Abstract
Depression is a common co-morbid condition most often observed in subjects with mild cognitive impairment (MCI) and during the early stages of Alzheimer’s disease (AD). Dysfunction of the central noradrenergic nervous system is an important component in depression. In AD, locus coeruleus (LC) noradrenergic neurons are significantly reduced pathologically and the reduction of LC neurons is hypothesized to begin very early in the progression of the disorder; however, it is not known if dysfunction of the noradrenergic system due to early LC neuronal loss is involved in mediating depression in early AD. Therefore, the purpose of this study was to determine in an animal model if a loss of noradrenergic LC neurons results in depressive-like behavior. The LC noradrenergic neuronal population was reduced by the bilateral administration of the neurotoxin 6-hydroxydopamine (6-OHDA) directly into the LC. Forced swim test (FST) was performed three weeks after the administration of 6-OHDA (5, 10 and 14 μg/μl), animals administered the 5 μg/μl of 6-OHDA demonstrated a significant increase in immobility, indicating depressive-like behavior. This increase in immobility at the 5 μg/μl dose was observed with a minimal loss of LC noradrenergic neurons as compared to LC neuronal loss observed at 10 and 14 μg/μl dose. A significant positive correlation between the number of surviving LC neurons after 6-OHDA and FST immobile time was observed, suggesting that in animals with a minimal loss of LC neurons (or a greater number of surviving LC neurons) following 6-OHDA demonstrated depressive-like behavior. As the 6-OHDA-induced loss of LC neurons is increased, the time spent immobile is reduced. Depressive-like behavior was also observed with the 5 μg/μl dose of 6-OHDA with a second behavior test, sucrose consumption. FTS increased immobility following 6-OHDA (5 μg/μl) was reversed by the administration of a single dose of L-1-3-4-dihydroxyphenylalanine (DOPA) or L-threo-3,4-dihydroxyphenylserine (DOPS) prior to behavioral assessment. Surviving LC neurons 3 weeks after 6-OHDA (5 μg/μl) demonstrated compensatory changes of increased firing frequency, a more irregular firing pattern, and a higher percentage of cells firing in bursts.
These results indicate that depressive-like behavior in mice is observed following the administration of 6-OHDA and the loss of LC noradrenergic neurons; however, the depressive-like behavior correlates positively with the number of surviving LC neurons with 6-OHDA administration. This data suggests the depression observed in MCI subjects and in the early stages of AD may due to the hypothesized early, minimal loss of LC neurons.
Keywords: locus coeruleus, 6-hydroxydopamine, forced swim test, electrophysiology, depression
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with the hallmark symptom of cognitive impairment. The majority of AD patients also exhibit neuropsychiatric symptoms (NPS) with depression being the most common NPS (Bhalla et al., 2009; Benoit et al., 2012; Lebedev et al., 2014). Depression associated with AD is mainly diagnosed in mild cognitive impaired (MCI) subjects, or very early in the prognosis of AD. As AD progresses and cognitive impairment continues, depression appears to diminish (Lee et al., 2007; Lyketsos et al., 2011; Wang et al., 2012; Lebedev et al., 2014; Van der Mussele et al., 2014). The reason for this remittance of depression with continued cognitive impairment in AD is unclear.
Neuropathological markers associated with AD occur many years before the onset of cognitive impairment, with the LC being one of the earliest regions affected (Braak and Del Tredici, 2011a,b, 2012; Braak et al., 2011, 2013). The number of noradrenergic neurons located in the LC are substantially reduced (80–90%) in postmortem assessment of AD brains (Tomlinson et al., 1981; Marcyniuk et al., 1986; Chan-Palay and Asan, 1989; Bondareff et al., 1982; German et al., 1992; Zarow et al., 2003; Szot et al., 2006; McMillan et al., 2011), and LC neuronal loss in AD presumably is progressive over the course of the illness. The CNS noradrenergic system has been implicated in the pathobiology of depression (Chandley and Ordway, 2012). Therefore, a common factor between AD and depression is the involvement of the central noradrenergic system, comprised of the pontine locus coeruleus (LC) and its widespread projections throughout the central nervous system (CNS). The focus of this study was to determine if a loss of LC neurons results in depressive-like behavior in mice.
2. Materials and Methods
2.1. Animals and surgical procedures
One hundred and thirty five adult (3 months of age; ~ 25g) male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) for all studies, except for electrophysiological analysis studies (Charles River Laboratories, Barcelona, Spain). All animals were housed singly in standard enriched environment cages in a temperature-controlled room with a 12-h light/dark cycle. Animals were housed individually because of the aggressive nature of this strain of animal; housing singly results in a similar degree of stress to all animals. Food and water were provided ad libitum. The animals were given at least 2 weeks’ acclimation to the facility before the LC administration of vehicle or 6-OHDA. All animal procedures were in accordance with the Animal Care Committee at the VA Puget Sound Health Care System, Seattle, WA, National Institute of Health guidelines and Local Committee for Animal Experimentation at the University of the Basque Country. The minimum number of animals was used for these studies and care was taken to minimize any suffering. Injection of LC 6-OHDA or vehicle (0.2% ascorbic acid/saline) bilaterally is described in detail in previously published work (Szot et al., 2012b, c). Briefly, animals were anesthetized with isofluorane and mounted into a stereotaxic apparatus. After skull was exposed 6-OHDA or vehicle was injected bilaterally into the LC at the following coordinates: AP: −5.4 mm from bregma; ML: ± 0.9 mm; DV: −3.25 mm from the surface of the skull, at a rate of 0.13 μl/min over ~ 8 min. The needle was left in place for an additional 4 min after the injection and then the needle was slowly withdrawn. Animals were sutured, removed from the stereotaxic apparatus and allowed to recover. Previous work indicated injection of 6-OHDA into the LC specifically affected the LC noradrenergic neurons (Szot et al., 2012b, c).
2.2. Experimental design
2.2.1. Forced Swim Test (FST)
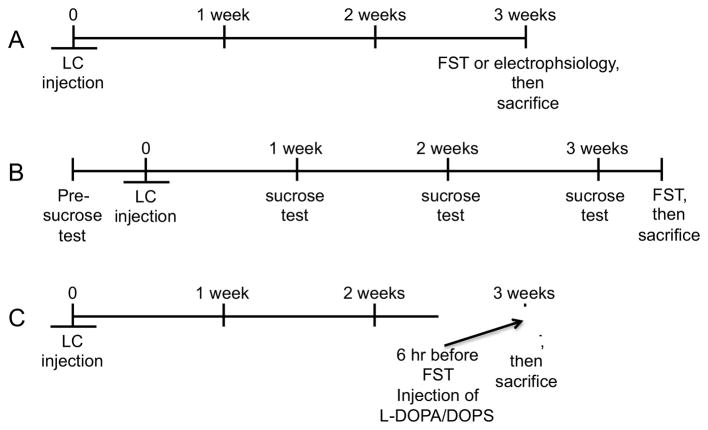
Mice were tested for depressive-like behavior in a modified version of the forced swim test (Porsolt et al., 1978) 3 weeks after LC injection of either vehicle or 6-OHDA (Figure 1A). Mice were individually placed into a container (W:24cm; L:45cm; D:20cm water level to ~14–15cm) containing tepid water (~27°C) for 8 min. A blinded reviewer scored the animal’s behavior for only the last 6 min. Immobility was considered when the animal was floating passively or with subtle movement of feet or tail required to keep the head above the surface of the water. In mice, an increase in the duration of time immobile is interpreted as depressive-like behavior. Results were expressed as average time (sec) ± standard error of the mean (SEM) of the animal’s time immobile for vehicle- or 6-OHDA-treated animals. Animals were killed 20 min after the FST. Brains were removed and the hindbrain portion containing the LC was dissected free and frozen on dry ice. The LC region was cut on a cryostat (16 μm) to assess neuronal loss by tyrosine hydroxylase-immunohistochemistry (TH-IH).
