Abstract
Over 90 percent of patients with Parkinson's disease experience speech‐motor impairment, namely, hypokinetic dysarthria characterized by reduced pitch and loudness. Resting‐state functional connectivity analysis of blood oxygen level‐dependent functional magnetic resonance imaging is a useful measure of intrinsic neural functioning. We utilized resting‐state functional connectivity modeling to analyze the intrinsic connectivity in patients with Parkinson's disease within a vocalization network defined by a previous meta‐analysis of speech (Brown et al., 2009). Functional connectivity of this network was assessed in 56 patients with Parkinson's disease and 56 gender‐, age‐, and movement‐matched healthy controls. We also had item 5 and 18 of the UPDRS, and the PDQ‐39 Communication subscale available for correlation with the voice network connectivity strength in patients. The within‐group analyses of connectivity patterns demonstrated a lack of subcortical–cortical connectivity in patients with Parkinson's disease. At the cortical level, we found robust (homotopic) interhemispheric connectivity but only inconsistent evidence for many intrahemispheric connections. When directly contrasted to the control group, we found a significant reduction of connections between the left thalamus and putamen, and cortical motor areas, as well as reduced right superior temporal gyrus connectivity. Furthermore, most symptom measures correlated with right putamen, left cerebellum, left superior temporal gyrus, right premotor, and left Rolandic operculum connectivity in the voice network. The results reflect the importance of (right) subcortical nodes and the superior temporal gyrus in Parkinson's disease, enhancing our understanding of the neurobiological underpinnings of vocalization impairment in Parkinson's disease. Hum Brain Mapp 36:1951–1962, 2015. © 2015 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: Parkinson's disease, neuroimaging, resting‐state, functional connectivity, voice network
Abbreviations
- BG
basal ganglia
- BOLD
blood‐oxygenation‐level‐dependent
- fMRI
functional MRI
- HD
hypokinetic dysarthria
- PDQ‐39
Parkinson's Disease Questionaire‐39
- RO
Rolandic operculum
- ROI
region of interest
- SMA
supplementary motor area
- STG
superior temporal gyrus
- UPDRS
Unified Parkinson's Disease Rating Scale
- vPM
ventral premotor
INTRODUCTION
Disruption of speech motor control is a very frequent and at the same time very disabling symptom of Parkinson's Disease [Duffy, 2005]. It is estimated that over 90% of patients with Parkinson's disease develop a speech disorder known as hypokinetic dysarthria [HD; Ramig et al., 2011]. Among the many speech symptoms associated with HD, impairments in vocalization are perhaps the most prominent and frequently observed [Duffy, 2005]. Vocalization deficits associated with HD involve an overall reduction as well as reduced variability of pitch and loudness during speech [Ramig et al., 2011; Rektorova et al., 2012]. In addition, self‐monitoring of speech is abnormal in Parkinson's disease [Rektorova et al., 2012].
Understanding the neurobiological basis of voice production in Parkinson's disease would provide a better understanding of these deficits, and provide important information for the development of new treatment approaches. The functional neural mechanisms that give rise to the voice disorders in Parkinson's disease, however, are poorly understood. Pinto et al. [2011] used functional MRI (fMRI) to compare limb versus speech movement activations in patients with Parkinson's disease. Both speech and hand movement tasks revealed globally reduced activation levels in Parkinson's disease relative to healthy controls. While a simultaneous speech and hand movement task induced activations representing the sum of the isolated hand plus isolated speech tasks in healthy participants, activations of the simultaneous tasks in patients with Parkinson's disease resulted in activations similar to that of the hand movement task. Furthermore, patients with Parkinson's disease exhibited greater activation in the dorsolateral prefrontal cortex and cingulate cortex. This study thus pointed towards aberrant and generally reduced neural activation during speech in patients with Parkinson's disease, as well as the additional effort and neural recruitment that are necessary for patients with Parkinson's disease while performing dual motor tasks.
Recently, a seed‐to‐whole‐brain correlational analysis to assess task‐related functional connectivity of the periaquaductal gray matter in Parkinson's disease related to healthy controls showed increased functional connectivity of periaquaductal gray matter to the right basal ganglia (BG), posterior superior temporal gyrus (STG), supramarginal and fusiform gyri, as well as the inferior parietal lobe [Rektorova et al., 2012]. These authors also noted that connection strength with the right putamen and supramarginal gyri was correlated with pitch variability in Parkinson's disease. In addition, connectivity strength of periaquaductal gray matter to right posterior STG and inferior parietal lobe was correlated with voice loudness.
An important development in clinical neuroimaging research using fMRI has been the emergence of resting‐state functional connectivity analyses. The clinical value of analyzing brain connectivity patterns based on inter‐regional correlations of activity patterns in a task free state emerges from several important aspects: (1) the subject only has to be still in the scanner, removing performance confounds, (2) the average time required to collect resting state data is about 10 min, (3) particularly pertinent to the current study, resting‐state functional connectivity patterns have been shown to be highly sensitive to disease states [e.g., Clos et al., 2014; Sommer et al., 2012], and (4) resting‐state functional connectivity corresponds to task‐related activation patterns underlining the physiological validity of these measurements [e.g., see Eickhoff and Grefkes, 2011; Rehme et al., 2012; Rottschy et al., 2013; Smith et al., 2012 for a comparison]. Though a known confound in functional resting state analyses is spurious trends in connectivity patterns due to movement artifact, there are well‐established experimental design and statistical methods to control for invalid findings, such as matching subjects based on similar estimates of movement [Dijk et al., 2012; Power et al., 2012; Satterthwaite et al., 2013a].
