Summary
To identify genes and signaling pathways that initiate Neurofibromatosis type 1 (Nf1) neurofibroma, we used unbiased insertional mutagenesis screening, mouse models, and molecular analyses. We mapped an Nf1-Stat3-Arid1b/β-catenin pathway which becomes active in the context of Nf1 loss. Genetic deletion of Stat3 in Schwann cells progenitors (SCPs) and Schwann cells (SCs) prevents neurofibroma formation, decreasing SCP self-renewal and β-catenin activity. β-catenin expression rescues effects of Stat3 loss in SCPs. Importantly, P-STAT3 and β-catenin expression correlate in human neurofibromas. Mechanistically, P-Stat3 represses Gsk3β and the SWI/SNF gene Arid1b, to increase β-catenin. Knock-down of Arid1b or Gsk3β in Stat3fl/f;Nf1fl/fl;DhhCre SCPs rescues neurofibroma formation after in vivo transplantation. Stat3 represses Arid1b through histone modification in a Brg1 dependent manner, indicating that epigenetic modification plays a role in early tumorigenesis. Our data map a neural tumorigenesis pathway, and support testing JAK/STAT and Wnt/β-catenin pathway inhibitors in neurofibroma therapeutic trials.
eTOC Blurb
Wu et al map an Nf1-Stat3-Arid1b/β-catenin pathway that initiates Neurofibromatosis type 1 (Nf1) neurofibroma, using unbiased insertional mutagenesis screening. Stat3 transcriptionally represses Gsk3β and Arid1b, thereby activating β-catenin in Schwann cell precursors - resulting in neurofibroma initiation and maintenance. Stat3 mediated epigenetic modification plays a role in early tumorigenesis.
Introduction
Neurofibromas are benign peripheral nerve tumors that cause significant morbidity by disfigurement and tissue compression, and mortality if they compress vital organs (Boyd et al., 2009). Neurofibromas are a major feature of Neurofibromatosis type 1 (NF1), a common autosomal dominant disorder affecting about 1 in 3500 individuals. Surgery remains the mainstay of neurofibroma therapy but is rarely curative, so new treatments are urgently needed.
Peripheral nerve Schwann cells (SCs) are the primary pathogenic cell in neurofibromas, as biallelic mutation/loss of NF1 occurs uniquely in neurofibroma SCs (Serra et al., 1997). Neurofibromas may develop from SCs or SCPs, because inactivation of Nf1 at the SCP stage or in adult mice results in neurofibroma formation (Chen et al., 2014; Wu et al., 2008; Zhu et al., 2002). NF1 encodes the RasGAP protein neurofibromin, and Ras signaling is elevated in neurofibroma SCs (Cichowski and Jacks, 2001). Other genes and signaling pathways that drive neurofibroma initiation and growth are largely unknown.
STAT3 is a latent transcription factor implicated in cancer, which regulates cell-cycle progression and apoptosis. STAT3 phosphorylation at Y705 is essential for STAT3 dimerization, required for STAT3 binding to DNA-promoter regions and transcriptional activation (Battle and Frank, 2002). Fewer benign lesions formed when Stat3 was absent in prostate and skin tumors in vivo, implicating it tumor initiation (Kim et al., 2009; Kroon et al., 2013). Stat3 also regulates self-renewal and growth of glioma stem cells (Sherry et al., 2009). Recent studies on MPNST, aggressive nerve sarcomas, implicate Stat3 in their growth (Banerjee et al., 2010; Wu et al., 2014). The role of Stat3 in the benign nerve tumors (neurofibromas) has not been studied.
Stat3 activated β-catenin through GSK3b in hepatocytes (Moh et al., 2008). B-catenin is a developmental signaling pathway re-activated in many cancers. How β-catenin becomes elevated and if β-catenin plays a role in neurofibroma is unknown, although neurofibroma β-catenin expression was reported (Luscan et al., 2014; Mo et al., 2013; Watson et al., 2013). Supporting possible roles for β-catenin in nerve tumorigenesis, in vivo activation of β-catenin in developing SCs delays SC differentiation and results in sustained proliferation (Grigoryan et al., 2013).
Multi-subunit SWI/SNF chromatin remodeling complexes modulate transcription factor access to target genes, resulting in activation or repression of transcription (Tolstorukov et al., 2013). Recent studies demonstrate mutation/loss of chromatin remodeling genes in progression to MPNST (De Raedt et al., 2014; Lee et al., 2014), but are unstudied in neurofibromas. Mutational inactivation of SWI/SNF complex genes, including BRG1/SMARCA4, encoding a SWI/SNF ATPase, and SWI/SNF subunit genes ARID1A and ARID1B, are increasingly implicated in development and cancer (Helming et al., 2014; Sausen et al., 2013). When an ARID1B-containing SWI/SNF complex is present, interaction of STAT3 with DNA activates c-myc transcription in pre-osteoblast MC3T3-E1 cells (Nagl et al., 2007). Also, BRG1 interacts with β-catenin to promote target-gene activation in colon cancer cells (Barker et al., 2001). In patients with intellectual disability, ARID1B represses BRG1-dependent Wnt/β-catenin signaling (Vasileiou et al., 2015).
We report results of an unbiased in vivo Sleeping Beauty insertional mutagenesis transposon screen. We demonstrate a critical role of Stat3 in driving neurofibromas. Stat3 transcriptionally represses Gsk3β and the SWI/SNF complex subunit Arid1b, thereby activating β-catenin in SCPs - resulting in neurofibroma initiation and maintenance.
Results
A transposon system and pathway analysis implicate the Wnt and Stat3 pathways in neurofibroma formation
To identify mechanisms underlying neurofibroma growth and/or tumor progression, we used insertional mutagenesis. We generated quadruple transgenic animals (Nf1fl/fl;DhhCre;Rosa26-lsl-SB11;T2/Onc) (Figure S1A). Survival and onset of tumorigenesis did not differ from control Nf1fl/fl;DhhCre animals (not shown), and neurofibroma size was similar (p= 0.1996) (Figure S1B, upper panel). However, the number of neurofibromas isolated from experimental animals was higher (5.4 vs. 2.8; p = 0.1017) (Figure S1B, lower panel). The trend toward significance in this small sample set suggests that transposition-related genes might play roles in increasing neurofibroma numbers and/or growth.
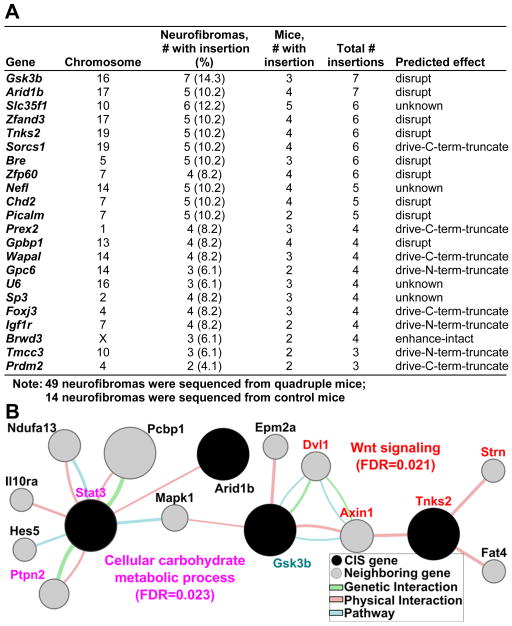
To identify potential genes responsible for neurofibroma tumorigenesis, we used high-throughput pyrosequencing of neurofibromas isolated from experimental quadruple transgenic animals. We identified 31 common transposon insertion sites (CIS). We removed CISs identified in control insertion-site mapping experiments in 3-week-old transgenic mouse tail DNA carrying both the T2/Onc and Rosa26-SB11 transgenes, and CISs identified in tumors from single mice. The remaining 22 CISs identified for neurofibroma tumorigenesis are shown in Figure 1A.
Figure 1. Genes identified by Sleeping Beauty Transposon System predict STAT3 and WNT pathway activation in neurofibroma.