Figure 1.
Timeline of LC injection of vehicle or 6-OHDA to (A) FST and electrophysiological studies, (B) sucrose consumption test, and (C) administration of L-DOPA and DOPS.
2.2.2. Sucrose Consumption
Four days before (pre-test) LC injection of vehicle or 6-OHDA (5 μg/μl) and 1, 2 and 3 weeks after LC injection of either vehicle or 6-OHDA, the sucrose consumption test was performed (Figure 1B). Mice were given a choice of their regular water or 1% sucrose solution for 3 hours (17:00hr – 20:00hr) on test days described above. The amount of 1% sucrose consumed during the 3 hr time was recorded for each animal. A decrease in the consumption of sucrose is interpreted as depressive-like behavior. Results were expressed as the average amount of sucrose consumed (g) ± SEM for each animal in vehicle- and 6-OHDA-treated groups. The FST was performed 4 days after the 3-week sucrose consumption test (Figure 1B) and scored as described above. Animals were killed after the FST as described above to measure the number of LC neuronal number by TH-IH.
2.2.3. Electrophysiological Analysis
Three weeks after the bilateral LC administration of either vehicle or 6-OHDA (5 μg/μl) (Figure 1A), single-unit extracellular recordings were performed as previously described (Torrecilla et al., 2013). Mice were anesthetized with chloral hydrate (400 mg/ kg i.p.) and placed in a stereotaxic frame with the skull positioned horizontally. A burr hole was drilled and the recording electrode was placed in the right LC (relative to lambda, AP: −1.5 mm, ML: +0.2 to +1.2 mm, DV: −2.7 to 4.0 mm). The recording electrode was filled with 2% solution of Pontamine Sky Blue in 0.5% sodium acetate with a tip diameter of 1–2 mM. The body temperature was maintained at 37°C for the entire experiment using a heating pad. LC neurons were identified by standard criteria, which included spontaneous activity displaying a regular rhythm and firing rate between 0.5 and 5 Hz, characteristic spikes with long-lasting (>2 ms), positive–negative waveform action potentials, and the biphasic excitation–inhibition response to pressure applied on the contralateral hind paw (paw pinch) (Gobbi et al., 2007). The extracellular signal from the electrode was pre-amplified, and then amplified with a high-input impedance amplifier and then monitored on an oscilloscope and an audio monitor. This activity was processed using computer software (Spike2 software; Cambridge Electronic Design, UK) and the following patterns were calculated: firing rate; the coefficient of variation (percentage ratio of standard deviation to the mean interval value of an interspike time-interval histogram); percentage of spikes in burst; mean spikes/burst; percentage of cells exhibiting burst firing and number of active neurons per track. Basal electrophysiological activity was measured for 180 s. Burst-firing of LC neurons was detected as a train of at least two spikes with the first interspike interval <80 ms and a termination interval >160 ms (Miguelez et al., 2011; Torrecilla et al., 2013). The number of spontaneously active noradrenergic neurons was determined in 5–10 stereotaxic electrode tracks 50 μm concentrically to the initial track. The pattern was constant for all animals. Animals were killed, brains were removed and frozen and shipped to Seattle, WA to assess LC neuronal loss by TH-IH.
2.3. Administration of L-DOPA and DOPS
To determine if the depressive-like behavior observed 3 weeks after LC neuronal loss induced by 6-OHDA (5 μg/μl) was due to reduced NE concentrations, L- 1-3-4-dihydroxyphenylalanine (DOPA) (3 mg/kg, sc) + benserazide (15 mg/kg, sc) (Sigma-Aldrich, St. Louis, MO) or L-threo-3,4-dihydroxyphenylserine (DOPS) (500 mg/kg, sc) + benserazide (0.25 mg/kg, sc) were administered to animals approximately 6 hr before the FST (Figure 1C). Control animals for the L-DOPA or DOPS group received the appropriate dose of benserazide alone. L-DOPA is the precursor to NE (and DA) and is considered the gold standard of treatment for Parkinson’s disease (Cotzias et al., 1969). DOPS is converted to NE by aromatic L-amino acid decarboxylase, which is present in all biogenic amine neurons. The doses of L-DOPA + benserazide and DOPS + benserazide, and time of exposure were chosen based on previously published work (Thomas et al., 1998; Francardo et al., 2011). As described above, the animals were killed after the FST, the brains were removed and the hippocampus (HP; bilaterally), frontal cortex (FC) and the hindbrain portion containing the LC region were dissected free, and the degree of LC neuronal loss was determined by TH-IH. Catecholamine concentrations were determined in the HP and FC.
2.4. Tyrosine hydroxylase Immunohistochemistry (TH-IH) and catecholamine HPLC measurements
Catecholamine concentrations were measured by HPLC using a slightly modified version previously described (Szot et al., 2012b, c) to include analysis of serotonin (5-HT) and its metabolite 5-hydroxy indole acetic acid (5-HIAA) along with NE, dopamine (DA), the NE/DA precursor DOPA; and metabolites of NE and DA: 3-methoxy-4-hydroxy-phenethyleneglycol (MHPG), dihydroxyphenylglycol (DHPG), dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA). The catecholamines from the L-DOPA treated group were analyzed together with the animals that received DOPS.
TH-IH was performed in the LC to assess the number of noradrenergic neurons in vehicle- and 6-OHDA-treated animals as previously described (Szot et al., 2012b, c). The number of TH-immunoreactive (IR) cell bodies in the LC were counted in three consecutive atlas-matched sections bilaterally in all animals and expressed as the average ± SEM for each group.
2.5. Statistical analysis
All experiments were analyzed using the computer program GraphPad Prism (Graph-Pad Software, Inc) and the level of significance was considered as p<0.05. When more than two groups were compared, a one-way ANOVA was used to assess statistical difference followed by a post hoc Tukey’s test. Simple linear regressions were performed between the number of TH-IR neurons in the LC versus time (sec) spent immobile in the FST. In the electrophysiological experiments the sample size (n) represents the number of recorded neurons for the basic electrophysiological parameters, and the number of animals for the parameter of number of neurons per tract. Differences in the percentage of neurons presenting burst firing were statistically evaluated by Fisher’s exact test. Parameters derived from burst pattern were analyzed by Mann-Whitney test. Spontaneous firing rate, coefficient of variation and number of neurons per track were compared by unpaired Student’s t test.