As we were explicitly interested in the network of regions associated with the control of vocalization in general (as opposed to specifically speech production), we focused on the connectivity in a vocalization network. The regions constituting this network have already been well defined by a recent meta‐analysis [Brown et al., 2009; Table 1]. In particular, the regions forming the meta‐analytically defined network were supplementary motor area (SMA), left and right Rolandic operculum (RO), left and right ventral premotor cortex (vPM), left and right STG, left and right putamen, left and right thalamus, and left and right cerebellum VI. We analyzed the functional connectivity between these regions within and between patients with Parkinson's disease and healthy subjects. Furthermore, in order to explore the relationship between the resting‐state functional connectivity in this voice network and clinical measures of voice pathology in Parkinson's disease, we correlated resting‐state functional connectivity to clinical‐behavioral measures, in particular disease duration, communication‐relevant items of the Unified Parkinson's Disease Rating Scale (UPDRS), and the Parkinson's Disease Questionnaire – 39 (PDQ‐39). We proposed that the Parkinson's disease group would have generally reduced connectivity in the voice network relative to the control group, in particular among the putamen and thalamus, due to the their role in the direct and indirect motor loops, and evidence in their increased activation following voice treatment for hypophonia [Cerasa et al., 2014; Gorges et al., 2013; Hacker et al., 2012; Jellinger, 2002; Krajcovicova et al., 2012; Liotti et al., 2003; Tessitore et al., 2012a, 2012b]. Following the same notion, we did not expect cerebellum connectivity to differentiate patients with Parkinson's disease, as it is not a region related to vocalization in this population (Narayana et al., 2010). Furthermore, we expected connectivity to be associated with the communication‐relevant UPDRS and PDQ‐39 items.
Table 1.
Network seed coordinates in MNI space
| Regions of Interest | MNI‐Space (x,y,z) | ||
|---|---|---|---|
| Rolandic Operculum (4/6/43) | 66 | 0 | 21 |
| −61 | 1 | 21 | |
| Ventral premotor cortex (4/6) | 60 | 1 | 37 |
| −52 | −5 | 41 | |
| Supplementary motor area (6) | 8 | 8 | 62 |
| Superior temporal gyrus (22) | 64 | −27 | 8 |
| −64 | −22 | 3 | |
| Putamen | 27 | 3 | −8 |
| −23 | 6 | 0 | |
| Ventral thalamus | 16 | −19 | 4 |
| −10 | −18 | −3 | |
| Cerebellum VI | 27 | −64 | −21 |
| −36 | −55 | −32 | |
MATERIALS AND METHODS
Participants
Fifty‐six patients diagnosed with Parkinson's disease (39 males, 17 females; age M = 62.07 (SD = 9.16); disease duration in years M = 4.93 (SD = 3.98); See Table 2 for clinical and demographic details for each site) and 56 age‐, gender‐, and movement‐matched [39 males, 17 females; age M = 60.73 (SD = 9.30)] healthy volunteers without any record of neurological or psychiatric disorders from three sites were included in the within‐group and between‐group analyses of resting state connectivity of the voice network. All 112 participants provided data that met quality threshold for movement parameters, yet were also matched between groups based on head‐movement (see Table 3). Subject matching between groups was possible by selecting healthy controls from a large pre‐existing database. Forty‐one of the 56 patients had symptom measures available for the behavioral correlation analysis. Patients were recruited from in‐ and out‐patient departments of University Hospitals in Aachen, Cologne, and Düsseldorf, Germany in order to obtain a more realistic assessment of the overall PD population, as well as to boost statistical power to detect any effects, even in the presence of potentially increased variance. Diagnoses were made by the attending neurologist based on a physical exam in the hospital, according to established ICD‐10 criteria for idiopathic Parkinson's disease. Symptoms were furthermore quantified by the UPDRS‐III. All subjects gave written consent to participate in the study as approved by the particular site's ethics committee. The ethics committee of the University of Düsseldorf approved the joint analysis of data from all combined sites. Patients with Parkinson's disease had not received any voice therapy or surgical implants (deep brain stimulation). All Parkinson's disease measures were administered while patients were on their medication. All patients were treated with Levadopa or dopaminergic agonists, though there was a marked difference in the exact compounds used, relative to the dosage and comedication. The reason for this was that patients were recruited in a naturalistic setting, and hence, each patient's treatment plan was the result of the ongoing evaluation of the attending neurologist. As a heterogeneous factor, medication may be considered a partial source of variance in the Parkinson's disease group's results. By not controlling for this source of variance, our analyses may be considered a conservative approach to identifying aberrant resting state network properties in Parkinson's disease.
Table 2.
Patient and Control characteristics. UPDRS = Unified Parkinson's Disease Rating Scale; PDQ‐39 = Parkinson's Disease Questionnaire‐39
| Healthy controls | Parkinson's patients | Disease | Motor assessment | Speech assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | n (female) | Age (SD) | n (female) | Age (SD) | Duration (SD) | Onset (SD) | UPDRS Item 5 (SD) | UPDRS Item 5 (SD) | UPDRS ltem 18 (SD) | PDQ‐39 Com. (SD) |
| Aachen | 28 (11 ♀) | 63.4 ± 5.2 | 26 (8 ♀) | 64.6 ± 8.8 | 3.9 ± 3.4 | 60.0 ± 8.4 | 25.7 ± 16.4 | 1 ± 0.8 | 1 ± 0.8 | 2.1 ± 1.9 |
| Cologne | 13 (0 ♀) | 62.2 ± 6.1 | 11 (0 ♀) | 62.5 ± 8.0 | 6.6 ± 3.0 | 57.6 ± 9.5 | 13.8 ± 7.4 | ‐ | 0 ± 0.4 | ‐ |
| Duesseldorf | 15 (10 ♀) | 54.4 ± 14.0 | 19 (9 ♀) | 58.4 ± 9.5 | 6.1 ± 6.3 | ‐ | ‐ | ‐ | ‐ | ‐ |
Table 3.