(A) Common insertion sites (CIS) identified from neurofibromas. The positions were based on the Ensembl NCBI m37 April 2007 mouse assembly. (B) Genemania pathway analysis using 22 CIS (black) and 20 genes (grey) connected to CIS by genetic, physical or pathway analysis identifies two significantly deregulated networks. WNT signaling related gene names are shown in Red and Cellular carbohydrate metabolic process related genes are in pink. GSK3b (turquoise) connects these pathways to those in Supplemental Figure 2. Circle size correlates to network association probabilities.
We used the Genemania prediction server (http://www.genemania.org) to predict pathways, interactions, and functions of the 22 CIS genes, and identified networks including CIS genes. The most significantly deregulated pathways were Wnt signaling (FDR=0.021) including CIS genes Tnks and Gsk3β, and major neighboring genes: Strn, Axin1, and Dvl1, and Stat3-associated cellular carbohydrate metabolic process (FDR=0.023) including the CIS genes Gsk3β and Arid1b and major neighboring genes Stat3 and Ptpn2 (Figure 1B). Interestingly, Gsk3β connected these two pathways in this in silico analysis. No tumor tissue from these mice was available for confirmatory analysis. The other signaling pathways identified by Genemania are shown in Figure S1C.
STAT3/β-catenin signaling is activated in mouse and human neurofibromas
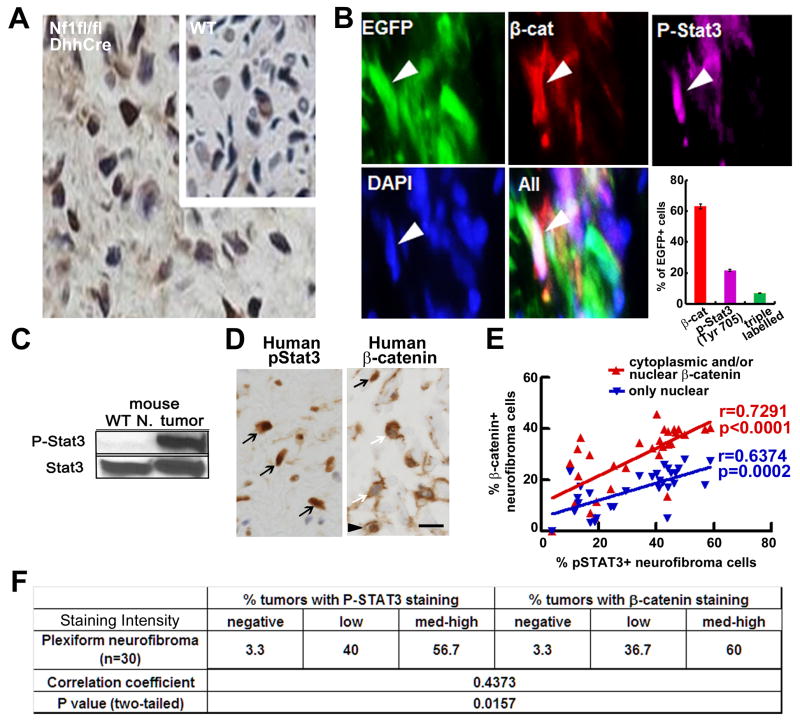
We focused on STAT3, a known oncogene and therapeutic target unstudied in neurofibroma. Antibodies recognizing P-Stat3-Y705 detected positive cells in all mouse GEM-neurofibromas (n=19) (Figure 2A), but not wild type mouse sciatic nerves (Figure 2A, insert). In contrast, P-Stat3-Ser727 was detectable in 1/4 mouse and 1/5 human neurofibromas (not shown). Given the link between the Stat and Wnt pathways identified by transposon mutagenesis, we tested if β-catenin is co-activated with P-Stat3 in neurofibroma SCs. DhhCre activates EGFP expression in the context of DhhCreNf1fl/fl mice in 40–50% of SCs, in a reporter mouse in which the CMV-β actin promoter and loxP flanked CAT gene are upstream of the Egfp cassette (Nakamura et al., 2006). We stained frozen sections of Nf1fl/fl;DhhCre;EGFP mouse neurofibromas with anti-P-Stat3 and anti-β-catenin. Of EGFP-expressing SCs, 21% were P-Stat3+ only, 62% expressed only β-catenin+ and, importantly, 6.7% of EGFP+ SCs were P-Stat3+ and β-catenin+ (Figure 2B). Thus, P-Stat3 and β-catenin can be co-activated in SCs in neurofibromas. We also detected Iba1+ macrophages that are P-Stat3+;EGFP− (not shown). Western blots confirmed robust P-Stat3-Y705 in mouse neurofibroma lysates versus wild-type peripheral nerve (Figure 2C).
Figure 2. P-STAT3 and β-catenin expression correlate in mouse and human neurofibromas.
(A) P-Stat3 immunostaining in mouse neurofibromas and wild type sciatic nerves (insert), visualized with DAB (brown). Nuclei are counterstained with hematoxylin (blue). (B) Representative immunofluorescent images show EGFP+ SCs (white arrows); some are also P- Stat3+ (purple) and β-catenin+ (red). Neurofibromas from 3 mice (4 sections/tumor) were stained. 350–500 EGFP+ cells were counted per section. DAPI (blue) staining highlights nuclei. Mean ± SEM is shown. (C) Western blot of P-Stat3-Y705 (P-Stat3) and total Stat3 (Stat3) in mouse neurofibroma (tumor) and wild-type sciatic nerve (WT nerve); blot is representative of neurofibromas (n=5) and wild-type nerves (n=3). (D) Representative pictures of immunostaining of P-STAT3(Y705) (left) and β-catenin (right) in human plexiform neurofibroma. In right, black arrow indicates nuclear β-catenin, white arrow indicates cytoplasmic β-catenin, black arrowhead indicates both. (E) Distribution of % P-STAT3 positive neurofibroma cells vs % nuclear β-catenin positive only (blue) or cytoplasmic and/or nuclear β-catenin positive (red) in 30 human plexiform neurofibromas. Spearman correlation coefficient analysis between P-STAT3(Y705) and β-catenin distribution (P-STAT3 vs total beta catenin shown in red, r=0.7219, p<0.0001; P-STAT3 vs nuclear beta catenin shown in blue, r=6374, p=0.0002, two tailed). (F) Quantification of intensity of P-STAT3(Y705) and β-catenin immune-positive cells in NF1 human plexiform neurofibromas and Spearman correlation coefficient analysis between P-STAT3(Y705) and β-catenin intensity (r=0.4373; p=0.0157, two-tailed). Bar=10μm.
We immunolabeled human plexiform neurofibroma sections to test if P-STAT3 and β-catenin expression correlate. Most (29/30) contained P-Y705-STAT3+ and β-catenin+ cells (Figure 2D–F). Some neurofibroma cells showed cytoplasmic staining (43%), others showed nuclear staining (23%) and 34.2% showed both. There was a significant correlation between P-STAT3 and total β-catenin (Figure 2E, F). Thus, the STAT3 and β-catenin pathways are activated in human neurofibromas, and highly correlated.
Targeted genetic deletion of Stat3 in SCs and SCPs decreases neurofibroma numbers and delays neurofibroma formation in vivo
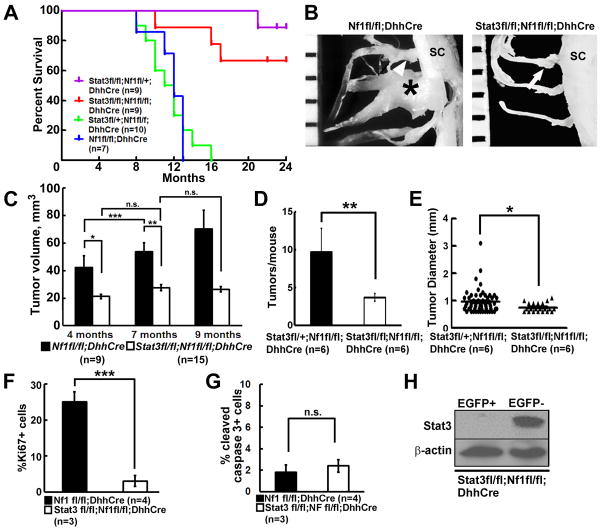
We then tested if activation of Stat3 in SCs/SCPs is necessary for neurofibroma initiation and/or maintenance. Loss of Stat3 in Stat3 fl/fl;DhhCre mice had no influence on peripheral (saphenous) nerve structure, as shown by electron microscopy; Remak bundles and myelinated axons showed normal differentiated morphology (Figure S2A.S2B). We bred Stat3fl/fl mice onto Nf1fl/fl;DhhCre mice; this required generating recombinants, as both Nf1 and Stat3 reside on mouse chromosome 11. Kaplan-Meier survival analysis revealed a significant difference in mouse survival between Stat3fl/fl;Nf1fl/fl;DhhCre mice and Stat3fl/+;Nf1fl/fl;DhhCre mice (p<0.001) or between Stat3fl/fl;Nf1fl/fl;DhhCre mice and Nf1fl/fl;DhhCre mice (p<0.001). Loss of one Stat3 allele does not influence survival (Stat3fl/+;Nf1fl/fl;DhhCre mice versus Nf1fl/fl;DhhCre mice p=0.66) (Figure 3A).