3. Results
3.1. Depressive-like behavior is observed with FST only with a moderate loss of LC neurons
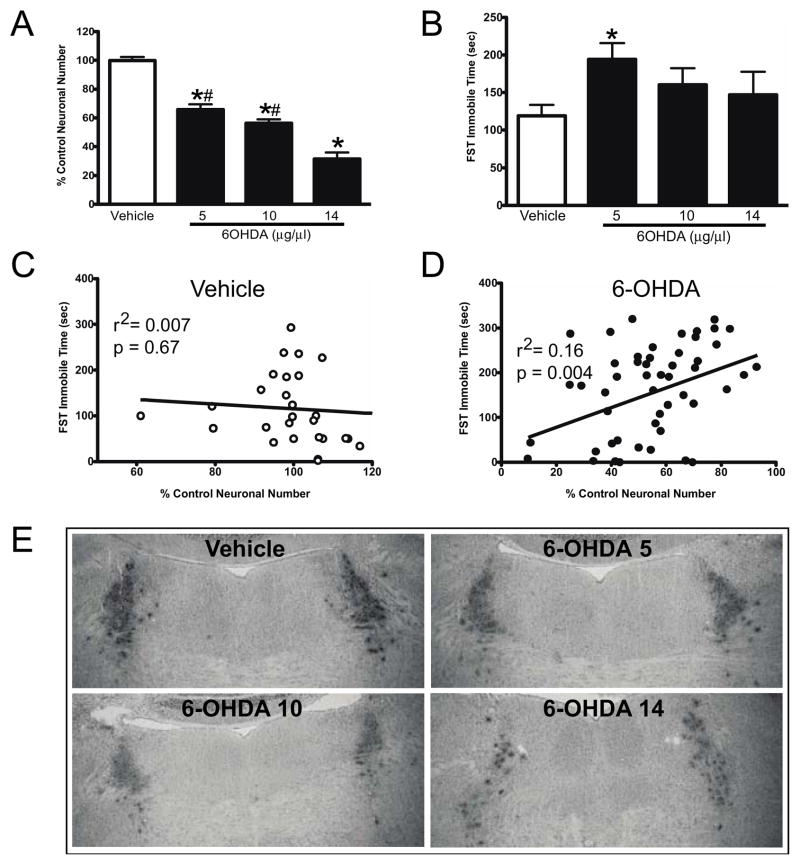
Vehicle (n = 29) or 6-OHDA was administered directly (bilaterally) into the LC of mice at the following doses: 5 (n = 18), 10 (n = 24) and 14 (n = 10) μg/1 μl. Animal’s weight was monitored during the next three weeks; no significant differences were observed between treatment groups (data not shown). Three weeks after administration of vehicle or 6-OHDA FST was performed, animals were killed shortly after FST to assess the number of TH-IR neurons in the LC. Three weeks after the administration of 6-OHDA a dose-dependent reduction in the number of TH-IR neurons in the LC was observed (Figure 2A histogram and Figure 2E TH-IR in LC); 5 μg/μl dose of 6-OHDA resulted in a 34% reduction, the 10 μg/μl dose with a 44% reduction, and 69% reduction with 14 μg/μl dose. All three doses of 6-OHDA resulted in a significant reduction in LC neurons compared to vehicle-treated animals, and the effect of the 14 μg/μl dose was significantly greater than that of the 5 and 10 μg/μl doses, results comparable to our previously published work (Szot et al., 2012c). However, only the 5 μg/μl dose of 6-OHDA resulted in a significant increase (63%) in the FST immobility time (i.e., depressive-like behavior). Although the 10 and 14 μg/μl dose of 6-OHDA significantly reduced the number of LC noradrenergic neurons, the increase in immobility time was not significant (10 μg/μl dose of 6-OHDA resulted in a 33% increase and the 14 μg/μl dose resulted in a 24% increase from vehicle-treated animals) (Figure 2B).
Figure 2.
Effect of vehicle (n=29) and dose response of 6-OHDA at 5 (n=18), 10 (n=24) and 14 μg/μl (n=10) on LC neuronal number and FST. A) 6-OHDA dose-response effect on LC neuronal number. *Indicates significant difference from vehicle-treated animals. #Indicates significant difference to 6-OHDA 14 μg/μl treated animals. B) 6-OHDA dose-response effect on FST immobile time (sec). *Indicates significant difference from vehicle-treated animals. Correlation of FST immobilization time (sec) to percent of control of LC neuronal number in vehicle- (C) and 6-OHDA- (D) treated animals. E) TH-IR labeling of LC neurons in vehicle and 6-OHDA treated animals at 5, 10 and 14 μg/μl.
When the number of LC neurons (% of control neuronal number) was compared to the FST immobile time in the vehicle-treated animals there was no correlation (Figure 1C). However, for animals that received 6-OHDA (all doses), a significant positive correlation was observed (Figure 2D) between the number of surviving LC neurons and the amount of time spent in the immobile phase in the FST. This data indicates that animals with a minimal loss of LC neurons due to LC 6-OHDA (or had a larger number of surviving LC neurons) had longer FST immobile times, while animals with a greater loss of LC neurons (or less surviving neurons) spent less time in the immobile phase. Since the 6-OHDA 5 μg/μl dose resulted in a significant increase in immobility time (Figure 2B), the remaining studies used this dose of 6-OHDA. LC neuronal numbers were determined in each experiment by TH-IH to determine the amount of LC loss.
3.2. Depressive-like behavior is also observed with the sucrose consumption test
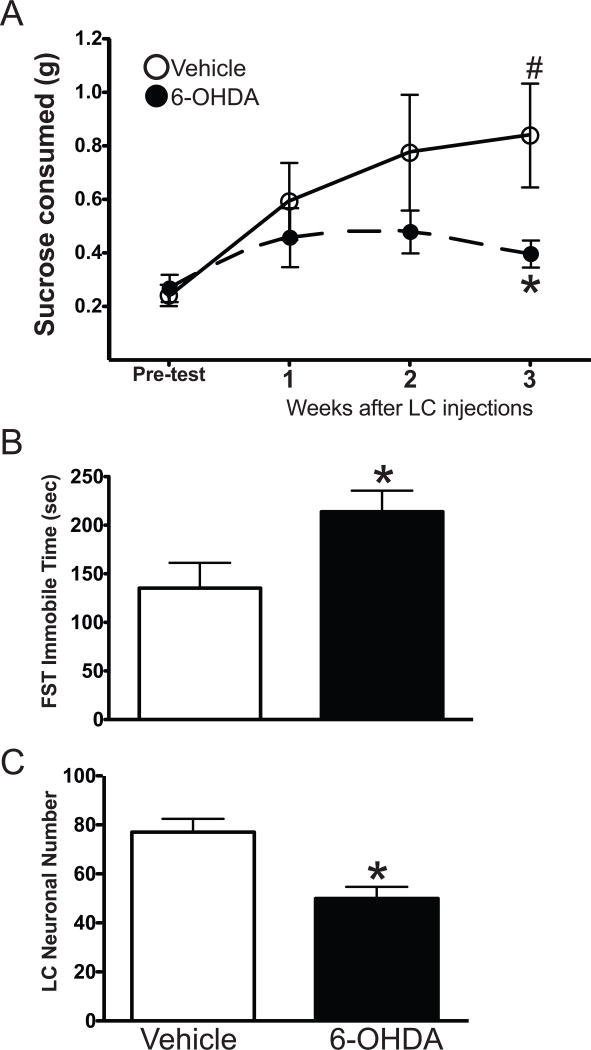
In another group of animals, the loss of LC neurons induced by 6-OHDA (5 μg/μl) resulted in depressive-like behavior in another behavior model, the sucrose consumption test (Figure 3A). Over the course of the 3 weeks the weight of the animals did not differ significantly between vehicle- and 6-OHDA-treated animals (data not shown). Vehicle-treated animals (n=9) increased their consumption of sucrose over the 3 weeks with a significant increase at the 3-week time point compared to the pre-test consumption (glucose consumption at: pre-test = 0.24 ± 0.04 g; 1 week = 0.59 ± 0.14 g; 2 weeks =0.77 ± 0.22 g, and 3 weeks = 0.84 ± 0.19 g). 6-OHDA-treated animals (n=8) one week after LC administration of 6-OHDA demonstrated an increase in sucrose consumption compared to pre-test consumption (glucose consumption at: pre-test = 0.27 ± 0.05 g; 1 week = 0.46 ± 0.11 g); although the increase was not as great as that observed in vehicle-treated animals. Two weeks after LC administration of 6-OHDA the amount of sucrose consumed peaked at a 0.48 ± 0.08 g; three weeks after LC administration of 6-OHDA the amount of sucrose consumed is reduced to 0.40 ± 0.05 g. Three weeks after LC administration of 6-OHDA the amount of sucrose consumed was significantly lower than vehicle-treated animals, indicating depressive-like behavior. The FST 4 days after the last sucrose consumption test also indicated the mice with 6-OHDA to exhibit depressive-like behavior (Figure 3B). The 6-OHDA-treated animals spent more time immobile (a 58% increase) than vehicle-treated animals. TH-IH in the LC of these animals demonstrated neuronal loss at approximately 35% (Figure 3C).