Between‐group matching on measures of head movement, by site. DVARS = derivative of RMS variance over voxels; FD = frame‐wise displacement; RMS = root mean squared movement
| Site | Subject Group | DVARS Mean (SD) | P (t‐test) | FD Mean (SD) | P (t‐test) | RMS Mean (SD) | P (t‐test) |
|---|---|---|---|---|---|---|---|
| 1 | Controls | 1.38 (0.26) | 0.503 | 0.35 (0.16) | 0.937 | 0.25 (0.12) | 0.826 |
| Patients | 1.42 (0.26) | 0.35 (0.11) | 0.25 (0.08) | ||||
| 2 | Controls | 2.04 (0.52) | 0.243 | 0.41 (0.19) | 0.250 | 0.30 (0.13) | 0.414 |
| Patients | 2.27 (0.39) | 0.49 (0.15) | 0.34 (0.10) | ||||
| 3 | Controls | 1.53 (0.37) | 0.732 | 0.30 (0.14) | 0.237 | 0.22 (0.10) | 0.366 |
| Patients | 1.57 (0.29) | 0.37 (0.17) | 0.26 (0.12) | ||||
| Overall | Controls | 1.57 (0.45) | 0.419 | 0.35 (0.16) | 0.284 | 0.26 (0.12) | 0.530 |
| Patients | 1.64 (0.43) | 0.38 (0.15) | 0.27 (0.10) |
Parkinson's Disease Measures
Vocalization impairment in the Parkinson's disease group was measured by items of the UPDRS and the PDQ‐39. The UPDRS is a clinical rating scale divided into five subscales measuring cognition, behavior and mood, activities of daily living (ADL), motor function, and symptom severity. Items 5 (speech impairment rating within the context of ADL) and 18 (clinical rating of speech impairment within the context of motor function) were chosen for their relation to voice complaint. The PDQ‐39 is a self‐completed questionnaire divided into eight subscales measuring mobility, activities of daily living, emotional well‐being, stigma, social support, cognition, communication, and bodily discomfort. At each site, the same experienced physicians acquired structured interviews and questionnaires immediately before the fMRI session.
Data acquisition and Preprocessing
Participants were instructed to lie still during the scan and not to think of anything in particular. The latter was confirmed during a post‐scan debriefing interview. For each subject, resting state EPI images were acquired using blood‐oxygen‐level‐dependent (BOLD) contrast using highly similar sequences at each site. All sites had parameters of 2200 ms TR, 30 ms TE, 90° flip angle, and 3.1 × 3.1 × 3.1 voxel sizes. In Aachen, a Siemens 3T scanner was used to acquire 36 slices and 165 images. In Cologne, a Siemens 3T Trio scanner was used to acquire 33 slices and 183 images. In Düsseldorf, a Siemens Magnetom Trio Tim Syngo MR B17 scanner was used to acquire 36 slices and 300 images. These differences should not have a substantial impact on the estimation of functional connectivity in the current approach of estimating stationary connection parameters by time‐series correlation. Though it would have been advantageous, it was not possible to have identical imaging parameters by retrospectively pooling three different sub‐samples. Nonetheless, we controlled for variance due to site before making statistical inferences about connectivity patterns (see below).
Patients and controls were matched for age, gender, and head movement at all three sites. In particular, given the sensitivity of intrinsic connectivity and the nature of this patient population, we found it necessary to control for recruitment site and in‐scanner movement. Site was statistically determined to not have an impact on inter‐individual variance within the patient group (Table 2). Finally, in order to control for expected head movement in all subjects beyond movement regression during preprocessing (see below), we created the largest sample pool as possible in which all patients' estimates of head movement [root mean squared movement (RMS), derivative of RMS variance over voxels (DVARS), and frame‐wise displacement (FD)], age, and gender were not significantly different to those of healthy control subjects [Dijk et al., 2012; Power et al., 2012; Satterthwaite et al., 2013b]. For each site, we randomly assembled equal groups of patients and controls. Subjects were considered matched when all five parameters were not significantly different at P > 0.20 following 107 repetitions of this procedure.
Prior to further processing (using SPM8, http://www.fil.ion.ucl.ac.uk/spm) the first four images were discarded allowing for magnetic field saturation. The EPI images were first corrected for head movement by affine registration using a two‐pass procedure. The mean EPI image for each subject was then spatially normalized to the MNI single subject template using the “unified segmentation” approach [Ashburner and Friston, 2005]. The ensuing deformation field was then applied to the individual EPI volumes and a 5‐mm FWHM Gaussian kernel smoothed the output images. In order to reduce spurious correlations by confounds such as physiological noise and motion [cf. Bandettini and Bullmore, 2008], variance that could be explained by first‐ or second‐order effects of the following nuisance variables was removed from each voxel's time series: (i) the six motion parameters derived from the image realignment, (ii) their first derivative, and (iii) global signal intensity per time‐point [(Jakobs et al., 2012; Reetz et al., 2012; Satterthwaite et al., 2012, 2013b)]. Data was then band‐pass filtered, preserving frequencies between 0.01 and 0.08 Hz [Fox and Raichle, 2007; zu Eulenburg et al., 2012). Prior to statistical analyses, variance that could be explained by the potentially confounding factors site and gender were removed from the data.
Data analysis: Individual and Group Level Analysis
Functional connectivity was then investigated using a network of regions based on a meta‐analysis of vocalization [see Table 1; Brown et al., 2009]. To determine regions of the brain that are reliably activated during vocalization, Brown et al. [2009] contrasted overt speech production with tongue movement, lip movement, and vowel phonation, which reflected neural activation of pure vocalization. Furthermore, the authors performed a functional meta‐analysis comparing activations from syllable singing versus activations due to overt reading. The resulting MNI coordinates of each region are displayed in Table 1. The time course for each ROI identified in Brown et al.'s [2009] meta‐analysis was then extracted for each subject as the first eigenvariate of the resting‐state signal time‐series of all grey‐matter voxels located within 5 mm of the respective peak coordinate. For each subject we then computed linear (Pearson) correlation coefficients between the extracted time series of each of the seed regions. These voxel‐wise correlation coefficients were then transformed into Fisher's Z‐values representing the functional connectivity strength for each connection in each subject. First, network models of connectivity were analyzed within each group in vocalization ROIs using a single sample t‐test thresholded at FDR‐corrected P < 0.001. Functional network connectivity was then assessed for significant differences between patients and controls using independent samples t‐tests thresholded at FDR‐corrected P < 0.05, and then transforming values to z‐scores.