Figure 3. Targeted genetic deletion of Stat3 in SCs and SCPs delays neurofibroma formation in vivo.
(A) Kaplan-Meier survival curve. Purple: Stat3 fl/fl;Nf1fll+;DhhCre; Red, Stat3fl/fl;Nf1fl/fl;DhhCre; Blue: Stat3 fl/+;Nf1fl/fl;DhhCre. Green Nf1fl/fl;DhhCre. (B) Representative gross dissections of thoracic paraspinal neurofibromas and nerve roots in 9 month old Nf1fl/fl;DhhCre (left) and Stat3fl/fl;Nf1fl/fl;DhhCre (right) mice. Ruler shows 1 mm markings. (C) Neurofibroma volumes at 4, 7, and 9 months of age, measured in MRI images. Nf1fl/fl;DhhCre mice (n=12, black bars) and Stat3fl/fl;Nf1fl/fl;DhhCre mice (n=15, white bars) at 4, 7 and 9 months of age. (D) Average tumor number per mouse at 5 months in the Stat3fl/fl;Nf1fl/fl;DhhCre (white bar, n=6) and littermates Stat3fll+;Nf1fl/fl;DhhCre mice (black bar, n=6). (E) Tumor diameter in the Stat3fl/fl;Nf1fl/fl;DhhCre (circle, n=6 mice with 57 tumors) and littermates Stat3fll+;Nf1fl/fl;DhhCre mice (triangle, n=6 mice with 21 tumors). (F) Cell proliferation shown as percent Ki67+ cells in Nf1fl/fl;DhhCre mice (n=4, black bar) and Stat3fl/fl;Nf1fl/fl;DhhCre mice (n=3, white bar). (G) Cell death shown as percent cleaved caspase 3+ cells in Nf1fl/fl;DhhCre mice (n=4, black bar) and Stat3fl/fl;Nf1fl/fl;DhhCre mice (n=3, white bar). (H) Western blot on FACS-sorted EGFP+, EGFP- cells dissociated from adult Stat3fl/fl;DhhCre;EGFPfl/fl mouse sciatic nerves. Statistics: c: Ordinary one way ANOVA, d-g: unpaired student t test. *=p<0.05, **=p<0.01, **=p<0.001, n.s.=not significant.
Nf1fl/fl;DhhCre nerves continually and significantly increased in size, corresponding to neurofibroma initiation and neurofibroma growth reported in this model (Wu et al., 2008). Representative tumors at 9 months are shown in a Nf1fl/fl;DhhCre versus a Stat3fl/fl;Nf1fl/fl;DhhCre mouse (Figure 3B). We quantified total neurofibroma burden by volumetric measurement of MRI scans, followed by mixed effects analysis of tumor volume. Strikingly, double mutant nerves (average 20mm3) were similar to wild type nerves (8–19 mm3) (Figure 3C). The difference between controls (Nf1fl/fl;DhhCre or Stat3fl/+;Nf1fl/fl;DhhCre; n=9) and Stat3fl/fl;Nf1fl/fl;DhhCre mice (n=15) was significant (p<0.001 at 4, 7 and 9 months; Figure S2C, S2D).
In the Nf1fl/fl;DhhCre model each mouse develops 4–20 neurofibromas. If Stat3 contributes to neurofibroma initiation, then tumor number should be reduced in Stat3 mutants. Indeed, Stat3fl/fl;Nf1fl/fl;DhhCre mice had significant fewer tumors/mouse versus Stat3fll+;Nf1fl/fl;DhhCre littermates at spinal cord dissection at 5 months old mice (Figure 3D). Confirming volumetric MRI scan results, neurofibroma diameter measured at spinal roots on dissection was significantly smaller in Stat3fl/fl;Nf1fl/fl;DhhCre versus Stat3fll+;Nf1fl/fl;DhhCre mice (Figure 3E). Rare small neurofibromas showed hyperplasia or GEM-grade 1 neurofibroma histology (Figure S3). Ki67+ proliferating cells in neurofibroma tissue sections significantly decreased in Stat3fl/fl;Nf1fl/fl;DhhCre neurofibromas (Figure 3F); numbers of dying cells were unchanged (CC3+) (Figure 3G). Importantly, Stat3 protein was absent in flow cytometry-sorted EGFP+ SCPs and SCs from Stat3fl/fl;Nf1fl/fl;DhhCre,EGFP+ mice (Figure 3H); we did, however, detect increased levels of Stat1 and Stat5 (not shown). Therefore, glial cell Stat3 regulates tumor cell proliferation in neurofibromas, and activation of Stat3 in SCs and/or SCPs is important for neurofibroma initiation and growth.
Stat3 contributes to neurofibroma initiation
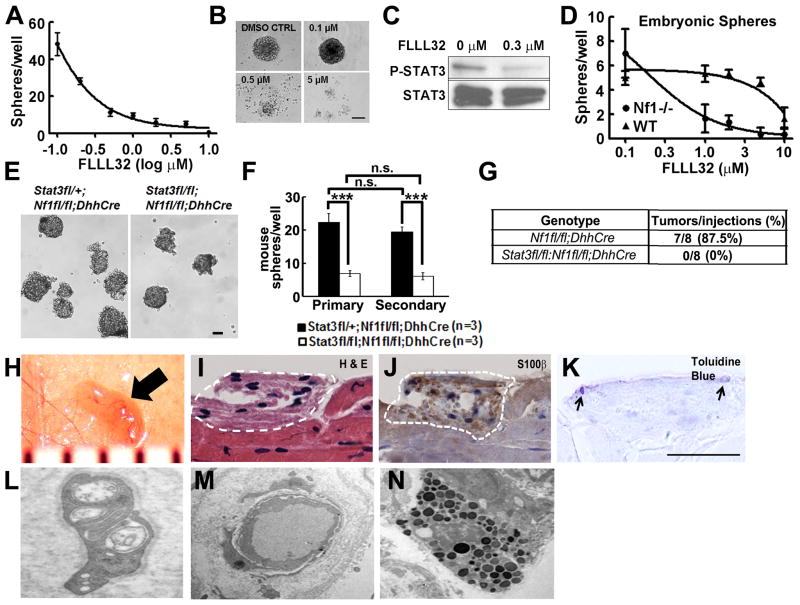
Our in vivo analyses could not distinguish function(s) of P-Stat3 in neurofibroma SCs versus SCPs, because the genetic loss-of-function targets both. To confirm that Stat3 is relevant to neurofibroma SCP-like cells we used sphere culture, enabling detection of growth and self-renewal of nervous system stem/progenitors. We isolated cells directly from human plexiform neurofibromas and cultured them as floating spheres at clonal density. We blocked STAT3 signaling with FLLL32, a JAK2/STAT3 inhibitor (Lin et al., 2010). FLLL32 inhibited human neurofibroma sphere formation (IC50 0.3 μM) (Figure 4A, 4B). Western blot confirmed decreased STAT3-Y705 phosphorylation and slightly reduced total STAT3 (Figure 4C). Importantly, FLLL32 inhibited the formation Nf1−/− SCP spheres (IC50 0.5–1μM), yet affected wild-type SCPs only at 10x higher concentration (Figure 4D). We also dissociated DRG/early neurofibromas from 6 week old mice. Stat3fl/fl;Nf1fl/fl;DhhCre cells formed significantly fewer primary and secondary spheres than Nf1fl/fl;DhhCre cells (Figure 4E–F); sphere size was similar (Figure 4E and not shown). We also depleted Stat3 from Nf1fl/fl;DhhCre neurofibroma SCP-spheres with shRNAs. Sphere numbers were significantly reduced 4 days after shStat3 infection, versus non-target controls (Figure S4A); we confirmed decreased Stat3 by Western blot (Figure S4B). Thus, Stat3 increases Nf1 mutant SCP self-renewal. In many cancers, self-renewing stem/progenitor-like cells contribute to tumorigenesis. Neurofibroma like lesions were detected in 7 of 8 nu/nu mice that were subcutaneously transplanted with Nf1fl/fl;DhhCre mice derived sphere cells (Figure 4G, 4H), while no lesion was detected in Stat3fl/fl;Nf1fl/fl;DhhCre derived sphere cells (Figure 4G). Hematoxylin and eosin staining of tissue sections showed no features of malignancy (Figure 4I), and anti-S100β+ cells SCs (Figure 4J) and mast cells (Figure 4K). On EM, these lesions contained SCs identified by their continuous basal lamina and wrapping of small axons (Figure 4L), blood vessels (Figure 4M) and mast cells (Figure 4N)—all features of neurofibroma. These in vitro and in vivo genetic results support the conclusion that Nf1−/− SCP self-renewal and neurofibroma tumorigenic potential are regulated by Stat3.