Figure 3.
Sucrose consumption in vehicle- (n=9) and 6-OHDA-treated (n=8) (5 μg/μl) animals. (A) Sucrose consumed (g) at the pre-test time and 1, 2 and 3 weeks after administration of vehicle or 6-OHDA. * Indicates significant difference from vehicle-treated animals at the pre-test point. # Indicates significant difference to vehicle- treated animals at the 3-week time point. (B) FST performed on the animals 4 days after the 3-week sucrose consumption test and (C) number of LC noradrenergic neurons in vehicle- and 6-OHDA-treated animals by TH-IH. * Indicates significant difference from vehicle-treated animals.
3.3. Basal electrophysiological properties of LC neurons in vehicle and 6-OHDA-lesioned mice
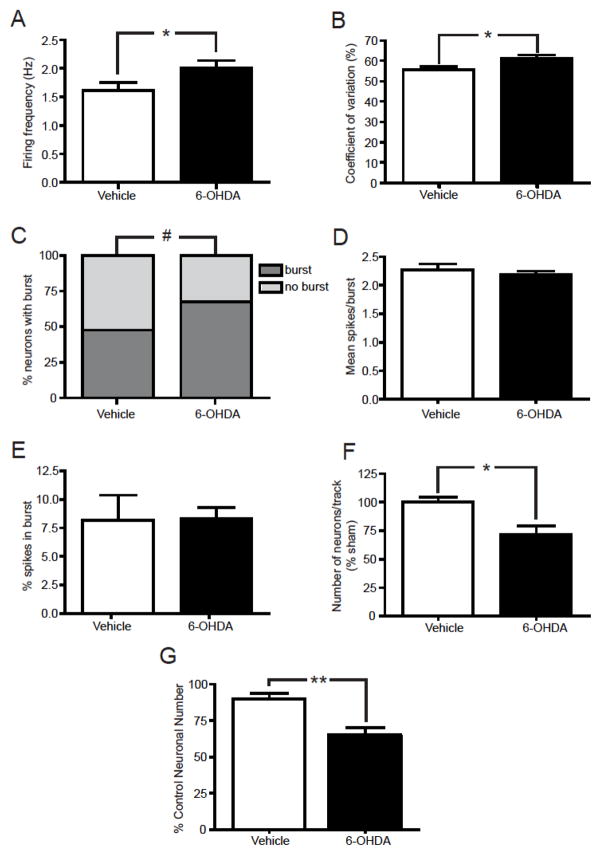
Electrophysiological characterization of LC cells was performed 3 weeks after the bilateral LC administration of vehicle or 6-OHDA (5 μg/μl). LC administration of 6-OHDA (5 μg/μl) evoked several electrophysiological modifications in activity (Figure 4) compared with that of vehicle-treated animals. The firing frequency of LC neurons from 6-OHDA-treated animals was significantly higher than that measured in vehicle-treated animals (vehicle: 1.62 ± 0.13 Hz; 6-OHDA: 2.00 ± 0.14 Hz) (Figure 4A). The coefficient of variation was also higher in 6-OHDA-treated animals compared to vehicle-treated animals (vehicle: 56 ± 2 %; 6-OHDA: 61 ± 2 %) (Figure 4B), indicating a more irregular firing pattern in the surviving LC noradrenergic neurons. In addition, the number of neurons discharging in burst was significantly higher in 6-OHDA-treated animals compared to vehicle-treated animals (vehicle: 47 % and 6-OHDA: 67 %) (Figure 4C). Further analysis of the burst activity did not demonstrate any difference between groups (Figure 4E). Mean spikes per burst and % spikes in burst were similar in vehicle- and 6-OHDA-treated animals (Figure 4D). However, the number of spontaneously active cells was significantly lower in the LC of 6-OHDA-treated animals than in vehicle-treated animals (29% reduction) (Figure 4F). The reduction in LC neuronal number induced in the 6-OHDA-treated animals was confirmed with TH-IH (Figure 4G); LC loss determined by TH-IH (27% reduction) is similar to that observed with electrophysiological methods.
Figure 4.
LC neuronal loss induced by 6-OHDA (5 μg/μl) altered electrophysiological properties of LC neurons. (A) Firing frequency, (B) coefficient of variation, (C) percent of LC neurons demonstrating burst activity, (D) mean number of spikes/burst activity, (E) percent of the number of spikes in a burst activity, (F) the number of spontaneously active LC neurons identified per tract and (G) LC neuronal number in vehicle- and 6-OHDA-treated animals by TH-IH. Seventy nine neurons were recorded in 4 vehicle-treated mice and 67 neurons in 6 mice bilaterally lesioned with 6-OHDA. * Indicates significant difference from vehicle-treated animals.
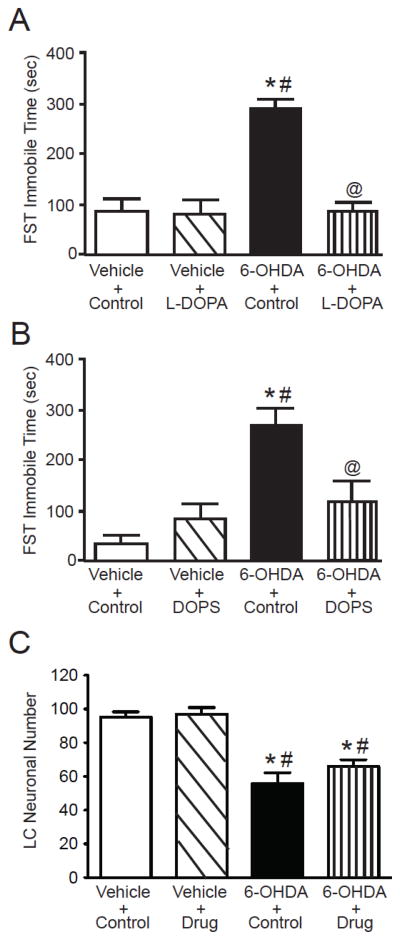
3.4. Prior administration of L-DOPA or DOPS reverses the depressive-like behavior in the FST
To determine if the depressive-like behavior observed with a low dose (5 μg/μl) of 6-OHDA could be reversed, the NE precursors L-DOPA and DOPS were administered subcutaneously approximately 6 hours prior to the FST performed 3 weeks after the LC administration of vehicle or 6-OHDA (Figure 5A and B). Three weeks after administration of vehicle or 6-OHDA there was no significant difference in the weight of animals (data not shown). The administration of 6-OHDA resulted in a significant increase (average increase ~350%) in FST immobile time compared to animals that received LC vehicle with or without L-DOPA or DOPS. The administration of L-DOPA or DOPS to LC vehicle-treated animals did not affect the FST immobile time. However, administration of L-DOPA or DOPS to animals that received LC 6-OHDA resulted in FST immobilization time significantly reduced compared to animals with LC 6-OHDA + control. The FST immobilization time in LC 6-OHDA-treated animals with either L-DOPA or DOPS was not significantly different from LC vehicle-treated animals with or without L-DOPA or DOPS (average increase ~72%). LC neuronal number was assessed by TH-IR. LC neuronal numbers in the LC vehicle-treated with either L-DOPA and DOPS were combined (vehicle + drug) and the 6-OHDA-treated animals with either L-DOPA and DOPS (6-OHDA + drug) were because there wasn’t significant difference between the tow drug treatments as to neuronal number. These data indicate that the prior administration of L-DOPA or DOPS to animals that demonstrate depressive-like behavior after LC 6-OHDA can normalize the FST behavior of the animals. The administration of L-DOPA or DOPS did not affect the reduction in LC neuronal number induced by the LC administration of 6-OHDA (Figure 5C); administration of 6-OHDA with or without L-DOPA or DOPS resulted in a reduction of approximately 37%.