To assess possible relationships between functional connectivity and voice impairment in Parkinson's disease patients, Spearman rank‐correlations of the individual connectivity strengths' between the different nodes of our network and on data from patients whom it was collected. The UPDRS item scores, PDQ‐39 Communication subscale, and disease duration network correlation results were thresholded at P < 0.05.
RESULTS
Figure 1 shows significant resting state connectivity for the vocalization network in healthy subjects (1a) and those with Parkinson's disease (1b). The intrinsic voice network in healthy subjects involved strong functional connectivity between left and right RO and between right RO and right vPM. Within subcortical structures, there was strong connectivity between left and right putamen and between left and right thalamus. Bilateral thalamus was also connected to the left RO. Also seen in the figure is connectivity between left and right vPM, and left RO. Bilateral vPM and bilateral RO were connected both within and between hemispheres, as well as bilateral RO and bilateral STG. Though the only subcortical co‐activation of the right STG is with the left thalamus, the bilateral thalamus is also connected with the left RO and the right vPM. Finally, the left cerebellum is connected to the right cerebellum, as well as negatively related to BOLD fluctuation in the right RO.
Figure 1.
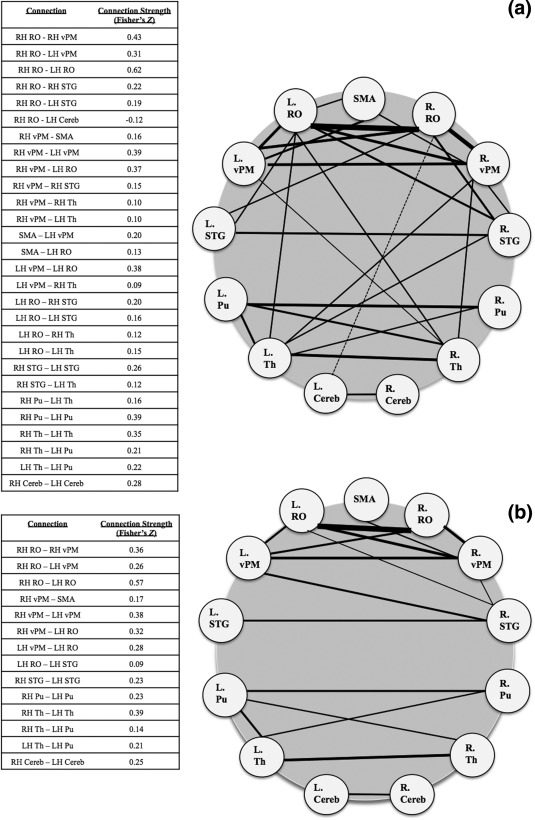
Resting state connectivity of the voice network in healthy subjects (1a, top) and patients with Parkinson's disease (1b, bottom). (FDR‐corrected p <. 001). SMA = supplemental motor area; RO = Rolandic operculum; vPM = ventral premotor area; STG = superior temporal gyrus; Pu = putamen; Th = thalamus; Cereb = cerebellum VI.
Connectivity in patients with Parkinson's disease (Fig. 1b) reveals a somewhat different voice network pattern. In particular, at the subcortical level, the group with Parkinson's disease shows left and right putamen connectivity, left and right thalamus connectivity, left and right cerebellum connectivity, and left thalamus and left putamen connectivity. There is no connectivity between subcortical and cortical structures. At a hemispheric level, the Parkinson's disease group has few intrahemispheric functional connections and limited STG connectivity. The left putamen to left thalamus, left vPM to left RO, and right vPM to right RO are connections that are common between groups.
Group Differences in Voice Motor Network
The predominate finding when comparing healthy control connectivity to subjects with Parkinson's disease (FDR‐corrected P < 0.05) is the difference in subcortical‐cortical functional connectivity (see Fig. 2). The left thalamus and right STG (z = 3.39) and left thalamus and right RO (z = 3.74) were different between groups. Likewise, functional connectivity between the right RO and left STG (z = 3.10) was different between groups. Within the basal ganglia, there was different connectivity between bilateral putamen (z = 4.15) between groups. Cortically, there is different connectivity between the right STG and right and left RO (z = 3.60; 3.31).
Figure 2.
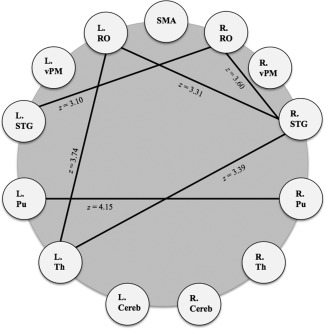
Group differences in the voice network (Healthy controls > PD, FDR‐corrected p < .05). SMA = supplemental motor area; RO = Rolandic operculum; vPM = ventral premotor area; STG = superior temporal gyrus; Pu = putamen; Th = thalamus; Cereb = cerebellum VI.
Correlation to Clinical Phenotypes
To test whether connectivity within the vocalization network is significantly related to clinical phenotype, that is, symptom severity, in Parkinson's disease patients, we correlated the inter‐individual variability in the strength of each connection with disease duration, UPDRS item 5 (patient‐rated speech impairment within the construct of ADL), UPDRS item 18 (clinician‐rated speech impairment within the construct of motor dysfunction), and PDQ‐39 Communication Sub‐Scale (Fig. 3; P < 0.05 uncorrected). Our analysis revealed significant associations between connectivity and symptom‐levels for subcortical, subcortical‐cortical, and cortical interactions. Specifically, disease duration was positively correlated with SMA‐left RO connectivity (rs = 0.37) and right RO‐right putamen connectivity (rs = 0.32). Patients who had been living with Parkinson's disease for longer had stronger connectivity between the SMA and left RO as well as the right RO and right putamen.
Figure 3.