Figure 4. Stat3 contributes to neurofibroma initiation.
(A) The JAK2/STAT3 inhibitor FLLL32 inhibits formation of human neurofibroma spheres. DMSO (0) served as control. (B) Phase contrast images of human neurofibroma spheres treated with FLLL32 for 5 days. (C) Western blot of P-STAT3-Y705 and STAT3 in human neurofibroma spheres, +/−0.3 μM FLLL32. (D) Low doses of FLLL32 inhibit formation of mouse E12.5 Nf1−/− spheres; effects on E12.5 wild-type spheres are observed only at higher concentrations. (E) Phase contrast images of primary neurofibroma/DRG spheres from Stat3fl/+;Nf1fl/fl;DhhCre mice (left; control) and Stat3fl/fl;Nf1fl/fl;DhhCre mice (right). (F) Primary and secondary neurofibroma/DRG sphere number is reduced in the absence of Stat3 (n=3/group). (G) Neurofibroma-like lesions form after subcutaneous injection of Nf1fl/fl;DhhCre neurofibroma sphere cells but not in Stat3fl/fl;Nf1fl/fl;DhhCre mouse derived neurofibroma sphere cells. (H) Gross photograph of a lesion (black arrow) under reflected skin in a mouse injected with Nf1fl/fl;DhhCre neurofibroma sphere cells. Ruler shows 1 mm markings. (I) H & E stained section of F; lesion is indicated by white dotted line. (J) Immunohistochemistry showing S100β+ cells (brown) in tumor. Blue is hematoxylin counterstain. (K) Toluidine blue staining showing mast cells (black arrow). On EM, lesions contain SCs, identified by continuous basal lamina and wrapping of small axons (L), blood vessels (M) and mast cells (N). Bar=50μm B and E, others bar=20μm. Statistics: Ordinary one way ANOVA.
Stat3 activates β-catenin signaling
Data in Figures 1 and 2 suggest a Stat3-β-catenin link in neurofibroma SCs. Stabilized β-catenin translocates to the nucleus and alters target gene transcription (Liu et al., 2002). Stat3fl/fl;Nf1fl/fl;DhhCre neurofibromas showed significantly decreased active (nuclear) β-catenin (45%), increased inactive β-catenin (P-β-catenin, S33, S37, and T41) (591% in cytoplasm, 166% in nucleus) and decreased cyclin D1 expression (>90%) in cytoplasm and nucleus versus controls (Figure 5A). In contrast, P-Erk did not change (Figure 5A). Thus, neurofibroma β-catenin expression, localization, and target gene expression are regulated by Stat3.
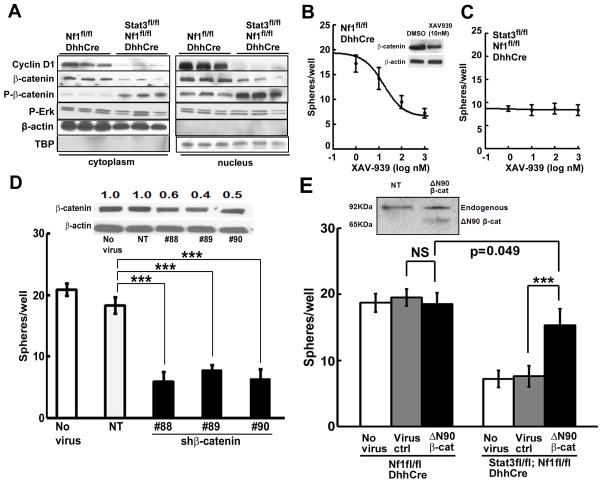
Figure 5. β-catenin signaling is a critical indicator of Stat3 in neurofibroma.
(A) Western blots of nuclear and cytoplasmic proteins from Stat3fl/fl;Nf1fl/fl;DhhCre or Nf1fl/fl;DhhCre mouse neurofibromas/DRGs with indicated antibodies. (B) β-catenin tankyrase inhibitor XAV-939 inhibits formation of Nf1fl/fl;DhhCre mouse neurofibroma spheres. DMSO (0) was control. Insert: 10nM XAV-939 inhibited β-catenin expression by 3 days. (C) Low dose XAV-939 has no effect on the formation of Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma spheres. (D) Three shβ-catenin shRNAs (#88, #89, and #90) each decrease mouse neurofibroma sphere formation, versus non-target lentivirus YFP (NT) or no virus controls. Insert shows Western blot confirming shβ-catenin-mediated knock down of total β-catenin. Anti-β-actin is loading control. Numbers show the ratio of β-catenin to β-actin loading control, then to no virus expression level. (E) Overexpression of β-catenin deltaN90 (ΔN90) in Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma/DRG spheres increased sphere numbers (black) versus virus (grey, p<0.001) or no virus controls (white, p<0.001). Overexpression of ΔN90 β-cat in Nf1fl/fl;DhhCre mouse neurofibroma/DRG spheres did not significantly increase sphere numbers (black) versus virus (grey, p=0.15) or no virus (white, p=0.43). Insert: Western blot detects endogenous 92KDa β-catenin and ~60KDa of overexpression of mutated ΔN90 β-cat in Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma spheres. Statistics: Ordinary one way ANOVA. Three independent experiments were performed in B, C and E.
To verify this conclusion in a system amenable for mechanistic analysis, we isolated SCPs from Nf1fl/fl;DhhCre neurofibromas and cultured them with or without blocking β-catenin signaling with the tankyrase inhibitor XAV-939, which stabilizes axin, a component of the β-catenin destruction complex. XAV-939 inhibited mouse neurofibroma sphere formation (IC50 0.1μM). Western blot confirmed a 50% decrease in total β-catenin with 3 days of drug exposure (10nM) (Figure 5B). Supporting the hypothesis that Stat3 is critical for β-catenin expression, remarkably, Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma spheres were insensitive to 100X higher concentrations of XAV-939 (Figure 5C). Depleting β-catenin with shRNAs in Nf1fl/fl;DhhCre neurofibroma SCP-like spheres also significantly reduced sphere numbers versus controls (Figure 5D); we confirmed decreased β-catenin by Western blot (Figure 5D insert). Thus β-catenin stabilization requires Stat3 in Nf1−/− neurofibroma derived SCP-like cells.
If β-catenin is critical in Stat3-driven neurofibromagenesis, then Stat3fl/fl;Nf1fl/fl;DhhCre spheres, which do not form tumors, should be rescued by β-catenin. To test this, we infected spheres with a stable, active, β-catenin mutant (ΔN90 β-cat) or virus control. Remarkably, overexpression of ΔN90 β-catenin significantly increased the number of Stat3fl/fl;Nf1fl/fl;DhhCre, but not Nf1fl/fl;DhhCre, neurofibroma spheres (Figure 5E). Importantly, 7/9 mice injected with ΔN90 β-catenin infected Stat3fl/fl;Nf1fl/fl;DhhCre spheres developed neurofibroma like lesions, while no lesions were detected in vector controls. Thus β-catenin is a major effector of Stat3 signaling in SCP, and necessary for neurofibromagenesis.
