Abstract
The human insula has been parcellated on the basis of resting state functional connectivity and diffusion tensor imaging. Little is known about the organization of the insula when involved in active tasks. We explored this issue using a novel meta-analytic clustering approach. We queried the BrainMap database asking for papers involving normal subjects that recorded activations in the insular cortex, retrieving 1305 papers, involving 22,872 subjects and a total of 2957 foci. Data were analyzed with several different methodologies, some of which expressly designed for this work. We used meta-analytic connectivity modeling and meta-analytic clustering of data obtained from the BrainMap database. We performed cluster analysis to subdivide the insula in areas with homogeneous connectivity, and density analysis of the activated foci using Voronoi tessellation. Our results confirm and extend previous findings obtained investigating the resting state connectivity of the anterior–posterior and left–right insulae. They indicate, for the first time, that some blocks of the anterior insula play the role of hubs between the anterior and the posterior insulae, as confirmed by their activation in several different paradigms. This finding supports the view that the network to which the anterior insula belongs is related to saliency detection. The insulae of both sides can be parcellated in two clusters, the anterior and the posterior: the anterior is characterized by an attentional pattern of connectivity with frontal, cingulate, parietal, cerebellar and anterior insular highly connected areas, whereas the posterior is characterized by a more local connectivity pattern with connections to sensorimotor, temporal and posterior cingulate areas. This antero–posterior subdivision, better characterized on the right side, results sharper with the connectivity based clusterization than with the behavioral based clusterization. The circuits belonging to the anterior insula are very homogeneous and their blocks in multidimensional scaling of MACM-based profiles are in central position, whereas those belonging to the posterior insula, especially on the left, are located at the periphery and sparse, thus suggesting that the posterior circuits bear a more heterogeneous connectivity. The anterior cluster is mostly activated by cognition, whereas the posterior is mostly activated by interoception, perception and emotion.
Keywords: Meta-analytic clustering, Voxelwise meta-analysis, Meta-analytic connectivity modeling, Functional connectivity, Attention, Salience, Activation likelihood estimation, fMRI, Data driven parcellation, K-means clustering, Hierarchical clustering, Voronoi parcellation
Introduction
The insular cortex consists of a distinct lobe located deep inside the lateral sulcus of the Sylvian fissure, hidden by the frontal and temporal opercula (Ture et al., 1999). Cytoarchitectonically, using myelin staining techniques, the insular cortex in humans and primates has been divided in three major regions: i) an agranular area that occupies the ventralmost anterior third of the insula (Mesulam and Mufson, 1982a, b); ii) a dorsal posterior granular (Augustine, 1985) iii) a dysgranular intermediate area that represents, both anatomically and functionally, a transition between the other two regions (Bonthius et al., 2005; Ture et al., 1999). Functionally, the ventral anterior agranular region is considered part of the paralimbic belt, whereas the dorsal posterior granular region is linked to the somatomotor system (Cauda et al., 2011; Mesulam and Mufson, 1982a). In contrast, histochemical staining for cytochrome oxidase, acetylcholinesterase and nicotinamide dinucleotide phosphate (NADPH)-diaphorase (Rivier and Clarke, 1997) reveals two distinct patterns: i) a lightly-stained, antero-inferior region, and ii) a darkly stained region located in the posterior insula. The anterior subdivision displays a connectivity profile that is intermediate between that of primary sensory and higher order association cortices, whereas the posterior subdivision displays a connectivity profile compatible with the pattern typical of primary sensory cortices. These two zones correspond to the two histological areas, the ventral anterior and the dorsal posterior (Raichle and Snyder, 2007).
The insular cortex, together with the anterior prefrontal, dorsolateral prefrontal, dorsomedial superior frontal and inferior parietal lobules, forms a ‘frontoparietal control network’ (Spreng et al., 2009; Vincent et al., 2008). This group of areas is commonly engaged in tasks that require controlled processing of information (Botvinick et al., 2004; Ramnani and Owen, 2004) and has been placed anatomically and functionally between the dorsal attentional (DAN) and the default mode (DMN) networks (Fox et al., 2005a; Raichle and Snyder, 2007).