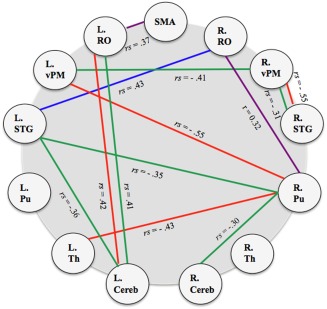
Parkinson's disease voice network correlates (p < .05). Purple—Disease duration; Blue—PDQ‐39 Communication Scale; Red—UPDRS item 5 (Communication impairment/ADL); green—UPDRS item 18 (Motor Examination); SMA = supplemental motor area; RO = Rolandic operculum; vPM = ventral premotor area; STG = superior temporal gyrus; Pu = putamen; Th = thalamus; Cereb = cerebellum VI.
Parkinson's Disease Questionnaire 39 Communication scores were positively associated with right RO‐left STG connectivity (rs = 0.43). Furthermore, this was the only positive association to a voice score. Patients who perceived more communication impairment thus had increased connectivity between the right RO and left STG. Item 5 of UPDRS‐II (rating of speech impairment within the context of ADL) was negatively related to connectivity between right vPM and right STG (rs = −0.55), the left vPM and right putamen (rs = −0.55), and the right putamen and left thalamus (rs = −0.43). That is, patients who experienced more difficulty with communication had decreased connectivity between the right vPM and right STG, left vPM and right putamen, and between the right putamen and left thalamus. However, item 5 was positively related to left cerebellum connectivity with the left RO (rs = 0.42). Increased complaint of speech impairment was related to increased connectivity between left cerebellum and left RO. Finally, item 18 on the Motor Scale (UPDRS‐III; clinician rated speech impairment) was negatively associated with the connectivity of right and left vPM (rs = −0.41), right vPM‐right STG (rs = −0.31), left STG and right putamen (rs = −0.35), left STG and left cerebellum (rs = −0.36), and right putamen and right cerebellum (rs = −0.30). Patients with more speech symptoms thus had reduced connectivity between the right and left vPM, the right vPM and right STG, left STG and right putamen, left RO and left cerebellum, and right putamen and right cerebellum.
DISCUSSION
We identified resting‐state functional connectivity among selected seed regions in a voice network [Brown et al., 2009] in patients with Parkinson's disease and healthy control subjects and the different connectivity patterns between the groups. Thus, identifying the final voice network specific to non‐speech phonation informed the hypothesis that those with Parkinson's disease who suffer from hypokinetic dysarthria have reduced intrinsic connectivity in this network in comparison to healthy subjects. Furthermore, we identified connectivity patterns for patients with Parkinson's disease that were related to communication‐specific variables. Various connections related to speech impairment ratings were altered in patients with Parkinson's disease.
The Healthy Voice Network
The resting‐state functional connectivity of an a priori, robustly defined vocalization network [Brown et al., 2009] demonstrated extensive bilateral, subcortical, subcortical‐cortical, and cortical connectivity in healthy subjects. Most notably present in the healthy voice network that is not seen in the PD network is the subcortical‐cortical connectivity. Though there were no putamen‐premotor connections as described in other studies [Choi et al., 2012; Kell et al., 2011; Simonyan et al., 2013], this may be a result of a more conservative threshold, different methodology in defining networks, as well as the prominence of dopamine‐driven thalamic‐cortical excitation linked to BOLD activation. Likewise, the connectivity between the thalamus and cortical motor and temporal areas that demonstrate interhemispheric homologue connectivity is in agreement with previous thalamic nucleus connectivity with the cortex [Zhang et al., 2008]. This thalamo‐cortical connectivity has also been demonstrated in post‐mortem axonal connections [Morel et al., 1997], and agrees with neurotransmitter action models of the basal ganglia. Furthermore, the left and right thalamus was connected with left and right vPM, left RO, and right STG.
Specific laterality of the observed thalamic‐RO resting‐state connectivity is critical given the nature of task‐driven speech representation in the brain in disease‐free populations [Bohland and Guenther, 2006]. Using an ROI activation analysis, Bohland and Guenther [2006] also demonstrated bilateral motor and thalamus activation during overt speech production (non‐word syllables), but strong left lateralized operculum (around inferior frontal gyrus) activation specific to complex syllable sequences. Guenther et al. [2006] relate the left lateralized opercular activity to the Speech Sound Map of the DIVA model, which suggests that the left lateralized cells activate well‐known speech chunks.
Finally, BOLD fluctuation in the left cerebellum was negatively related to that of the right RO. Similarly, Kipping et al. [2013] noted connectivity of left lobule VI to the right IFG (as well as left IFG), and numerous other cortical sensorimotor regions. The extensive cerebellar‐cerebral connectivity, unlike the current work, may be a factor of only studying a single group of subjects, as well as different ROI identification techniques. Furthermore, the authors demonstrate cerebellar‐IFG connectivity to be relevant in an executive control network, though this IFG connectivity was particularly unique to right lobule VI, not found in the current study [Kipping et al., 2013].
Parkinson's Disease Reductions in Voice Network Resting‐State Functional Connectivity
The between‐group contrast analysis revealed reduced left thalamus, putamen, STG, and RO connectivity in patients with Parkinson's disease. The most pronounced difference in connectivity is that of the bilateral putamen. Given the role of the putamen in motor initiation and Parkinson's disease, this finding is expected [Hornykiewicz, 2001]. Over 80% of dopamine in the brain is located in the caudate and putamen. Given the 95% decrease in dopamine in the putamen in patients with Parkinson's disease, it is possible that the reduced bilateral putamen and subcortical‐cortical connectivity is due to the overly inhibitory nature of dopamine activity from the putamen and pallidum, and sequentially, a lack of excitatory left thalamus connections to the cortex, particular to this sample, to the right STG and left RO. For example, Wu et al. [2012] most clearly demonstrated this possibility in both resting state and task‐induced activity in most of the ROI's selected in the current study. The authors showed that the SN positively affected the bilateral putamen and thalamus at rest in healthy subjects. However, Parkinson's disease depletes the dopaminergic neurons in the BG, most notably in the SN, which affects putamen, Globus pallidus (GP), and thalamus functioning. Wu et al. [2012] further reported reduced causal connectivity from SN to thalamus, BG, GP, and putamen at rest compared to healthy controls. With the administration of Levodopa, the reduced connectivity partially normalized. Furthermore, areas of the right pallidum that are anatomically and functionally connected to the thalamus display slight shape differences in early‐stage Parkinson's disease compared to healthy, matched controls [Menke et al., 2013].