Stat3 alters Gsk3β inhibitory phosphorylation
We wondered how Stat3 activates β-catenin in neurofibromas. Gsk3β was a frequent CIS identified in our insertional mutagenesis screen, which predicted reduced GSK3β (Figure 1). GSK3β(Ser9) phosphorylation inhibits GSK3β activity, allowing β-catenin stabilization/activation (Wu and Pan, 2010). STAT3 can negatively regulate GSK3β transcription (Moh et al., 2008). Supporting the idea that Stat3 transcriptionally represses Gsk3β in neurofibromas, Gsk3β mRNA expression increased in Stat3fl/fl;Nf1fl/fl;DhhCre versus Nf1fl/fl;DhhCre neurofibromas (Figure S5A). ChIP using anti-Stat3 detected Stat3 bound to the Stat3 binding motif in the Gsk3β 5′ UTR in neurofibroma DNA (Figure S5B, S5C). Furthermore, in the absence of Stat3 total Gsk3β increased in neurofibromas (Figure S5D). Thus, in neurofibromas Stat3 represses Gsk3β transcription, correlating with a reduction in Gsk3β protein. However, targeting Gsk3β with shRNA did not significantly rescue sphere formation in Stat3fl/fl;Nf1fl/fl;DhhCre SCP spheres (Figure S5E), suggesting the existence of additional pathways.
Stat3 transcriptionally represses Arid1b expression
Arid1b was another frequent CIS identified by insertional mutagenesis (Figure 1A). Transposon insertions into the Arid1b locus predicted disrupted C-termini or truncated N-termini, e.g. inactivating insertions, and anti-Arid1b immunofluorescence was strong in WT SCs but reduced in neurofibroma SCs (Figure S6A). QRT-PCR confirmed negative regulation of Arid1b mRNA expression in neurofibromas by Stat3 (e.g. Arid1b mRNA is low in neurofibroma and increases in the absence of Stat3; Figure 6A). Similar results were observed in FACS-sorted EGFP+ neurofibroma SCs vs WT SCs (Figure S6B). We identified a putative Stat3 binding site 7kb downstream of the mouse Arid1b transcriptional start site (Figure 6B and Figure S7). When Nf1fl/fl;DhhCre neurofibroma DNA was subjected to ChIP using anti-Stat3, we detected Stat3 bound to Arid1b at this site by PCR (Figure 6C).
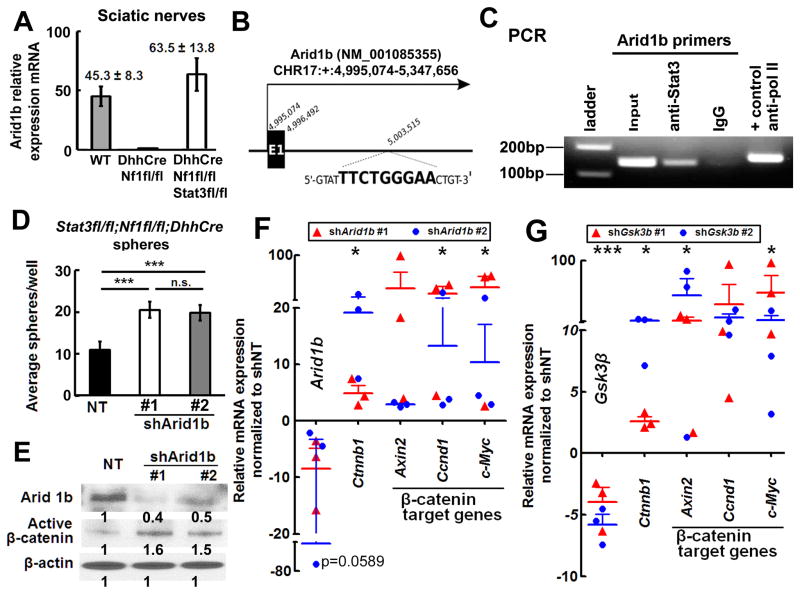
Figure 6. Stat3 transcriptionally represses Arid1b expression, activating β-catenin.
(A) qRT-PCR shows high Arid1b mRNA in Stat3fl/fl;Nf1fl/fl;DhhCre mouse sciatic nerve (white, n=6) versus WT (n=6) and Nf1fl/fl;DhhCre nerve (n=6). (B) Schematic, exon 1 mouse Arid1b gene. A putative Stat3 binding motif is between Exon 1 and Exon 2; the binding motif sequence is shown in bold. (C) Stat3 on the Arid1b promoter. PCR amplified a 139-bp Arid1b DNA fragment after ChIP with anti-Stat3. IgG was negative control. (D) Two Arid1b shRNAs increase numbers of Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma spheres. (E) Western blot shows knockdown of Arid1b and increased β-catenin in Stat3fl/fl;Nf1fl/fl;DhhCre mouse neurofibroma spheres 4 days after shArid1b infection in two different shRNA clones. (F–G) β-catenin target gene expression increases after shArid1b (F) or shGsk3β (G) infection of Stat3fl/fl;Nf1fl/fl;DhhCre neurofibroma spheres. Mean ± SEM is shown for 3 independent experiments and 2 clones of shRNA in D, F and G. A representative experiment (of 3) is shown in C. Statistics: Ordinary one way ANOVA.
Arid1b expression was low in Nf1fl/fl;DhhCre and high in Stat3fl/fl;Nf1fl/fl;DhhCre tumor-derived neurofibroma spheres (not shown). We exposed Stat3fl/fl;Nf1fl/fl;DhhCre neurofibroma spheres to shArid1b. Decreasing Arid1b expression significantly increased sphere numbers (Figure 6D), correlating with elevated levels of activated β-catenin protein (Figure 6E) and mRNA expression (Figure 6G), and elevated levels of the β-catenin target genes Axin2, Ccnd1, and Myc (Figure 6G). ShGsk3β increased β-catenin and its target gene mRNA expression in Stat3fl/fl;Nf1fl/fl;DhhCre spheres (Figure 6F), but did not rescue numbers of Stat3fl/fl;Nf1fl/fl;DhhCre spheres (Figure S5E, Figure 7B). Knockdown of Gsk3β and Arid1b simultaneously with shRNA showed similar effects to shArid1b alone (Figure 7A).
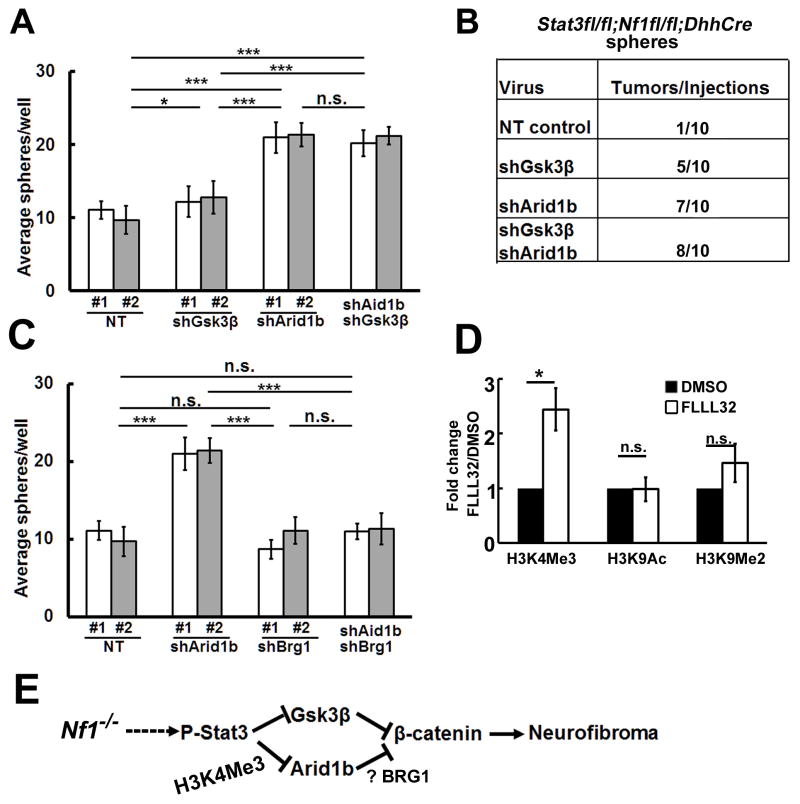
Figure 7. Arid1b and Gsk3β contribute to Stat3 mediated neurofibromagenesis.