The insula participates in a circuit responsible for the detection of salience (for a review see Menon and Uddin, 2010): it integrates external elements about the stimuli and internal information about individual cognitive, homeostatic and emotional states to organize behavior. Dosenbach and colleagues (Dosenbach et al., 2006, 2007, 2008) showed that the insula, together with the dorsal anterior cingulate cortex, is part of the “core network” which is activated for the maintenance of the task-level control and the focal attention. Cauda et al. (2011) recently showed that the anterior insula represents also a hub that connects the pain-related areas to several other networks involved in the processing and integration of sensory data from external and internal sources in order to obtain a coherent representation of pain and other salient conditions.
Recent findings (Cauda et al., 2011; Cauda et al., 2012; Taylor et al., 2009) using resting state functional connectivity (RSFC) and probabilistic tract-tracing (Cerliani et al., in press) documented a rostrocaudal trajectory of connectivity that can be subdivided in two (in the paper of Deen et al. (2011); three) major complementary networks involving the ventral-anterior and dorsal-posterior insula: one network links the anterior insula to the middle and inferior temporal cortex and the anterior cingulate cortex, and is primarily related to attentional and limbic regions, which play a role in salience detection and other emotional aspects; the other network links the middle-posterior insula to premotor, sensorimotor, supplementary motor and middle-posterior cingulate cortices, indicating a role in sensorimotor integration.
To the best of our knowledge, the insular cortex has been only functionally parcellated on the basis of resting state functional connectivity and diffusion tensor imaging (DTI): little is known about its connectivity when subjects are involved in active tasks. Since the insula is involved in several behavioral functions and tasks, and since it exerts a complex integrative function, we hypothesized that during active tasks different or more complex parcellation aspects may arise. Given these premises, it becomes fundamental to investigate its connectivity starting from a vast variety of functional data, i.e. data coming from the execution of an active task.
Databases such as BrainMap and Brede (Nielsen, 2003, 2009; Nielsen and Hansen, 2000; Yarkoni et al., 2011) offer the possibility of performing meta-analysis on a wide number of papers. In particular, the BrainMap database (Laird et al., 2005a, b, 2009) is a large repository of data from functional studies performed with PET and fMRI techniques. It currently contains 2092 papers, involving 9871 experiments regarding 83 functional paradigm classes leading to 78,477 locations. For each paper included in the database, specific data regarding the functional paradigm employed and the behavioral class are associated. BrainMap allows searches by keyword and by anatomical area. The latter possibility has recently led to the development and evolution of the so-called structure-based meta-analysis (Laird et al., 2005b). Such a meta-analysis searches all the papers showing at least one location (focus) in a specific region of interest (ROI) rather than a particular behavioral class or paradigm. This meta-analytical technique, although not entirely new (Koski and Paus, 2000; Lancaster et al., 2005; Postuma and Dagher, 2006; Toro et al., 2008), has been recently described and formalized by Robinson et al. (2010), using activation likelihood estimation (ALE) (Laird et al., 2005a) as a statistical method to estimate the co-occurrence of foci. This method has been called ‘Meta-analytic connectivity modeling’ (MACM), and the results of this analysis have been interpreted as a form of functional connectivity or task based co-activations (Robinson et al., 2010).