Additionally, the associations of left hemisphere and right hemisphere cortical nodes with the thalamus that were significantly greater in healthy controls are representative of the lateralization of particular aspects of speech and vocalization. The left hemisphere is known to be active while detecting temporal errors in vocalization, whereas the right hemisphere is known to be active while detecting spectral and prosodic errors in vocalization [Duffy, 2005; Johnsrude et al., 2000; Zatorre and Belin, 2001]. Generally, the reduced connectivity between the thalamus and STG cortical nodes reinforces findings from speech‐related functional activation studies in the past decade that reveal their role in motor and sensorimotor dysfunction in Parkinson's disease. Of particular interest is the role of the STG in this patient population. A recent study has shown the directional influences to and from the bilateral STG during a pitch‐shifting paradigm, albeit in a healthy population and in a different vocalization network [Flagmeier et al., 2014]. Reduced prosody and loudness are certainly core vocal symptoms of Parkinson's disease [Duffy, 2005], and the inability to easily correct for these errors is most likely a result of reduced STG connectivity. However, a causal modeling analysis would be most helpful in clarifying the STG's implication in error detection and correction in Parkinson's disease. Currently, the symptom correlates of intrinsic connectivity provide additional understanding of the functionality of the voice network.
Relationship of Intrinsic Connectivity within the Voice Network to Clinical Symptoms
First, longer disease duration was related to increased connectivity between the SMA and left RO, as well as the right RO and right putamen. This association is interesting as neither of these connections was decreased in the PD group, nor are they present in the PD group alone. The SMA is functionally connected to the left RO in healthy controls, while connectivity between the right RO and right putamen is entirely unanticipated. A hypothesis may be that dopaminergic medication (and possibly voice therapy) is not administered until mid‐ to late‐ stages of the disease [Sapir, 2014]. Increased duration would necessitate prescribed dopamine, which may increase connectivity in these areas. However, this cannot be presently emphasized because of the lack of dopaminergic drug information in this sample.
Behaviorally specific to the voice network, patients' higher ratings of communication impairment (higher scores = worse impairment) were related to increased coupling of the right RO and left STG, and left cerebellum and left RO, but decreased connectivity between the right STG and right vPM, the right putamen and left vPM, and the right putamen and left thalamus. The increased connectivity of the left STG possibly alludes to the increased effort of integrating the external feedback to modulate oral aspects of vocal control [Obeso et al., 2012; Parkinson et al., 2012; Solomon and Robin, 2005]. Obeso et al. [2012] showed that executive dysfunction explained partial variance of patients' phonemic fluency scores. Executive control is hypothesized to be a large component of internal sense of effort, although inhibition of inappropriate word generation could also play a significant role in decreased fluency. We also emphasize the importance of the STG in relaying auditory feedback in response to unexpected shifts in vocal pitch [Flagmeier et al., 2014; Parkinson et al., 2012]. The STG is suppressed when auditory feedback matches the efference copy, yet uninhibited when the expected auditory feedback does not match that in the efference copy [Greenlee et al., 2011].
The RO in relation to cerebellar lobule VI may also play a role in implementing kinesthetic feedback during vocalization in tandem with the auditory feedback that failed to match that of the predicted vocal output. Lobule VI receives direct facial, kinesthetic afferent projections from the spinal cord [Stoodley and Schmahmann, 2010]. The relationship between left lobule VI and left RO (in association with perceived communication impairment) may represent the reception of reduced kinesthetic feedback. The RO, similar to auditory feedback, is possibly cued to corollary discharge.
Lenfeldt et al. [2013] also found that increased UPDRS‐II scale scores were related to increased mean diffusivity in the putamen and the thalamus. The loss of axonal integrity, as well as decreased dopamine uptake in these regions, may help explain the decreased connectivity between the right putamen and the left thalamus. Furthermore, the same may be said about the reduced connectivity between right putamen and left vPM in relation to difficulty with communication. Though in the off‐medication state, Wu et al. [2012] also demonstrated a relationship between increased UPDRS scores and decreased positive effect of the SN on both the putamen and motor cortex.
Interestingly, the clinician's rating of patients' motor‐speech impairment on UPDRS item 18 indicated similar locations of decreased connectivity. Increased scores of motor‐speech impairment (higher scores = worse impairment) were associated with increased connectivity between left cerebellum and left RO, but decreased connectivity between the right STG and right vPM, right and left vPM, right putamen and left STG, right cerebellum and right putamen, as well as left cerebellum and left STG. The positive relationship of clinician rating of speech impairment and connectivity between left cerebellum and left RO, as well as the negative relationship of clinician impairment rating and right STG – right vPM connectivity, most likely reveals similar implications as the patients' perception of their impairment.
Clinician‐reported speech impairment was also related to decreased connectivity among right and left cerebellum, right putamen, left STG and bilateral vPM. Wu et al. [2012] similarly reported that the decreased effect of the SN on the putamen, temporal lobe, and motor cortex was related to increased UPDRS scores [Wu et al., 2012]. Due to the origin of reduced dopamine, the reduced activity of the SN, and yet the partial benefit of Levodopa, are most likely the cause of the abnormal resting state connectivity in these regions. As Wu et al. [2012] demonstrated a heightened effect of the SN on the motor cortex during motor execution, the reduced availability of dopamine in Parkinson's disease decreases the ability to surpass the necessary threshold of dopaminergic activity for motor initiation. In the present case, this may be in reference to perceived auditory feedback error. Including the right and left STG, this disturbed processing would make perceptions of self‐vocalization and movement distorted, or correction more effortful.