Figure 5.
L-DOPA and DOPS normalize FST immobilization time in animals with a moderate loss of LC neurons induced by 6-OHDA (5 μg/μl). FST immobilization time (sec) in LC vehicle or 6-OHDA animals which received either (A) L-DOPA or (B) DOPS; L-DOPA and DOPA treatment consisted of 4 groups of animals: (1) vehicle in the LC + peripheral control injection (n=5 for L-DOPA and DOPS), (2) vehicle in the LC + either L-DOPA or DOPS peripherally (n=5 for L-DOPA and DOPS), (3) 6-OHDA in the LC + peripheral control injection (n=4 for L-DOPA and DOPS) and (4) 6-OHDA in the LC + either L-DOPA or DOPS peripherally (n=7 for L-DOPA and DOPS). (C) Combined LC neuronal number in the 4 groups for L-DOPA and DOPS treatment. * Indicates significant difference from vehicle + control treated animals. # Indicates significant difference to vehicle + L-DOPA or DOPS treated animals. @ Indicates significant difference to 6-OHDA + control animals.
3.5. Catecholamine levels following FST with Prior administration of L-DOPA or DOPS
Catecholamine levels in the PFC and HP of the animals sacrificed approximately 20 min after FST (Figure 5) are shown in Table 1 (L-DOPA) and Table 2 (DOPS). Table 1 shows the NE, MHPG, DHPG, DOPA and DA concentrations in the PFC (Table 1A) and HP (Table 1B) of LC vehicle- or 6-OHDA-treated animals with or without L-DOPA. Control animals received benserazide 15 mg/kg, sc, the typical dose co-administered with L-DOPA (Francardo et al., 2011). The acute administration of L-DOPA to animals with LC vehicle (LC vehicle + L-DOPA) did not significantly alter the concentrations of NE, MHPG, DHPG, DOPA or DA in the PFC or HP from LC vehicle + control animals; however, there was a trend for a reduction in NE, MHPG, DHPG and DA in the L-DOPA treated animals (LC vehicle + L-DOPA) in both the PFC and HP. Administration of 6-OHDA (LC 6-OHDA + control) resulted in a significant reduction in NE and its metabolites MHPG and DHPG in both the PFC and HP from the LC vehicle + control animals. DOPA and DA concentration in the PFC and HP were not significantly affected by the loss of LC noradrenergic neurons, although there was a trend for a reduction in the HP. The administration of an acute dose of L-DOPA to animals with LC 6-OHDA (LC 6-OHDA + L-DOPA) did not significantly alter the effects of 6-OHDA on catecholamine concentrations in the PFC or HP; however there was a trend for an increase. The concentrations of 5-HT, DOPAC, HVA and 5-HIAA in the PFC and HP of LC vehicle- or 6-OHDA-treated animals with or without L-DOPA were not statistically different among the four groups (data not shown).
Table 1.
Catecholamine concentrations (ng per catecholamine/mg protein) in the (A) prefrontal cortex (PFC) and (B) hippocampus (HP) of LC treated animals with either vehicle or 6-OHDA (5 μg/μl) and peripherally administered with or without L-DOPA.
| A | PFC | ||||
|---|---|---|---|---|---|
| Group | NE | MHPG | DHPG | DOPA | DA |
| LC vehicle + control (5) | 4.12 ± 0.30 | 0.72 ± 0.04 | 0.61 ±0.04 | 0.20 ±0.04 | 0.87 ±0.38 |
| LC vehicle + L-DOPA (5) | 3.51 ±0.39 | 0.63 ±0.05 | 0.54 ±0.07 | 0.24 ±0.01 | 0.54 ±0.16 |
| LC 6-OHDA +control (4) | 1.65 ±0.60* | 0.39 ± 0.08* | 0.35 ±0.09* | 0.17 ±0.01 | 1.05 ±0.76 |
| LC 6-OHDA +L-DOPA (7) | 2.15 ±0.42* | 0.48 ± 0.06* | 0.39 ±0.05* | 0.22 ±0.01 | 1.05 ±0.36 |
| B | HP | ||||
|---|---|---|---|---|---|
| Group | NE | MHPG | DHPG | DOPA | DA |
| LC vehicle + control (5) | 4.10 ±0.34 | 0.39 ± 0.03 | 0.31 ±0.02 | 0.21 ±0.03 | 0.72 ± 0.48 |
| LC vehicle + L-DOPA (5) | 3.28 ±0.35 | 0.30 ±0.04 | 0.24 ±0.02 | 0.22 ±0.01 | 0.28 ± 0.09 |
| LC 6-OHDA +control (4) | 1.84 ±0.56* | 0.19 ±0.05* | 0.17 ±0.04* | 0.15 ±0.02 | 0.20 ± 0.06 |
| LC 6-OHDA+L-DOPA (7) | 2.00 ±0.51* | 0.19 ±0.04* | 0.18 ±0.03* | 0.22 ±0.01 | 0.23 ±0.03 |
Each catecholamine concentration is the average ± SEM.
Indicates significant difference from the LC vehicle + control.
Table 2.
Catecholamine concentrations (ng per catecholamine/mg protein) in the (A) prefrontal cortex (PFC) and (B) hippocampus (HP) of LC treated animals with either vehicle or 6-OHDA (5 μg/μl) and peripherally administered with or without DOPS.
| A | PFC | ||||
|---|---|---|---|---|---|
| Group | NE | MHPG | DHPG | DOPA | DA |
| LC vehicle + control (5) | 3.82 ±0.11 | 0.67 ±0.09 | 0.29 ± 0.02 | 1.16 ±0.08 | 0.75 ± 0.15 |
| LC vehicle + DOPS (5) | 3.57 ±0.18 | 0.66 ±0.07 | ND | 1.22 ±0.12 | 0.65 ±0.18 |
| LC 6-OHDA+control (5) | 1.68 ±0.51*# | 0.37 ± 0.05*# | 0.17 ±0.04* | 0.66 ± 0.15*# | 0.37 ±0.05 |
| LC 6-OHDA+ DOPS (7) | 1.47 ±0.42*# | 0.43 ±0.05 | ND | 1.12±0.05a | 0.43 ± 0.05 |
| B | HP | ||||
|---|---|---|---|---|---|
| Group | NE | MHPG | DHPG | DOPA | DA |
| LC vehicle + control (5) | 3.95 ±0.14 | 0.70 ± 0.04 | 0.27 ±0.01 | 0.96 ±0.09 | 0.84 ± 0.47 |
| LC vehicle + DOPS (5) | 3.39 ±0.14 | 0.61 ±0.10 | ND | 1.03 ±0.12 | 0.45 ±0.14 |
| LC 6-OHDA + control (5) | 1.68±0.54*# | 0.35 ± 0.08* | 0.15 ±0.03* | 0.50 ± 0.12*# | 0.87 ±0.41 |
| LC 6-OHDA +DOPS(7) | 1.47±0.45*# | 0.39 ±0.05* | ND | 0.89 ± 0.05a | 0.44 ± 0.13 |
Each catecholamine concentration is the average ± SEM.