In order to investigate the pattern of MACM of the insula during the widest number of active tasks, we developed a new technique that, starting from meta-analytic data, aims at discovering the MACM-based and behavioral domain-based parcellation of the insular cortex. We called this method ‘meta-analytic clustering’ (MaC). In fact, this data driven method retrieves, through a database query, all the experiments performed on normal subjects reporting at least an activation in the area under investigation. It then calculates the meta-analytic connectivity, using MACM; in other words, for each voxel, it calculates the co-occurrence of foci: considering the experiments activating that insular voxel, which other voxels did they activate? Then, the patterns obtained from MACM are voxelwise submitted to a clustering algorithm that assigns each voxel to the appropriate insular cluster on the basis of its specific MACM, i.e. on a coactivation basis: in this way we obtained a functional parcellation of the insula using an extensive dataset of studies in which subjects are involved in active tasks. In addition, for each cluster in which the insula has been parcellated, a specific statistical test is performed to investigate which behavioral classes tend to generate activity in that area. To compare the functional clusterization to the behavioral domain results, we also clusterized the insular surface using the behavioral domain data and compared this result with the functional (MACM-based) parcellation results. All these connectivity data were then submitted to several data mining and network analysis techniques to inspect how the insular networks are structured and connected to each other. To discover which voxels possess the biggest discriminative power to differentiate between anterior insular and posterior insular connectivity patterns we employed a multivariate technique: the Multivoxel Pattern Analysis (MVPA) (De Martino et al., 2008; Norman et al., 2006).
Moreover, given that some insular areas may exert a hub function connecting different insular subnetworks to each other and to other brain networks, we investigated the density of foci in each voxel of the insular surface. Indeed we argued that, if a particular insular area exerts a hub function, this area is likely to be activated more often than non-hub areas. To do so we adapted a tessellation technique (Voronoi polygons/Dirichlet tessellation) to derive voxelwise the density of foci.
Using meta-analytic connectivity modeling and meta-analytic clustering of data obtained from the BrainMap database we show that the insulae of both sides are characterized by a rostrocaudal organization in two clusters, one anterior and one posterior, which are different for connectivity and function. Nevertheless, left and right insulae show different patterns of activation and connectivity. The data are new and are obtained with new ad hoc methodologies from a very high number of studies, which strengthens our conclusions.
Materials and methods
Database search
We queried the BrainMap database (Laird et al., 2005a) asking for papers involving normal subjects that recorded activations in the insular surface. The BrainMap database contains, at the moment, 2092 papers generated by 9871 experiments expressing 83 paradigm classes; those papers indicate 78,477 local maxima of activations (locations).
A systematic search was conducted for studies involving normal subjects that reported at least one activation (focus) in the insular cortex.
The insular cortex VOI was manually drawn by an anatomist (A.V.) on the Colin 27 template (http://neuro.debian.net/pkgs/mni-colin27-nifti.html). The MNI insular coordinates were then converted to Talairach coordinates using the icbm2Tal transform (http://brainmap.org/icbm2tal/).
Results from this search were saved in a series of files containing locations, papers and behavioral domains. In BrainMap, metadata are organized under three experiment-level fields: context, paradigm class and behavioral domain. To limit the search to the corresponding category we used the following query: Normal subjects AND (the functional area under study). For further information about the papers included in each meta-analysis see Supplementary Table S1.
Meta-analytic clustering (MaC)
Data preparation
All clustering analyses were performed using MATLAB (Mathworks, Natick, MA, USA) and its toolbox, where necessary. To parcellate the surface of the insular cortex we employed the meta-analytic connectivity modeling (MACM) (Cauda et al., 2010; Robinson et al., 2010; Torta and Cauda, 2011). To calculate this type of connectivity in a voxelwise manner and to allow a proper comparison of areas, the activation likelihood estimation (ALE) algorithm needs a sufficient and constant number of peak coordinates of activations (foci) in each subarea in which it calculates the MACM.
To obtain a completely data driven and unbiased subdivision of the insular cortex in which each subregion has a sufficient number (in our case 50) of foci, we employed the quad tree algorithm (Ballard and Brown, 1982; Laird et al., 2005a).
The number of 50 foci was chosen on the basis of the personal experience of the authors and after a simulation in which the stability of the parcellation results using 10 to 100 foci in steps of 10 was evaluated. The results were stable with blocks of n>40 foci.