(A) In vitro, shGsk3β does not fully rescue Stat3fl/fl;Nf1fl/fl;DhhCre sphere numbers. Simultaneous knockdown of Gsk3β and Arid1b shows similar effects to shArid1b alone. (B) Neurofibroma-like tumors form in Stat3fl/fl;Nf1fl/fl;DhhCre sphere cells infected with shArid1b, shGsk3β, or both and transplanted into nu/nu mice. (C) Brg1 is necessary for Stat3fl/fl;Nf1fl/fl;DhhCre sphere formation in cells treated with shArid1b. (D) CHIP shows enhancement of the H3K4Me3 mark at the Arid1b promoter. (F) Schematic shows a model of neurofibroma initiation: Loss of Nf1 in SCP causes activation of P-Stat3. P-Stat3 transcriptionally represses Arid1b and Gsk3β, increasing β-catenin activity. Mean ± SEM is shown for 3 independent experiments in Panels A, C, and D. Two different shRNA clones (#1 and #2) were used in A and C, For combination, we used shArid1b #1+shGsk3β #1 (white bar) or shArid1b #2+shGsk3β #2 (grey bar) in A. We used shArid1b #1+shBrg1 #1 (white bar) shArid1b #2+shBrg1 #2 (grey bar) on C. Statistics: Ordinary one way ANOVA.
To test whether tumor formation is affected by reduction in Arid1b, Gsk3β, or both, we transplanted sphere cells into nude mice. Stat3fl/fl;Nf1fl/fl;DhhCre spheres rarely formed tumors. Tumors formed in Stat3fl/fl;Nf1fl/fl;DhhCre sphere cells infected with shGsk3β, shArid1b or shArid1b + shGsk3β by 8 weeks after transplantation (p=0.0157; Figure 7B). There were no significant differences between the three experimental groups (p=0.3669), suggesting both Gsk3β and Arid1b are involved in Stat3-mediated neurofibromagenesis.
ARID1B acts in SWI/SNF complexes containing BRG1, the central catalytic subunit of numerous chromatin-modifying enzymatic complexes (Trotter and Archer, 2008). BRG1 can be required for Stat3 recruitment to target genes (Ni and Bremner, 2007). To test whether Arid1b requires Brg1 to affect sphere numbers, we knocked down Brg1 and/or Arid1b in Stat3fl/fl;Nf1fl/fl;DhhCre spheres with shRNA. The low numbers characteristic of Stat3fl/fl;Nf1fl/fl;DhhCre spheres were rescued by shArid1b, but not shBrg1. The combination of shArid1b together with shBrg1 prevented the shArid1b effect, indicating that the effect requires Brg1 and likely chromatin remodeling (Figure 7C).
Stat3 binds the Arid1b promoter fragment, repressing Arib1b expression. Arid1b’s known function is in chromatin remodeling, and we wondered if the Arid1b gene itself might be subject to Stat3-dependent histone modification. We performed CHIP with antibodies recognizing the histone modifications H3K4Me3, H3K9Me2, and H3K9Ac in vehicle or Stat3 inhibitor (FLLL32) treated primary mouse neurofibroma SCs at the Arid1b promoter. We detected a significant enhancement in the H3K4Me3 marks at the Arid1b promoter in three independent experiments (p<0.05). In contrast, H3K9Me2 or H3K9Ac did not significantly change at this site after FLLL32 exposure (Figure 7D). Taken together, in the setting of Nf1 loss, Stat3 transcriptionally represses the SWI/SNF gene Arid1b through histone H3K4Me3 modification and this repression is BRG1 dependent (Figure 7E). This results in an increase in β-catenin.
Discussion
Through performing an unbiased insertional mutagenesis screen, use of mouse genetics and SCP culture we identified an Nf1/P-Stat3/Arid1b/β-catenin pathway in SCP that is critical for neurofibroma initiation. P-Stat3 is a major neurofibroma oncogene as targeted genetic deletion of Stat3 in nerve SCs and SCPs dramatically delays neurofibroma formation and tumors, once formed, grow very slowly (Figure 2A–C). Stat3 provides a first example of a genetic loss of function affecting neurofibroma initiation and growth in vivo. Loss of Stat3 decreased numbers of neurofibroma SCP-like cells, neurofibroma SCP self-renewal, and neurofibroma formation by SCP after transplantation, functions defining cancer stem/progenitor-like cells in tumor initiation (Figure 4). Importantly, β-catenin expression rescued all Stat3-loss of function driven phenotypes in Nf1 mutant SCPs (Figure 5E). Supporting the relevance of our findings to human neurofibroma, primary patient-derived neurofibroma SCP-like cell sphere formation, a surrogate of tumor initiation, was dramatically reduced by Jak2/Stat3 inhibition (Figure 4A), and P-Y705-STAT3 and β-catenin expression were highly correlated in human plexiform neurofibromas (Figure 2).
We confirmed that Stat3 and β-catenin are detectable in human plexiform neurofibroma, and demonstrated that Stat3 and β-catenin are critical for neurofibroma formation in transplantation (Figure 5G). The Sleeping Beauty system and pathway analysis defined mutations predicting loss of Gsk3β and Arid1b, identifying the WNT and STAT pathways as players that might cooperate with loss of Nf1 in neurofibromagenesis (Figure 1). GSK3β and β-catenin signaling were previously implicated in MPNST, sarcomas that are malignant derivatives of neurofibromas (Mo et al., 2013; Rahrmann et al., 2013; Watson et al., 2013). Other CIS genes (WAPAL, SP3, BTBD9, and IGFR1) are up-regulated in neurofibroma cells, suggesting roles as proto-oncogenes early in tumor progression. Down-regulated CIS genes (TMCC3, SLC35F1, and SORCS) may have tumor suppressor functions.
Stat3 is present on the Gsk3β promoter in neurofibromas (Figure S5), consistent with Stat3 repressing GSK3β transcription in hepatocytes (Moh et al., 2008). In SCP, decreasing Gsk3β also increased β-catenin target gene expression, and tumor formation, but did not rescue SCP sphere formation. Given that shGsk3β or shArid1b enable tumor formation and β-catenin expression, but only shArid1b rescues neurofibroma sphere number, these genes likely use different mechanisms to repress β-catenin function; loss of either is sufficient to drive neurofibroma formation in the absence of Stat3, suggesting that interference with β-catenin signaling by one of several mechanisms will interfere with tumor formation.
We identify Arid1b as a critical link between P-Stat3 and β-catenin. ARID1B is a tumor suppressor, mutated by deletion, in neuroblastoma (Sausen et al., 2013). The ARID1B promoter can be hyper-methylated, resulting in decreased ARID1B expression, as in pancreatic cancer cells (Khursheed et al., 2013). Arid1b also functions as a tumor suppressor in the context of Nf1 loss, with low expression in neurofibromas resulting in increased β-catenin, Wnt/β-catenin target gene expression, and tumorigenesis (Figure 6). There was variation in the extent of Wnt/β-catenin target gene expression among samples on shRNA exposure, which is likely due to our use of primary cells. The differentiation state of individual cells in spheres and/or different levels of shRNA expression after lentiviral infection may account for this variation. While our study was nearing completion, Vasileiou et al. (2015) showed that, as in our study, reducing ARID1B increases Wnt/β-catenin target gene expression. They also overexpressed ARID1B by transient transfection and inhibited Wnt/β-catenin activity in cell lines. We were unable to overexpress ARID1B, as the ARID1B cDNA is too large for the lentiviral infection required in our primary cells. Nevertheless, together the two studies strongly support critical roles for ARID1B in regulation of Wnt/β-catenin activity.