ND: Not determined.
Indicates significant difference from the LC vehicle + control.
Indicates significant difference from LC vehicle + DOPS.
Indicates significant difference from LC 6-OHDA + control.
Table 2 contains the concentrations of NE, MHPG, DHPG, DOPA and DA in the PFC (Table 2A) and HP (Table 2B) of animals that received LC vehicle or 6-OHDA with or without DOPS. Control animals received benserazide 0.25 mg/kg, sc, the dose typically administered with DOPS (Thomas et al., 1998). The acute administration of DOPS to animals with LC vehicle (LC vehicle + DOPS) did not alter the concentrations of NE, MHPG, DOPA or DA in the PFC or HP relative to those in the LC vehicle + control animals. Administration of 6-OHDA directly into the LC (LC 6-OHDA + control) resulted in a significant reduction of NE in both the PFC and HP compared to LC vehicle-treated animals with and without DOPS. MHPG concentration in the PFC of LC 6-OHDA-treated animals was also significantly reduced in the PFC compared to LC vehicle-treated animals with and without DOPS; MHPG in the HP was significantly different compared to the LC vehicle + control. DHPG in the PFC and HP of LC 6-OHDA-treated animals was significantly reduced compared to the LC vehicle-treated + control animals. Interestingly, DOPA concentration in the PFC and HP of LC 6-OHDA-treated animals (LC 6-OHDA + control) was also significantly lower than in LC vehicle-treated animals with and without DOPS. Administration of DOPS to either LC vehicle- or 6-OHDA-treated animals interfered with the detection of DHPG by HPLC. The administration of an acute dose of DOPS to animals with LC 6-OHDA (LC 6-OHDA + DOPS) did not significantly alter the effects of 6-OHDA on catecholamine concentrations except that the acute administration of DOPS normalized the concentration of DOPA in both the PFC and HP and MHPG in the PFC. DOPA concentration in DOPS-treated LC 6-OHDA-treated animals (LC 6-OHDA + DOPS) was significantly different from LC 6-OHDA + control animals. DOPS administration also normalized MHPG concentration only in the PFC, MHPG concentration in the LC 6-OHDA + DOPS animals was not significantly different from LC vehicle with or without DOPS. Therefore, the acute administration of DOPS to LC 6-OHDA-treated animals normalized specific catecholamine concentrations in the PFC and HP. The concentrations of 5-HT, DOPAC, HVA and 5-HIAA in the PFC and HP of LC vehicle- or 6-OHDA-treated animals with or without DOPS were not statistically different among the four groups (data not shown).
4. Discussion
4.1. Depressive-like behavior with moderate LC neuronal loss
These studies indicate that in mice singly housed, FST depressive-like behavior is observed in association with a loss of LC noradrenergic neurons; specifically, the behavior is observed with a minimal loss of LC neurons (approximately 25–40%). As the degree of LC neuronal loss increases, the animals do not display FST depressive-like behavior (i.e., the time spent immobile is not significantly different to that of vehicle-treated animals). The lowest dose of 6-OHDA used (5 μg/μl) consistently resulted in animals exhibiting a significant increase in immobility time compared to vehicle-treated animals. It is unclear what effect an even lower dose of 6-OHDA would have on LC neuronal number and FST immobility time. This is the first study to indicate there is an effect of LC neuronal loss on depressive-like behavior. Our findings may explain previously published work in which CNS NE concentrations were reduced but depressive-like behavior was not observed (Cryan et al., 2001, 2002, 2004; Togsverd et al., 2008). Depressive-like behavior may not have been observed in these studies because the reduction in CNS NE concentrations produced by deleting the dopamine-β-hydroxylase gene (Dbh knockout mice) or the administration of DSP-4 was extensive. The depressive-like behavior of a low dose of 6-OHDA seen with the FST was also observed with a second behavioral test, the sucrose consumption (‘anhedonia’) test.
The depressive-like behavior observed in animals administered 6-OHDA indicates a minimal amount of neuronal loss is required, a degree of LC loss that may be observed in the early stages of AD, a progressive neurodegenerative disorder. The association of a minimal loss of LC neurons to depressive-like behavior may explain the predominance of depression in MCI subjects and early AD subjects (Zubenko et al., 2003; Modrego and Ferrandez, 2004; Lee et al., 2007; Bhalla et al., 2009; Lyketsos et al., 2011; Lee et al., 2012; Wang et al., 2012; Geda et al., 2014; Lebedev et al., 2014; Van der Mussele et al., 2014). The loss of depressive-like behavior in mice as the degree of LC neuronal loss increases, also explains the remittance of depressive symptom as AD progresses to further cognitive impairment (Lee et al., 2007; Lyketsos et al., 2011; Wang et al., 2012; Lebedev et al., 2014; Van der Mussele et al., 2014) and presumably the loss of more LC neurons.
The mechanism(s) underlying depressive-like behavior with a minimal loss of LC neurons is(are) unclear. It can be hypothesized that when there is an initial loss of LC neurons, the surviving LC neurons respond to the loss by compensating in a manner that results in depressive-like behavior. However, when the loss exceeds a certain point, the surviving LC neurons are unable to compensate for the loss and without this ‘compensatory response’ the depressive-like behavior is not observed. This suggests the minimal loss of LC neurons triggers, or is associated with, an additional alteration in function that is required for the depressive-like behavior. Previous work with the administration of 6-OHDA directly into the LC documented that specific reduction in neuronal number was observed only in the LC; the lateral tegmental noradrenergic neurons and dopaminergic neurons in the substantia nigra/ventral tegmental region were unaffected (Szot et al., 2012c). However, noradrenergic terminals are consequently reduced throughout the brain in association with the neuronal loss (Szot et al., 2012c); and this reduced noradrenergic innervation could have consequences on other systems, which may result in the depressive-like behavior.
4.2. Alteration in electrophysiology of surviving LC neurons
In this study, the dose of 6-OHDA (5 μg/μl) that resulted in depressive-like behavior also resulted in specific alterations in the activity of the surviving LC neurons. The surviving LC neurons in the animals treated with 6-OHDA that resulted in a minimal loss of LC also demonstrated increased activity (increased firing frequency, more irregular firing pattern, and a higher percentage of cells firing in burst). At the present time it is unclear if the alteration in LC activity is observed when the loss of LC is increased. This pattern of LC activity is also observed in the Wistar Kyoto rat (Bruzos-Cidon et al., 2014), which is proposed to be an animal model of depression (Lahmame et al., 1997; Lopez-Rubalcava and Lucki, 2000).
Interestingly, recent work in AD has shown increased neuronal activity in multiple brain areas, including the frontal and temporal cortex and HP, particularly in MCI subjects (Stargardt et al., 2015). The cortex and HP receive sole noradrenergic innervation from the LC (Loughlin et al., 1986a,b), suggesting that in MCI and early stage AD subjects, when the loss of LC neurons is presumably minimal, there is increased neuronal activity in regions the LC innervates. This increase of neuronal activity is also observed in different animal models of AD, and precedes the deposition of β-amyloid (Stargardt et al., 2015). It may be linked to increased seizure activity observed in AD mouse models (Szot, 2012a). At the present time it is unclear if the there is a change in cortical or HP neuronal activity in animals with a minimal loss of LC neurons.