The quad tree is an algorithm that subdivides the two dimensional space by decomposing the region into four equal quadrants, subquadrants, and so on with each subregion containing the data corresponding to the specific criteria for the partition. Since we performed all parcellations on a 3D mesh we assumed a constant gray matter (GM) thickness and we operated only in 2D, projecting all foci within +1 and −5 mm from the GM plane to this surface.
The results of the quad tree decomposition consisted of 29 blocks for each insula including an equal number of foci but of different area. After that, we constructed a map composed of all activation coordinates of all articles associated to each block (see Supplementary Fig. S1).
Meta-analytic connectivity
To calculate the MACM, the foci in each quad tree block were pooled using the ALE (Laird et al., 2005a) algorithm. Each coordinate (focus) is modeled by a 3-DGaussian distribution, defined by a full-width half-maximum (FWHM) of 10 mm. This width is based on previous work (Turkeltaub et al., 2002). The analyses show robust results using widths between 10 and 20 mm. The ALE statistic is computed at every voxel in the brain. To make a valid assessment of the significance of the results, the values from the ALE images were tested against null distributions. An appropriate threshold was determined, while controlling the false discovery rate (FDR) (Genovese et al., 2002) at a significance level of p<0.05.
Clustering methods
To subdivide the insular cortex in areas with homogeneous connectivity, we employed the cluster analysis (Cauda et al., 2010; Frades and Matthiesen, 2010).
We employed two different types of clustering algorithms: hierarchical and k-means clustering (Filzmoser et al., 1999). Hierarchical clustering bears several advantages: i) the data are not divided into a predetermined number of partitions avoiding to specify a priori the number of clusters, and ii) it allows the creation of a dendrogram. In contrast, k-means clustering is a partitioning method that operates on actual observations, creates a single level of clusters and allows to use different objective functions as partition criteria but requires to specify the number of clusters.
In the first step, using as input the results of ALE analysis (MACM), we performed a hierarchical clustering and then, on the basis of the optimal cluster number obtained, we performed a k-means clustering. We employed hierarchical clustering also as a control for the k-mean clustering and most importantly, to obtain the dendrogram. Indeed we were interested to inspect the hierarchical structure inside each cluster. To verify the goodness of the hierarchical clustering we used the cophenetic distance, a measure concerning how well the cluster tree reflects the data for different distance measures and to verify the consistency of each link. This method, together with the visual inspection of the dendrogram and the reordered distance matrix, permits to find the optimal number of clusters of the data. The matrix was composed of rows, representing the blocks formed by the quad tree algorithm, and columns representing the probability of activation obtained by the ALE analysis of each voxel in the brain. This data matrix was used to create the distance matrix. There are different criteria to evaluate the distances between clusters in the hierarchical clustering literature. For our analysis we employed the Ward method that uses an analysis of variance approach (Ward, 1963). Subsequently, we employed the k-mean clustering to assess the results using as input the optimal number of clusters obtained from the techniques described above. The results of the k-means clustering were further verified using the average silhouette values. In addition to the MACM-based clusterization, we were interested in investigating whether we could obtain a clusterization similar to the MACM based on parcellating each block with its behavioral profile rather than on the basis of its MACM profile. BrainMap stores a series of behavioral data for each paper, thus allowing us to build, for each block, a profile in which the relative percent of each behavioral category is represented. Using this information it is possible to cluster together blocks with a similar behavioral profile, i.e. activated by similar behavioral classes. The BrainMap follows a coding scheme of the behavioral domain according to six main categories: cognition, action, perception, emotion, interoception and pharmacology. BrainMap stores also a second level of coding that however was not used for this clusterization. Since the clustering algorithm used to obtain the MACM based clusterization was ineffective in dealing with multilevel behavioral classification as present in BrainMap, we employed only the top-tier classification. Indeed, in order to compare the two clusterizations, we needed to employ the same algorithms for MACM-based and behavioral-based clusterization.
For each block we created a vector containing the behavioral domain metadata extracted from the papers retrieved from BrainMap for that block. The resulting matrix — 29 rows (blocks)×6 columns (behavioral categories) — was employed to create a distance matrix and, using the hierarchical and the k-mean clustering, to investigate the clusterization obtained in the behavioral domain.