We detected increased H3K4Me3 at the Arid1b promoter region after Stat3 inhibition. Given that the Stat3 binding site is in intron 1 of the Arid1b gene, it is likely that Stat3 binds to this intronic region and regulates Arid1b by antisense transcription, a mechanism by which mammalian genes regulate sense transcription (Faghihi and Wahlestedt, 2009; Magistri et al., 2012). Genome-wide mapping of chromatin modification will be necessary to further clarify this mechanism.
In summary, loss of Nf1 activates Stat3 SCPs, enabling tumor initiation by repressing the SWI/SNF gene Arid1b through histones H3K4Me3 and H3K27Me3 modification, thereby activating β-catenin. Knock-down of Arid1b in Stat3fl/f;Nf1fl/fl;DhhCre SCPs by shRNA is sufficient to recue neurofibroma formation in in vivo transplantation. Mouse and human NF1 mutant cells are significantly more sensitive than their wild type counterparts to treatment with a JAK2/STAT3 inhibitor (Figure 4A, 4D), suggesting that a therapeutic window will exist for neurofibroma therapy using JAK/STAT pathway inhibitors now in clinical trials (Lesina et al., 2011). Given that β-catenin targeted therapeutics are not yet proven, blocking β-catenin via STAT or targeting SWI/SNF complexes may be feasible strategies.
Experimental Procedures
Animals
Mice were housed in temperature- and humidity-controlled facilities on 12-hour dark-light cycles with free access to food and water. The animal care and use committees of Cincinnati Children’s Hospital Medical Center or University of Minnesota approved all animal procedures. See Supplemental Experimental Procedures for additional details.
Pyrosequencing and Genemania analysis
T2/Onc integration sites from 49 neurofibromas were cloned and sequenced using bar-coded primers and linker-mediated PCR, followed by pyrosequencing. Amplicon sequencing using the GS20 Flex pyrosequencing machine (Roche, Indianapolis, IN) was performed according to the manufacturer’s protocol. Primers used a unique 10-bp barcode-recognition sequence for each tumor sample. After removal of redundant and other non-specific noise as described (Keng et al., 2009), we obtained 6,353 non-redundant insertions. Next, we identified CISs with more Sleeping Beauty mutagenic transposon insertions than predicted based on Monte Carlo criteria for statistical significance. We defined CISs as regions in the genome with six insertions located within 185 kb of each other, five insertions within 95 kb, four insertions within 35 kb, or three insertions within 5 kb. We used the Genemania algorithm to generate gene-gene association networks (genetic interaction, physical interaction, and pathways), after inputting these CIS. This generated networks, each of which we extended using the top 20 related genes precomputed in the program. These are defined as neighboring genes. We similarly colored genes significantly enriched in a given “biological process” within a Gene-Ontology (GO) category, after applying a significance cutoff of FDR<0.05).
Embryonic and Neurofibroma sphere formation and SC culture
Embryonic mouse spheres, dissociated from E12.5 DRG with 0.25% Trypsin 20 min. at 37°C (Mediatech; Herndon, VA) produced single-cell suspensions with narrow-bore pipettes and a 70 μm strainer (BD-Falcon). For mouse or human neurofibroma spheres, we chopped tissue into 1–3 mm3 pieces, plated in 20mL L-15 (Mediatech) plus 0.5 mg/mL collagenase type 1 (Worthington; Lakewood, NJ), and 2.5 mg/mL dispase protease type II (Cambrex; East Rutherford, NJ) at 37°C for 4–6 hours. We plated trypan blue negative cells (Stem Cell Technologies, Vancouver, BC) at 1 × 104 cells in 1 mL per well in 24-well low-binding plates in medium containing DMEM:F-12 (3:1) + 20 ng/ml rhEGF (R&D Systems), 20 ng/ml rh bFGF (R&D Systems), 1% B-27 (Invitrogen), 2 μg/ml heparin (Sigma). We maintained cultures at 37°C and 5% CO2 and counted floating spheres after 4–7 days. To passage, we centrifuged sphere cultures, dissociated and plated at 1 × 104 cells/ml in fresh sphere medium as described (Williams et al., 2008). For each experiment, we show a representative of 3 independent experiments.
Immunohistochemistry
Tissue was embedded in paraffin, and 6-μm sections were cut and stained with either H&E, toluidine blue or incubated overnight at 4°C with antibody: anti-S100β, Dako, Carpinteria, CA), Ki67 (Novacastra Leica Microsystems, Buffalo Grove, IL) or anti-P-Stat3 (Y705), anti-cleaved caspase 3, β-catenin (Cell Signaling, Danvers, MA). Visualization methods were as described (Williams et al., 2008).
Western blots
Western blots were performed using antibodies recognizing P-Jak2, Jak2, P-Stat3, Stat3, P-β-catenin, β-catenin, P-GSK3β, GSK3β, and β-actin (Cell Signaling, MA). At least three different tumor/cell lysates were analyzed per antigen.
Tumorigenesis assay in nude mice
We injected 5×105 mouse sphere cells/injection subcutaneously into athymic female nude mice (Harlan, Indianapolis, IN). After two months we dissected tumors and fixed them in 4% paraformaldehyde overnight, then embedded in paraffin for histology.
Measurement of tumor number and tumor size
We perfused each mouse intracardially with 4% paraformaldehyde (w/v) in PBS, incubated overnight in 300 ml 4% paraformaldehyde, incubated overnight, again, in 50 ml decalcification solution (Cal-Rite, Richard Allan Scientific, Kalamazoo, MI), and then transferred to PBS. Using a Leica dissecting microscope, we dissected the spinal cord with attached DRG and nerve roots, and counted tumors. A tumor was defined as a mass surrounding the DRG and/or nerve roots, with a diameter greater than 1 mm, measured perpendicular to DRG/nerve roots.
Tumor volumetric measurement
MRI imaging and volumetric measurement of neurofibromas and statistical analyses using mixed effects modeling were as described (Wu et al., 2012).
Lentiviral infection
We infected secondary Nf1fl/fl;DhhCre neurofibroma spheres or Stat3fl/fl;Nf1fl/fl;DhhCre DRG/neurofibroma spheres with shRNAs and non-target control (Sigma, St Louis, MO, USA), β-catenin overexpression lentivirus ΔN90 (Addgene, Cambridge MA) or same backbone control (Sigma). We incubated lentiviral particles with neurofibroma spheres for 3–5 days and counted sphere numbers. For in vivo xenografts, Stat3fl/fl;Nf1fl/fl;DhhCre DRG/neurofibroma spheres were infected with lentivirus in the presence of polybrene (8 μg/ml; Sigma-Aldrich) for 16–20 hours, followed by selection in G418 (500mg/ml; Sigma-Aldrich). Spheres were collected and dissociated for xenograft injection.
Statistics
Kaplan Meier analysis used a Gehan-Breslow-Wilcox log-rank test. Neurofibroma growth was modeled by Mixed Effects Model Analysis. P values were generated with a random effects model analysis on log transformed tumor volume data using the SAS mixed procedure (Jessen et al., 2013). We used unpaired 2-tailed Student’s t tests to analyze significance of cell proliferation and cell death quantification in tissue sections when two samples were compared. Other experiments used Ordinary one way ANOVA, reported as mean ± SEM. P<0.05 was considered significant.
Supplementary Material
Highlights.
Insertional mutagenesis identifies STAT3 as a driver of benign neurofibroma.
Stat3 activates β-catenin to initiate neurofibroma formation.
Stat3 represses Gsk3β and Arid1b to increase beta-catenin.
Neurofibroma-initiating cells require Stat3 and β-catenin for tumorigenesis.
Acknowledgments
We thank the Minnesota Supercomputing Institute for computational resources for sequence analysis, Dr. Ari Melnick (Weil Cornell Medical School) for assistance in design of histone modification analysis, and Ms. Huiqing Li for animal husbandry. This work was supported by NIH R01 NS28840 (N.R.), NIH P50 NS057531 (N.R. and D.L.), a DAMD New Investigator Award (W81XWH-11-1-0259) and an Ohio State University Comprehensive Cancer Center Pelotonia Idea Grant (J.W.), and the American Cancer Society (IRG-67-003-44) (J.R.F.). The CHTN provided some benign neurofibromas used in this study (IRB approved).