Finally, reduced syncing of BOLD fluctuation in the cerebellum in relation to right putamen and left STG, regarding increased speech impairment, most likely reflect what occurs in task‐based functional neuroimaging data in healthy adults and those with Parkinson's disease. Sensorimotor tasks, such as speaking, often activate cerebellum and sensorimotor cortices, such as STG, in healthy populations [Brown et al., 2009; Soros et al., 2006; Stoodley and Schmahmann, 2010]. As Parkinson's patients with hypokinetic dysarthria suffer from primarily disordered voice and reduced dopamine in the basal ganglia, it is possible that the reduced connectivity between cerebellum and these regions is because cerebellar fluctuations in BOLD are relatively normal, but putamen and STG are aberrant. This notion is supported by the contrast analysis. There are no differences in cerebellar connectivity, but indeed reductions in putamen and STG connectivity. Furthermore, in idiopathic Parkinson's disease, cerebellar activation remains significant and unchanged following voice treatment [Narayana et al., 2010].
Limitations
In the current resting state functional connectivity analysis, global signal intensity was considered a nuisance variable and removed from the time‐series. Though there have been many publications that warn of the possible effects of doing so, this is still arguably up for debate [Satterthwaite et al., 2013b]. In particular, it may be noted that the global signal may in large parts be driven by non‐neuronal sources, ranging from physiological effects (such as cardiovascular and breathing patterns) to trivial technical confounds such as scanner drifts. In fact, these considerations and the observation of the ensuing (spurious) global correlation structure in resting‐state BOLD data have motivated global signal removal in the first place [Satterthwaite et al., 2013a]. Given that there is no comprehensive evaluation and consensus on the right approach to resting state functional connectivity, this is included as a possible limitation and may be tested further in the future.
As no further neuropsychological assessment scores were available for all patients, we did not correlate other clinically relevant variables to intrinsic connectivity strength in this network. However, it may be worth doing so in later research in order to isolate alterations in connectivity in this network that have also been related to other realms of cognitive decline in patients with Parkinson's disease [Lebedev et al., 2014].
In the correlation analyses, several relationships were found with the clinical scores that were available. None, however, survived correction for multiple comparisons. These findings should thus be seen as a first indication towards the existence of altered functional connectivity in patients with PD who do and do not have severe speech deficits. These may be followed up by analysis of a more targeted sample of not and severely impaired patients.
CONCLUSIONS
The current resting state connectivity analysis expands what is known about differences in the voice network in patients with Parkinson's disease. Resting state connectivity allows us to see intrinsic states in the brain that are not confounded by task activity. The ROIs that compose the healthy voice network were selected from a task‐related meta‐analysis for a comparison of resting‐state connectivity between healthy and Parkinson's disease. In using this technique, and by controlling for extraneous confounding variables, we provide evidence that the Parkinson's disease population exhibits vast reductions in left thalamo‐cortical, bilateral putamen, and cortical audio‐vocal connectivity in the voice network. However, the majority of cortical connectivity remains similar to that of controls. Furthermore, the majority of the behavioral correlates were largely associated with aberrant putamen, STG, and vPM coupling. In order to understand cause and effect of cortical and subcortical‐cortical connectivity, further research should encompass dynamic causal modeling in this network for healthy controls and patients with Parkinson's disease.
Anneliese B. New: Manuscript preparation writing of the first draft. Donald A. Robin: Research project conception; Statistical analysis design, review, and critique; Manuscript preparation writing of the first draft, review and critique. Amy L. Parkinson: Research project conception; Statistical analysis execution; Manuscript preparation review and critique. Claudia Rottschy: Research project organization and execution; Manuscript preparation review and critique. Kathrin Reetz: Research project organization and execution; Manuscript preparation review and critique. Felix Hoffstaedter: Data analysis; Manuscript review and critique. Christian Mathis: Data collection; Manuscript review and critique. Martin Sudmeyer: Data collection; Manuscript review and critique. Christian Grefkes: Data collection (Cologne site); Manuscript review and critique. Charles R. Larson: Manuscript preparation review and critique. Loraine O. Ramig: Manuscript preparation review and critique; Parkinson's disease clinical specialist. Peter T. Fox: Manuscript preparation review and critique. Simon B. Eickhoff: Research project conception and organization; Statistical analysis design, review and critique; Manuscript preparation review and critique.
Conflict of Interest: Dr. Loraine Ramig receives income for the development of LSVT LOUD for patients with Parkinson's disease.
Correction added on 7 January 2016, after first online publication.
REFERENCES
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Bullmore E (2008): Endogenous oscillations and networks in functional magnetic resonance imaging. Hum Brain Mapp 29:737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH (2006): An fMRI investigation of syllable sequence production. NeuroImage 32:821–841. [DOI] [PubMed] [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkletaub P, Liotti M (2009): The somatotopy of speech: Phonation and articulation in the human motor cortex. Brain Cognit 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Salsone M, Donzuso G, Chiriaco C, Realmuto S, Nicoletti A, et al. (2014): Neurofunctional correlates of attention rehabilitation in Parkinson's disease: An explorative study. Neurol Sci 35:1173–1180. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL (2012): The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Diederen KM, Meijering AL, Sommer IE, Eickhoff SB (2014): Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct Funct 219:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR (2005): Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 2nd ed, Mosby, St. Louis.