Density analysis
The insula is considered a hub area that exerts a pivotal function between different brain networks (Sridharan et al., 2008). In agreement with this hypothesis 62% papers in BrainMap display at least one activation in the insular cortex. We speculated that insular areas exerting hub functions are more likely to be activated often. To test this hypothesis, we calculated the density of foci of the insular surface. For density, we intended the number of foci per unit of area. In order to perform this analysis, we employed the Voronoi tessellation algorithm (Klein, 1989). Briefly, a Voronoi tessellation is a decomposition of metric space by distances between sets of points. The insular surface was divided into ‘cells’ each containing one focus.
An interesting property of the Voronoi diagrams is that the area of each polygon is inversely proportional to the density of points (foci) in that area. Therefore, by the Voronoi vertex we calculated the area of each patch and used the set of areas as color index map to represent the density. We employed the Voronoi tessellation as a further validation of previous analyses. Indeed, ALE is a kernel density algorithm that models each focus as a Gaussian probability distribution; conversely, the Voronoi algorithm uses a deterministic approach.
Network analysis
The insula is nested in a complex network of areas with a wide range of functions. In addition, also within the insular cortex different groups of areas interact with each other to form an insular network. To inspect this latter aspect we employed a series of network analysis techniques.
The distance matrix (that represents the dissimilarity between the connectivity pattern of each block) was submitted to a network analysis (Bullmore and Sporns, 2009). First of all, we reordered the matrix in order to place more edges closer to the diagonal. The reordering was obtained using a routine of the Brain Connectivity Toolbox that minimizes the cost function of the matrix (Rubinov and Sporns, 2010). Then, using the data in the reordered distance matrix, we constructed a network and we optimally represented the results using a force-directed algorithm. To this aim, we employed the Fruchterman–Reingold method (Fruchterman and Reingold, 1991). In this algorithm, the nodes are represented by steel rings and the edges are springs between them. The attractive force is analogous to the spring force and the repulsive force is analogous to the electrical force. The basic idea is to minimize the energy of the system by moving the nodes and changing the forces between them. A threshold was applied to the resulting image in order to represent as color-filled only the circles (blocks) with highest network connectivity (first quartile). (For a summary figure of the data analysis steps see Fig. 1.)
Fig. 1.
Summary figure of the analytic steps.
Multivariate pattern recognition
To inspect the pattern of brain areas having the highest discriminative power to differentiate the two insular patterns of connectivity (the anterior and the posterior) we employed the Multivoxel Pattern Analysis (MVPA). The rationale for MVPA use consists in the multivariate nature of the connectivity data, since each MACM map contains information about brain activation at thousands of measured voxels. MVPA searches through MACM data to identify patterns that are highly predictive of each of the two clusters. This method allows estimating maximally discriminative response patterns without a priori definition of regions of interest. In other words, this algorithm selects the optimal solution from the many possible, using a set of informative training examples (support vectors). In brief, starting from the entire set of measured voxels this method uses a training algorithm (least square support vector machine, ls-SVM) iteratively to eliminate irrelevant voxels and to estimate the informative spatial patterns. Correct classification of the test data increases, while features/voxels are pruned on the basis of their discrimination ability.
In our case, for each block, the ALE maps were labeled ‘anterior’ or ‘posterior’ on the basis of their membership to anterior or posterior clusters and then analyzed using the ls-SVM classification algorithm. The two classes of ALE maps were divided into a training and a test set. The training set was used for estimating the maximally discriminative pattern between anterior and posterior clusters with the iterative algorithm; the test set was only used to assess the correctness of classification (Bishop, 2006).
Results
Database search
The BrainMap query retrieved 1305 papers involving 22,872 subjects and a total of 2957 foci.