The exact cause of depression is still unclear, although it is well established that the noradrenergic nervous system is one of the neurotransmitter systems involved, in addition to serotonergic and dopaminergic systems. Initial belief was that depression was due to reduced noradrenergic function, but more recently the hypothesis of an increase in LC activity in depression has been postulated, this hypothesis has been supported mainly by studies that indirectly suggest an increase in LC activity in depression (Hoffman and Weiss, 1986; Simson and Weiss, 1988, 1989; Simson et al., 1986; Weiss and Simson, 1986, 1988; Stone et al., 2011). The most supportive data of an increased LC activity in depression is the decrease in LC activity observed with the administration of antidepressant drugs and electroconvulsive therapy (Grant and Weiss, 2001; West et al., 2009). There are few studies examining the number of LC neurons in depressed individuals because of the complication of antidepressant drugs (Chandley and Ordway, 2012); but there are several clinical studies that measured NE and its metabolites in the CSF of depressed subjects and the changes indicate an increase in LC activity (Coppen et al., 1979; Shaw et al., 1973; Wong et al., 2000; Ehnvall et al., 2003). At present, it is unknown if the 6-OHDA-treated animals with increased LC activity had altered synaptic NE levels compared to vehicle-treated animals. The reduced tissue content in the PFC and HP observed with 6-OHDA-induced neuronal loss does not necessarily reflect the amount of NE released from terminals. Following the administration of the neurotoxin DSP4 to animals, NE tissue content is severely reduced, but NE released from noradrenergic terminals is not significantly different from control animals (Hughes and Stanford, 1998; Kask et al., 1997). Future work will determine if NE release into the synapse in animals with a moderate loss of LC neurons is elevated compared to vehicle-treated animals.
The change in electrophysiological activity of the surviving LC neurons with a minimal loss of LC neurons may be considered a compensatory response. However, the shifts in specific parameters measured suggest that, still, baseline or stimulated LC neuronal activity is not ‘normal’. Importantly, assessment of a second depressive-like behavior, the sucrose consumption task, likewise was reduced in association with minimal LC neuronal loss, and swim-task increased immobility was observed in the same animals. Combined, the data from two different behavioral tasks strongly support a unique effect of minimal LC loss. Finally, and importantly, it should be pointed out that further loss of LC neurons did not result in increased immobility (Fig2B and D), suggesting behavioral specificity: mice were not immobile simply because they lost some critical source of stimulation for activity within the pool.
4.3. Administration of NE precursors
The depressive-like behavior observed with 6-OHDA was reversed with the prior acute administration of L-DOPA or DOPS. This normalization of FST in the 6-OHDA-treated animals was observed despite the inability of these NE precursors to normalize CNS NE concentrations. Administration of an acute dose of L-DOPA did not normalize NE concentration; however there is a trend for an increase in NE concentration in the PFC and HP. The acute administration of DOPS did not normalize NE concentration in the PFC and HP, but DOPS did normalize DOPA concentration in the HP and PFC, and MHPG in the PHC, indicating that a single injection of DOPS can normalize some catecholamine’s associated with NE synthesis and degradation.
One possibility for the apparent inability of L-DOPA or DOPS to elevate NE in this study may be related to the timing of the collection of PFC and HP tissue shortly after FST performance. The FST is a stressful behavioral test, and stress increases the activity of LC neurons and release of NE, which may reduce NE content (Koob, 1999). Another possibility is that very small (undetectable) changes in NE content can have an effect on behavioral outputs. Administration of DOPS to Dbh knockout mice 6 hr before seizure behavioral testing normalized the behavior (Szot et al., 1999); however, these behavioral changes were not necessarily associated with an increase of NE concentration in forebrain regions such as the FC and HP (Thomas et al., 1998).
4.4 Conclusion
These studies are the first to indicate that depressive-like behavior is observed in mice with a loss of LC neurons; however, the behavior is only observed when the loss of LC neurons is minimal. As LC neuronal loss increases, the depressive-like behavior is not observed (i.e. immobile time is similar to vehicle treated animals). The depressive-like behavior is reversed with the prior administration of NE precursors, and is associated with targeted changes in LC neuronal activity in the surviving neurons. The clinical implication of our findings is that the depression observed mainly in the early stages of AD can be attributed to a minimal loss of LC neurons. The progression of AD, with a progressive loss of LC neurons, may also explain why depression appears to remit in AD (Lee et al., 2007; Lyketsos et al., 2011; Wang et al., 2012; Lebedev et al., 2014; Van der Mussele et al., 2014).
Highlights.
5 μg/μl dose of 6-OHDA results LC neuronal loss than higher doses.
5 μg/μl dose of 6-OHDA results in depressive-like behavior
5 μg/μl dose of 6-OHDA alters electrophysiological properties of surviving LC neurons
NE precursors alleviate 5 μg/μl dose 6-OHDA induced depressive-like behavior
Acknowledgments
We thank Dr. David Weinshenker (Emory University, Atlanta, GA) for his generous donation of DOPS to perform the experiments outlined in this manuscript. We would like to thank SGIKer (UPV/EHU) for the animal care. We are grateful to Alison Michele Keeney (summer student from University of Washington) for her help. We are also grateful to Dianne Figlewicz, Ph.D. for her comprehensive review of the manuscript.
Footnotes
Funding and disclosure
This work was supported by Department of Veterans Affairs VISN 20 (Northwest Network) Mental Illness Research, Education, and Clinical Center (MIRECC; Patricia Szot, Allyn Franklin, Murray A Raskind), Geriatric Research, Education, and Clinical Center (GRECC; Carl Sikkema, Charles Wilkinson), University of Washington Department of Psychiatry and Behavioral Science (Patricia Szot, Murray A Raskind) and grants form the Basque Government (UFI 11/32) and the Spanish Government (FIS PI12/00613) (Cristina Miguelez, Luisa Ugedo). These institutions had no further competing financial interest in relation to the work described. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benoit M, Berrut G, Doussaint J, Bakchine S, Bonin-Guillaume S, Fremont P, et al. Apathy and depression in mild Alzheimer’s disease: a cross-sectional study using diagnostic criteria. J Alzheimer’s Dis. 2012;31:325–334. doi: 10.3233/JAD-2012-112003. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, et al. Reynolds CF. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/wnl.32.2.164. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011a;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011b;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer’s disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol Aging. 2013;34:286–297. doi: 10.1016/j.neurobiolaging.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Bruzos-Cidon C, Miguelez C, Rodriguez JJ, Gutierrez-Lanza R, Ugedo L, Torrecilla M. Altered neuronal activity and differential sensitivity to acute antidepressants of locus coeruleus and dorsal raphe nucleus in Wistar Kyoto rats: A comparative study with Srague Dawley and Wistar rats. Eur Neuropsychoparmacol. 2014;24:1112–1122. doi: 10.1016/j.euroneuro.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Chandley MJ, Ordway GA. Chapter 3: Noradrenergic dysfunction in depressed and suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (Fl): CRC Press; 2012. [Google Scholar]
- Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer’s type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- Coppen A, Rama Roa VA, Ruthven CR, Goodwin BL, Sandler M. Urinary 4-hydroxy-3-methoxyphenylglycol is not a predictor for clinical response to amitriptyline in depressive illness. Psychopharmacology. 1979;64:95–97. doi: 10.1007/BF00427352. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of parkinsonism: Chronic treatment with L-DOPA. N Engl J Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dalvi A, Jin S-H, Hirsch BR, Lucki I, Thomas SA. Use of dopamine-β-hydroxylase deficient mice to determine the role of norepinephrine in the mechanism of action of antidepressant drugs. J Pharmacol Expt Therap. 2001;298:651–657. [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidperessant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin S-H, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehnvall A, Sjogren M, Zachrisson OCG, Agren H. Lifetime burden of mood swings and activation of brain norepinephrine turnover in patients with treatment-refractory depressive illness. J Affect Dis. 2003;74:185–189. doi: 10.1016/s0165-0327(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkisnon’s disease. Neurobiol Dis. 2011;42:327–340. doi: 10.1016/j.nbd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJH, Pankratz VS, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, et al. Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neuropsychopharmacol. 2007;17:328–338. doi: 10.1016/j.euroneuro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophsiologic activity. Biol Psychiatry. 2001;49:117–129. doi: 10.1016/s0006-3223(00)00936-7. [DOI] [PubMed] [Google Scholar]
- Hoffman LJ, Weiss JM. Behavioral depression following clonidine withdrawal: a new animal model of long-lasting depression? Psychopharmacol Bull. 1986;22:943–949. [PubMed] [Google Scholar]
- Hughes ZA, Stanford SC. A partial noradrenergic lesion induced by DSP-4 increases extracellular noradrenaline concentration in frontal cortex: a microdialysis study in vivo. Pscyhopharmacology. 1998;136:299–303. doi: 10.1007/s002130050569. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, Tuomainen P, Rago L, Mannisto PT. Overflow of noradrenaline and dopamine in frontal cortex after [N-(2-chloroethyl)N-ethyl-2-bromobenzylamine] (DSP-4) treatment: in vivo microdialysis study in anesthetized rats. Naunyn Schmiedeberg’s Arch Pharmacol. 1997;355:267–272. doi: 10.1007/pl00004942. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Lahmame A, del Arco C, Pazos A, Yritia M, Armario A. Are Wistar-Kyoto rats a genetic animal model of depression resistant to antidepressants? Eur J Pharmacol. 1997;337:115–123. doi: 10.1016/s0014-2999(97)01276-4. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Beyer MK, Fritze F, Westman E, Ballard C, Aarsland D. Cortical changes associated with depression and antidepressants use in Alzheimer’s and Lewy body Dementia: an MRI surface-based morphometric study. Am J Geriatr Psychiatry. 2014;22:4–13. doi: 10.1016/j.jagp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Lee GL, Lu PH, Hua X, Lee S, Wu S, Nguyen K, et al. Depressive symptoms in mild cognitive impairment predict greater atrophy in Alzheimer’s Disease-related regions. Biol Psychiatry. 2012;71:814–821. doi: 10.1016/j.biopsych.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Waelsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatrics. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986a;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus noradrenaline neurons: morphologic subpopulations have different efferent targets. Neuroscience. 1986b;18:307–319. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcyniuk B, Mann DM, Yates PO. Loss of nerve cells from locus coeruleus in Alzheimer’s disase is topographically arranged. Neurosci Lett. 1986;64:247–252. doi: 10.1016/0304-3940(86)90336-8. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, White SS, Franklin A, Greenup JL, Leverenz JB, Raskind MA, et al. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res. 2011;1373:240–252. doi: 10.1016/j.brainres.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez C, Grandoso L, Ugedo L. Locus coeruleus and dorsal raphe neuron activity and response to acute antidepressant administration in a rat model of Parkinson’s disease. Int J Neuropsychopharmacol. 2011;14:187–200. doi: 10.1017/S146114571000043X. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer’s type: a prospective cohort study. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioral despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Blockade of alpha 2-adrenergic receptors, but not blockade of gamma-aminobutyric acid, serotonin, or opiate receptors, augments responsiveness of locus coeruleus neurons to excitatory stimulation. Neuropharmacology. 1989;28:651–660. doi: 10.1016/0028-3908(89)90147-0. [DOI] [PubMed] [Google Scholar]
- Simson PG, Weiss JM, Hoffman LJ, Ambrose MJ. Reversal of behavioral depression by infusion of an alpha-2 adrenergic agonist into the locus coeruleus. Neuropharmacology. 1986;25:385–389. doi: 10.1016/0028-3908(86)90232-7. [DOI] [PubMed] [Google Scholar]
- Shaw DM, O’Keeffe R, MacSweeney DA, Brooksbank BW, Noguera R, Coppen A. 3-Methoxy-4-hydroxyphenylglycol in depression. Psychol Med. 1973;3:333–336. doi: 10.1017/s003329170004962x. [DOI] [PubMed] [Google Scholar]
- Stargardt A, Swaab DF, Bossers K. The storm before the quiet: neuronal hyperactivity and Aβ in the presymptomatic stages of Alzheimer’s disease. Neurobiol Aging. 2015;36:1–11. doi: 10.1016/j.neurobiolaging.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67:193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P. Common factors among Alzheimer’s disease, Parkinson’s disease, and epilepsy: Possible role of the noradrenergic nervous system. Epilepsia. 2012a;53(Suppl 1):61–66. doi: 10.1111/j.1528-1167.2012.03476.x. [DOI] [PubMed] [Google Scholar]
- Szot P, Franklin A, Sikkema C, Wilkinson CW, Raskind MA. Sequential loss of LC noradrenergic and dopaminergic neurons results in a correlation of dopaminergic neuronal number to striatal dopamine concentration. Front Pharmacol. 2012b;3 doi: 10.3389/fphar.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Knight L, Franklin A, Sikkema C, Foster S, Wilkinson CW, et al. Lesioning noradrenergic neurons of the locus coeruleus in C57Bl/6 mice with unilateral 6-hydroxydopamine injection, to assess molecular, electrophysiological and biochemical changes in noradrenergic signaling. Neuroscience. 2012c;216:143–157. doi: 10.1016/j.neuroscience.2012.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, et al. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus coeruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine b-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Togsverd M, Werge TM, Tanko LB, Bagger YZ, Hansen T, Qin G, et al. Association of a dopamine beta-hydroxylase gene variant with depression in elderly women possibly reflecting noradrenergic dysfunction. J Affect Disord. 2008;106:169–172. doi: 10.1016/j.jad.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of the Alzheimer’s type. J Neurol Sci. 1981;49:419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Fernandez-Aedo I, Arrue A, Zumarraga M, Ugedo L. Role of GIRK channels on the noradrenergic transmission in vivo: an electrophysiological and neurochemical study on GIRK2 mutant mice. Int J Neuropsychopharmacol. 2013;16:1093–1104. doi: 10.1017/S1461145712000971. [DOI] [PubMed] [Google Scholar]
- Van der Mussele S, Marien P, Saerens J, Somers N, Goeman J, De Deyn PP, Engelborghs S. Behavioral syndromes in mild cognitive impairment and Alzheimer’s disease. J Alzheimer’s Dis. 2014;38:319–329. doi: 10.3233/JAD-130596. [DOI] [PubMed] [Google Scholar]
- Wang L, Potter GG, Krishnan RKR, Dolcos F, Smith GS, Steffens DC. Neural correlates associated with cognitive decline in late-life depression. Am J Geriatr Psychiatry. 2012;20:653–663. doi: 10.1097/JGP.0b013e31823e2cc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Simson PG. Depression in an animal model: focus on the locus ceruleus. Ciba Found Symp. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PE. Neurochemical and electrophysiological events underlying stress-induced depression in an animal model. Adv Exp Med Biol. 1988;245:425–240. doi: 10.1007/978-1-4899-2064-5_33. [DOI] [PubMed] [Google Scholar]
- West CHK, Ritchie JC, Boss-Williams K, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12:627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M-L, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry. 2003;160:857–866. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]