novel markers of inflammation in patients with stable coronary heart disease. Am J Cardiol. 2001;873:262–266. [DOI] [PubMed] [Google Scholar]
- 33. Ridker PM, Hennekens CH, Buring JE et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;34212:836–843. [DOI] [PubMed] [Google Scholar]
- 34. Sagastagoitia JD, Sáez Y, Vacas M et al. Association between inflammation, lipid and hemostatic factors in patients with stable angina. Thromb Res. 2007;1201:53–59. [DOI] [PubMed] [Google Scholar]
- 35. Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;566:1026–1034. [DOI] [PubMed] [Google Scholar]
- 36. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;208:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunderson EP, Lewis CE, Tsai AL et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;5612:2990–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holzman C, Bullen B, Fisher R et al. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15(suppl 2):136–158. [DOI] [PubMed] [Google Scholar]
- 39. Hauri P, ed. The International Classification of Sleep Disorders, Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 40. Berry RB, Budhiraja R, Gottlieb DJ et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;85:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marshall NS, Wong KK, Phillips CL et al. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;51:15–20. [PMC free article] [PubMed] [Google Scholar]
- 42. Newman AB, Nieto FJ, Guidry U et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;1541:50–59. [DOI] [PubMed] [Google Scholar]
- 43. Nieto FJ, Young TB, Lind BK et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;28314:1829–1836. [DOI] [PubMed] [Google Scholar]
- 44. Peppard PE, Young T, Palta M et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;34219:1378–1384. [DOI] [PubMed] [Google Scholar]
- 45. Reichmuth KJ, Austin D, Skatrud JB et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;17212:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(suppl 4):S122–S126. [PubMed] [Google Scholar]
- 47. Young T, Peppard P, Palta M et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;15715:1746–1752. [PubMed] [Google Scholar]
- 48. Punjabi NM, Caffo BS, Goodwin JL et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;68:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Redline S, Yenokyan G, Gottlieb DJ et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;1822:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gottlieb DJ, Yenokyan G, Newman AB et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;1224:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shahar E, Whitney CW, Redline S et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;1631:19–25. [DOI] [PubMed] [Google Scholar]
- 52. Facco FL, Kramer J, Ho KH et al. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;1151:77–83. [DOI] [PubMed] [Google Scholar]
- 53. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;277:1405–1417. [DOI] [PubMed] [Google Scholar]
- 54. Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003;96:477–483. [DOI] [PubMed] [Google Scholar]
- 55. Santiago JR, Nolledo MS, Kinzler W et al. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;1345:396–408. [DOI] [PubMed] [Google Scholar]
- 56. Louis JM, Auckley D, Sokol RJ et al. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;2023:261.e1–261.e5. [DOI] [PubMed] [Google Scholar]
- 57. Facco FL, Grobman WA, Kramer J et al. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;2032:142.e1–142.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen YH, Kang JH, Lin CC et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;2062:136.e1–136.e5. [DOI] [PubMed] [Google Scholar]
- 59. Bourjeily G, Raker CA, Chalhoub M et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;364:849–855. [DOI] [PubMed] [Google Scholar]
- 60. Haas DM, Parker CB, Wing DA et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). Am J Obstet Gynecol. 2015;2124:539.e1–539.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Facco FL, Parker CB, Reddy UM et al. NuMoM2b Sleep-Disordered Breathing study: objectives and methods. Am J Obstet Gynecol. 2015;2124:542.e1–542.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heckbert SR, Kooperberg C, Safford MM et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;16012:1152–1158. [DOI] [PubMed] [Google Scholar]
- 63. Yore MM, Ham SA, Ainsworth BE et al. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;398:1267–1274. [DOI] [PubMed] [Google Scholar]
- 64. Kriska AM, Knowler WC, LaPorte RE et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;134:401–411. [DOI] [PubMed] [Google Scholar]
- 65. Pettee Gabriel K, McClain JJ, Schmid KK et al. Reliability and convergent validity of the past-week Modifiable Activity Questionnaire. Public Health Nutr. 2011;143:435–442. [DOI] [PubMed] [Google Scholar]
- 66. Schulz LO, Harper IT, Smith CJ et al. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;26:541–548. [DOI] [PubMed] [Google Scholar]
- 67. Block G, Woods M, Potosky A et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;4312:1327–1335. [DOI] [PubMed] [Google Scholar]
- 68. Coates RJ, Eley JW, Block G et al. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am J Epidemiol. 1991;1346:658–671. [DOI] [PubMed] [Google Scholar]
- 69. Sinha R, Block G, Taylor PR. Determinants of plasma ascorbic acid in a healthy male population. Cancer Epidemiol Biomarkers Prev. 1992;14:297–302. [PubMed] [Google Scholar]
- 70. Block G, Sinha R, Gridley G. Collection of dietary-supplement data and implications for analysis. Am J Clin Nutr. 1994;59(1 suppl):232S–239S. [DOI] [PubMed] [Google Scholar]
- 71. Snook Parrott M, Bodnar LM, Simhan HN et al. Maternal cereal consumption and adequacy of micronutrient intake in the periconceptional period. Public Health Nutr. 2009;128:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aurélie L, Gilles F, Jean-Jacques D et al. Characterization of the Three-Factor Eating Questionnaire scores of a young French cohort. Appetite. 2012;592:385–390. [DOI] [PubMed] [Google Scholar]
- 73. Karlsson J, Persson LO, Sjöström L et al. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;2412:1715–1725. [DOI] [PubMed] [Google Scholar]