Footnotes
Author Contributions
Conception and design: N.R. and D.A.L.
Acquisition of data: J.W., V.K., D.M.P., J.K.K., A.V.P., E.J., W.J.J., K.C., B.R.T., K.T.S., D.F., E.D., G.H., J.A.C., A.O.S.
Provided resources: E.B.S. and J.R.F, FLLL32; D.E.L., Stat3fl/fl mouse, R.J.S., human samples.
Analysis and interpretation of data (including statistical analysis): J.W., V.K., Y.Z., M.K., N.R., D.A.L.
Writing and review the manuscript: J.W., V.K., N.R., D.A.L.
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee S, Byrd JN, Gianino SM, Harpstrite SE, Rodriguez FJ, Tuskan RG, Reilly KM, Piwnica-Worms DR, Gutmann DH. The neurofibromatosis type 1 tumor suppressor controls cell growth by regulating signal transducer and activator of transcription-3 activity in vitro and in vivo. Cancer Res. 2010;70:1356–1366. doi: 10.1158/0008-5472.CAN-09-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1–14. doi: 10.1016/j.jaad.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Liu C, Patel AJ, Liao CP, Wang Y, Le LQ. Cells of origin in the embryonic nerve roots for NF1-associated plexiform neurofibroma. Cancer Cell. 2014;26:695–706. doi: 10.1016/j.ccell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- De Raedt T, Beert E, Pasmant E, Luscan A, Brems H, Ortonne N, Helin K, Hornick JL, Mautner V, Kehrer-Sawatzki H, et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature. 2014;514:247–251. doi: 10.1038/nature13561. [DOI] [PubMed] [Google Scholar]
- Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10:637–643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Stein S, Qi J, Wende H, Garratt AN, Nave KA, Birchmeier C, Birchmeier W. Wnt/Rspondin/beta-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proc Natl Acad Sci U S A. 2013;110:18174–18179. doi: 10.1073/pnas.1310490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helming KC, Wang X, Wilson BG, Vazquez F, Haswell JR, Manchester HE, Kim Y, Kryukov GV, Ghandi M, Aguirre AJ, et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat Med. 2014;20:251–254. doi: 10.1038/nm.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim MO, Dombi E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123:340–347. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng VW, Villanueva A, Chiang DY, Dupuy AJ, Ryan BJ, Matise I, Silverstein KA, Sarver A, Starr TK, Akagi K, et al. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat Biotech. 2009;27:264–274. doi: 10.1038/nbt.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursheed M, Kolla JN, Kotapalli V, Gupta N, Gowrishankar S, Uppin SG, Sastry RA, Koganti S, Sundaram C, Pollack JR, Bashyam MD. ARID1B, a member of the human SWI/SNF chromatin remodeling complex, exhibits tumour-suppressor activities in pancreatic cancer cell lines. Br J Cancer. 2013;108:2056–2062. doi: 10.1038/bjc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Kataoka K, Rao D, Kiguchi K, Cotsarelis G, Digiovanni J. Targeted disruption of stat3 reveals a major role for follicular stem cells in skin tumor initiation. Cancer Res. 2009;69:7587–7594. doi: 10.1158/0008-5472.CAN-09-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon P, Berry PA, Stower MJ, Rodrigues G, Mann VM, Simms M, Bhasin D, Chettiar S, Li C, Li PK, et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013;73:5288–5298. doi: 10.1158/0008-5472.CAN-13-0874. [DOI] [PubMed] [Google Scholar]
- Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1227–1232. doi: 10.1038/ng.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski M, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E, Pandit B, Ihnat M, Shenoy S, Kulp S, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–2454. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Luscan A, Shackleford G, Masliah-Planchon J, Laurendeau I, Ortonne N, Varin J, Lallemand F, Leroy K, Dumaine V, Hivelin M, et al. The Activation of the WNT Signaling Pathway Is a Hallmark in Neurofibromatosis Type 1 Tumorigenesis. Clin Cancer Res. 2014;20:358–371. doi: 10.1158/1078-0432.CCR-13-0780. [DOI] [PubMed] [Google Scholar]
- Magistri M, Faghihi MA, St Laurent G, 3rd, Wahlestedt C. Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet. 2012;28:389–396. doi: 10.1016/j.tig.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W, Chen J, Patel A, Zhang L, Chau V, Li Y, Cho W, Lim K, Xu J, Lazar AJ, et al. CXCR4/CXCL12 mediate autocrine cell-cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell. 2013;152:1077–1090. doi: 10.1016/j.cell.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moh A, Zhang W, Yu S, Wang J, Xu X, Li J, Fu XY. STAT3 sensitizes insulin signaling by negatively regulating glycogen synthase kinase-3 beta. Diabetes. 2008;57:1227–1235. doi: 10.2337/db06-1582. [DOI] [PubMed] [Google Scholar]
- Nagl NG, Jr, Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 2007;26:752–763. doi: 10.1038/sj.emboj.7601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Colbert M, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bremner R. Brahma-related gene 1-dependent STAT3 recruitment at IL-6-inducible genes. J Immunol. 2007;178:345–351. doi: 10.4049/jimmunol.178.1.345. [DOI] [PubMed] [Google Scholar]
- Rahrmann EP, Watson AL, Keng VW, Choi K, Moriarity BS, Beckmann DA, Wolf NK, Sarver A, Collins MH, Moertel CL, et al. Forward genetic screen for malignant peripheral nerve sheath tumor formation identifies new genes and pathways driving tumorigenesis. Nat Genet. 2013;45:756–766. doi: 10.1038/ng.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausen M, Leary RJ, Jones S, Wu J, Reynolds CP, Liu X, Blackford A, Parmigiani G, Diaz LA, Jr, Papadopoulos N, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra E, Puig S, Otero D, Gaona A, Kruyer H, Ars E, Estivill X, Lazaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997;61:512–519. doi: 10.1086/515504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstorukov MY, Sansam CG, Lu P, Koellhoffer EC, Helming KC, Alver BH, Tillman EJ, Evans JA, Wilson BG, Park PJ, Roberts CW. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proc Natl Acad Sci U S A. 2013;110:10165–10170. doi: 10.1073/pnas.1302209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter KW, Archer TK. The BRG1 transcriptional coregulator. Nucl Recept Signal. 2008;6:e004. doi: 10.1621/nrs.06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiou G, Ekici AB, Uebe S, Zweier C, Hoyer J, Engels H, Behrens J, Reis A, Hadjihannas MV. Chromatin-Remodeling-Factor ARID1B Represses Wnt/beta-Catenin Signaling. Am J Hum Genet. 2015;97:445–456. doi: 10.1016/j.ajhg.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AL, Rahrmann EP, Moriarity BS, Choi K, Conboy CB, Greeley AD, Halfond AL, Anderson LK, Wahl BR, Keng VW, et al. Canonical Wnt/beta-catenin signaling drives human schwann cell transformation, progression, and tumor maintenance. Cancer Discov. 2013;3:674–689. doi: 10.1158/2159-8290.CD-13-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Wu J, Johansson G, Rizvi TA, Miller SC, Geiger H, Malik P, Li W, Mukouyama YS, Cancelas JA, Ratner N. Nf1 mutation expands an EGFR-dependent peripheral nerve progenitor that confers neurofibroma tumorigenic potential. Cell Stem Cell. 2008;3:658–669. doi: 10.1016/j.stem.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in biochemical sciences. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dombi E, Jousma E, Scott Dunn R, Lindquist D, Schnell BM, Kim MO, Kim A, Widemann BC, Cripe TP, Ratner N. Preclincial testing of sorafenib and RAD001 in the Nf1(flox/flox); DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2012;58:173–180. doi: 10.1002/pbc.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Patmore DM, Jousma E, Eaves DW, Breving K, Patel AV, Schwartz EB, Fuchs JR, Cripe TP, Stemmer-Rachamimov AO, Ratner N. EGFR-STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene. 2014;33:173–180. doi: 10.1038/onc.2012.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns D, Parada L. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.