- Eickhoff SB, Grefkes C, (2011): Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin EEG Neurosci 42:107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagmeier SG, Ray KL, Parkinson AL, Li K, Vargas R, Price LR, et al. (2014): The neural changes in connectivity of the voice network during voice pitch perturbation. Brain Lang 11:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neuroscience 8:700–711. [DOI] [PubMed] [Google Scholar]
- Gorges M, Muller HP, Lule D, Ludolph AC, Pinkhardt EH, Kassubek J (2013): Functional connectivity within the default mode network is associated with saccadic accuracy in Parkinson's disease: A resting‐state FMRI and videooculographic study. Brain Connect 3:265–272. [DOI] [PubMed] [Google Scholar]
- Greenlee JD, Jackson JW, Chen F, Larson CR, Oya H, Kawasaki H, et al. (2011): Human auditory cortical activation during self‐vocalization. Plos One e14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA (2006): Neural modelling and imaging of the cortical interactions underlying syllable production. Brain Lang 96:280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ (2012): Resting state functional connectivity of the striatum in Parkinson's disease. Brain 135:3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O (2001): Chemical neuroanatomy of the basal ganglia—normal and in Parkinson's disease. J Chem Neuroanaty 22:3–12. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, et al. (2012): Across‐study and within‐subject functional connectivity of a right temporo‐parietal junction subregion involved in stimulus‐context integration. NeuroImage 60:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA (2002): Recent developments in the Pathology of Parkinson's Disease. Vienna: Springer; pp. 347–376. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ (2000): Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123:155–163. [DOI] [PubMed] [Google Scholar]
- Kell CA, Morillon B, Kouneiher F, Giraud AL (2011): Lateralization of speech production starts in sensory cortices: A possible sensory origin of cerebral left dominance of speech. Cereb Cortex 21:932–937. [DOI] [PubMed] [Google Scholar]
- Kipping JA, Grodd W, Kumar V, Taubert M, Villringer A, Margulies DS (2013): Overlapping and parallel cerebello‐cerebral networks contributing to sensorimotor control: An intrinsic functional connectivity study. NeuroImage 83:837–848. [DOI] [PubMed] [Google Scholar]
- Krajcovicova L, Mikl M, Marecek R, Rektorova I (2012): The default mode network integrity in patients with Parkinson's disease is levodopa equivalent dose‐dependent. J Neural Trans 119:443–454. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Westman E, Simmons A, Lebedeva A, Siepel FJ, Pereira JB, Aarsland D (2014): Large‐scale resting state network correlates of cognitive impairment in Parkinson's disease and related dopaminergic deficits. Front Syst Neurosci 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfeldt N, Hansson W, Larsson A, Nyberg L, Birgander R, Forsgren L (2013): Diffusion tensor imaging and relation to Parkinson rating scales. J Neurol 260:2823–2830. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, Fox PT (2003): Hypophonia in Parkinson's disease neural correlates of voice treatment revealed by PET. Neurology 60: 432–440. [DOI] [PubMed] [Google Scholar]
- Menke RA, Szewczyk‐Krolikowski K, Jbabdi S, Jenkinson M, Talbot K, Mackay CE, et al. Comprehensive morphometry of subcortical grey matter structures in early‐stage Parkinson's disease. Hum Brain Mapp (in press):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D (1997): Multiarchitectonic and stereotactic atlas of the human thalamus. J Comput Neurosci 387:588–630. [DOI] [PubMed] [Google Scholar]
- Narayana S, Fox PT, Zhang W, Franklin C, Robin DA, Vogel D, et al. (2010): Neural correlates of efficacy of voice therapy in Parkinson's disease identified by performance‐correlation analysis. Hum Brain Mapp 31:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Casabona E, Bringas ML, Alvarez L, Jahanshahi M (2012): Semantic and phonemic verbal fluency in Parkinson's disease: Influence of clinical and demographic variables. Behav Neurosci 25:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson AL, Flagmeier SG, Manes JL, Larson CR, Rogers B, Robin DA (2012): Understanding the neural mechanisms involved in sensory control of voice production. NeuroImage 61:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Mancini L, Jahanshahi M, Thornton JS, Tripoliti E, Yousry TA, et al. (2011): Functional magnetic resonance imaging exploration of combined hand and speech movements in Parkinson's disease. Movement Disorders 26:2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder, AZ , Schlaggar BL, Petersen, SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Fox C (2011): Speech and voice disorders in Parkinson's disease In: Olanow WC, Stocchi F, Lang AE. editors Parkinson's Disease: Non‐Motor and Non‐Dopaminergic Features, 1st ed Blackwell Publishing: Hoboken. pp. 348–362. [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, et al. (2012): Investigating function and connectivity of morphometric findings: Exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17): NeuroImage 62:1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Grefkes C (2012): State‐dependent differences between functional and effective connectivity of the human cortical motor system: NeuroImage 67:237–246. [DOI] [PubMed] [Google Scholar]
- Rektorova I, Mikl M, Barrett J, Marecek R, Rektor I, Paus T (2012): Functional neuroanatomy of vocalization in patients with Parkinson's disease. J Neurol Sci 313:7–12. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Kleiman A, Dogan I, Langner R, Mirzazade S, Kronenbuerger M, et al. (2013): Diminished activation of motor working‐memory networks in Parkinson's disease. PLoS One 8:e61786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. (2013a): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. NeuroImage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. (2013b): Heterogenous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. NeuroImage 83:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir S (2014): Multiple factors are involved in the dysarthria associated with Parkinson's disease: A review with implications for clinical practice and research. J Speech, Lang, Hearing Res 57:1330–1343. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Herscovitch P, Horwitz B (2013): Speech‐induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: A combined PET, fMRI and DTI study. Neuroimage 70:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, et al. (2012): Temporally‐independent functional modes of spontaneous brain activity. Proc Natl Acad Sci USA 109:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NP, Robin DA (2005): Perceptions of effort during handgrip and tongue elevation in Parkinson's disease. Parkinsonism Related Disorders 11:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KMJ, Eickhoff SB (2012): Resting state functional connectivity in patients with chronic hallucinations. Plos One 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Sokoloff LG, Bose A, McInosh AR, Graham SJ, Stuss DT (2006): Clustered functional MRI of overt speech production. NeuroImage 32:376–387. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ and Schmahmann JD (2010): Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, Vitale C, et al. (2012a): Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. Am J Neuroradiol 33:1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Esposito F, Vitale C, Santangello G, Amboni M, Russo A, et al. (2012b): Default‐mode network connectivity in cognitively unimpaired patients with Parkinson's disease. Neurology 79:1–7. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, et al. (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X, et al. (2012): Basal ganglia circuits change in Parkinson's disease patients. Neurosci Lett 524:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ and Belin P (2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11:946–953. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME (2008): Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol 100:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Eulenberg P, Caspers S, Roski C, Eickhoff SB (2012): Meta‐analytical definition and functional connectivity of the human vestibular cortex. NeuroImage 60:162–169. [DOI] [PubMed] [Google Scholar]