- 74. Provencher V, Bégin C, Piché ME et al. Disinhibition, as assessed by the Three-Factor Eating Questionnaire, is inversely related to psychological well-being in postmenopausal women. Int J Obes (Lond). 2007;312:315–320. [DOI] [PubMed] [Google Scholar]
- 75. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;291:71–83. [DOI] [PubMed] [Google Scholar]
- 76. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 77. Cox JL, Chapman G, Murray D et al. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord. 1996;393:185–189. [DOI] [PubMed] [Google Scholar]
- 78. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;82:99–107. [Google Scholar]
- 79. Zelkowitz P, Milet TH. Screening for post-partum depression in a community sample. Can J Psychiatry. 1995;402:80–86. [DOI] [PubMed] [Google Scholar]
- 80. Edge D, Baker D, Rogers A. Perinatal depression among black Caribbean women. Health Soc Care Community. 2004;125:430–438. [DOI] [PubMed] [Google Scholar]
- 81. Spielberger CD. State-Trait Anxiety Inventory for Adults, Manual, Instrument and Scoring Guide. Palo Alto, CA: Mind Garden, Inc.; 1983. [Google Scholar]
- 82. Chaudron LH, Kitzman HJ, Peifer KL et al. Self-recognition of and provider response to maternal depressive symptoms in low-income Hispanic women. J Womens Health (Larchmt). 2005;144:331–338. [DOI] [PubMed] [Google Scholar]
- 83. Yonkers KA, Ramin SM, Rush AJ et al. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am J Psychiatry. 2001;15811:1856–1863. [DOI] [PubMed] [Google Scholar]
- 84. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;244:385–396. [PubMed] [Google Scholar]
- 85. Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol. 1993;641:131–140. [DOI] [PubMed] [Google Scholar]
- 86. Cohen S, Williamson GD, eds. Perceived Stress in a Probability Sample of the United States. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 87. Cole SR. Assessment of differential item functioning in the Perceived Stress Scale-10. J Epidemiol Community Health. 1999;535:319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ramírez MT, Hernández RL. Factor structure of the Perceived Stress Scale (PSS) in a sample from Mexico. Span J Psychol. 2007;101:199–206. [DOI] [PubMed] [Google Scholar]
- 89. Netzer NC, Stoohs RA, Netzer CM et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;1317:485–491. [DOI] [PubMed] [Google Scholar]
- 90. Levine DW, Kaplan RM, Kripke DF et al. Factor structure and measurement invariance of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;152:123–136. [DOI] [PubMed] [Google Scholar]
- 91. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;154:376–381. [DOI] [PubMed] [Google Scholar]
- 92. Allen RP, Picchietti D, Hening WA et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;42:101–119. [DOI] [PubMed] [Google Scholar]
- 93. Yu L, Buysse DJ, Germain A et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;101:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Quan SF, Howard BV, Iber C et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;2012:1077–1085. [PubMed] [Google Scholar]
- 95. Young T, Palta M, Dempsey J et al. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;1085:246–249. [PMC free article] [PubMed] [Google Scholar]
- 96. Hogue CJ, Parker CB, Willinger M et al. The association of stillbirth with depressive symptoms 6–36 months post-delivery. Paediatr Perinat Epidemiol. 2015;292:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tomeo CA, Rich-Edwards JW, Michels KB et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;106:774–777. [PubMed] [Google Scholar]
- 98. James PA, Oparil S, Carter BL et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;3115:507–520. [DOI] [PubMed] [Google Scholar]
- 99. Joint National Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. [PubMed] [Google Scholar]
- 100. Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;287:412–419. [DOI] [PubMed] [Google Scholar]
- 101. Mitchell A, Misialek JR, Folsom AR et al. Usefulness of N-terminal pro-brain natriuretic peptide and myocardial perfusion in asymptomatic adults (from the multi-ethnic study of atherosclerosis). Am J Cardiol. 2015;11510:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. National Heart, Lung, and Blood Institute. Risk assessment tool for estimating your 10-year risk of having a heart attack. http://cvdrisk.nhlbi.nih.gov/calculator.asp Updated November, 2014. Accessed March 16, 2015.
- 103. American Heart Association. 2013 prevention guidelines tools: CV risk calculator. https://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp Accessed March 16, 2015.
- 104. Smith GN, Walker MC, Liu A et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;2001:58.e1–58.e8. [DOI] [PubMed] [Google Scholar]
- 105. McMahan CA, Gidding SS, Fayad ZA et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;1658:883–890. [DOI] [PubMed] [Google Scholar]
- 106. Centers for Disease Control and Prevention. Blood pressure – BPQ. http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/BPQ_H.pdf Accessed March 16, 2015.
- 107. Lawrence JM, Contreras R, Chen W et al. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;315:899–904. [DOI] [PubMed] [Google Scholar]
- 108. Dyer AR, Liu K, Walsh M et al. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;131:13–21. [DOI] [PubMed] [Google Scholar]
- 109. Roberts JM, Myatt L, Spong CY et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;36214:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Roberts JM, Pearson G, Cutler J et al. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;413:437–445. [DOI] [PubMed] [Google Scholar]
- 111. Copen CE, Thoma ME, Kirmeyer S. Interpregnancy intervals in the United States: data from the birth certificate and the national survey of family growth. Natl Vital Stat Rep. 2015;644:1–11. [PubMed] [Google Scholar]
- 112. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;1225:1122–1131. [DOI] [PubMed] [Google Scholar]
- 113. Wen S, Tan H, Xie R et al. A pre-conception cohort to study preeclampsia in China: rationale, study design, and preliminary results. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;3711:1081–1087. [DOI] [PubMed] [Google Scholar]
- 114. Baker D, Park C, Sweeney C et al. Recruitment of women in the National Children's Study Initial Vanguard Study. Am J Epidemiol. 2014;17911:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]