Clustering
We inferred the optimal number of clusters by a visual inspection of the reordered distance matrix and by using the cophenetic correlation distance (ccd). The cophenetic correlation distance is a measure of the quality of the solution found for different numbers of clusters (Sokal and Rohlf, 1962). Both methods reported an optimal number of two clusters (ccd=0.75).
We performed the MACM-related spatial clustering of the insular cortex using the k-means method (see Fig. S2). To minimize the risk of inconsistent results obtained for the initial random placement of starting points, we computed the K-means 256 times, as recommended in Nanetti et al. (2009). Most frequently, the same two clusters were identified.
In the right insula, the first cluster, located in the ventral anterior insular cortex, has a volume that represents 41% of the total insular GM volume; the second cluster is located in the dorsal posterior insula but includes a small area also in the middle of cluster 1, with an overall volume corresponding to the 59% of the total insular GM. Similarly, in the left insula the first cluster, located in the ventral anterior insula, has a volume that represents 62% of the total insular GM; the second cluster, located in the dorsal posterior insula, has a small area also in the middle of cluster 1, with an overall volume that represents the 38% of the total insular GM. See Fig. 2.
Fig. 2.
Insular cortex clusterization results.
The behavioral domain-based clustering results are similar to the MACM-based clusters but the subdivision between anterior and posterior insulae is less clear (see also Fig. 3 for a conjunction analysis): in the right insula, the posterior cluster shows also a big separated portion in the dorsal anterior insular cortex; in the left insula, the posterior cluster occupies most of the insular surface and the anterior cluster is located in a more dorsal location than in the right insula and in the MACM-based parcellation.
Fig. 3.

Insular cortex MACM-based and behavioral-based clusterization results comparison. Comparison between the MACM-based and the behavioral domain-based clusterization results. The figure shows the areas of overlap and the differences between the results obtained with the use of the two methods.
Fig. 4 (left panels) shows the reordered distance matrix of the two insular MACM results, while the right insula shows a sharper subdivision in two clusters, the left one shows more homogeneous characteristics of meta-analytic connectivity. This result is further evidenced by the hierarchical clustering (Fig. 4 right panels) in which the dendrogram shows further subdivisions of the insular data.
Fig. 4.
Insular cortex MACM-based clusterization results. Left panels: distance matrix. Right panels: dendrogram of the insular cortex hierarchical clusterization.
Fig. 5 shows the mean MACM connectivity of the four clusters: both the right and left anterior clusters are characterized by an attentional pattern of connectivity with frontal, cingulate, parietal, cerebellar and anterior insular highly connected areas. The posterior clusters are characterized by a more local pattern of sensorimotor MACM connectivity with connection to sensorimotor, temporal, posterior cingulate and posterior insular areas (see also Supplementary Tables S2–S5).
Fig. 5.
Mean MACM of the two clusters. Mean meta-analytic connectivity of the two networks. ALE maps were computed at an FDR-corrected threshold of p<0.05; minimum cluster dimension k>100 mm3 and visualized using Mricron (http://www.cabiatl.com/mricro/mricron/index.htm). Upper panel shows the connectivity of the anterior cluster. Lower panel shows the connectivity of the posterior cluster.
Fig. 6 shows a multidimensional scaling of the network derived from the distance between blocks. Points represent blocks and are color-coded indicating the cluster to which each point belongs. In the starting image, the distance between points represents the Euclidean distance between the MACM maps of each block, and then the network representation is optimized using multidimensional scaling. The pattern of connectivity of each block is a multidimensional dataset better visualized using algorithms that involve a dimensionality reduction. Using MDS similar entities are placed together while dissimilar entities are placed apart. Blocks belonging to the anterior insula and in particular to the right anterior cluster show a central position with the highest similarity to all other three clusters, conversely, posterior clusters have a more peripheral position indicating a more dissimilar connectivity profile.
Fig. 6.

Multidimensional scaling of the MACM-based profiles. Multidimensional scaling of the MACM-based connectivity profiles. Points represent blocks and are coded with a color indicating the cluster to which they belong. Blocks with a similar MACM profile are connected with a straight line.
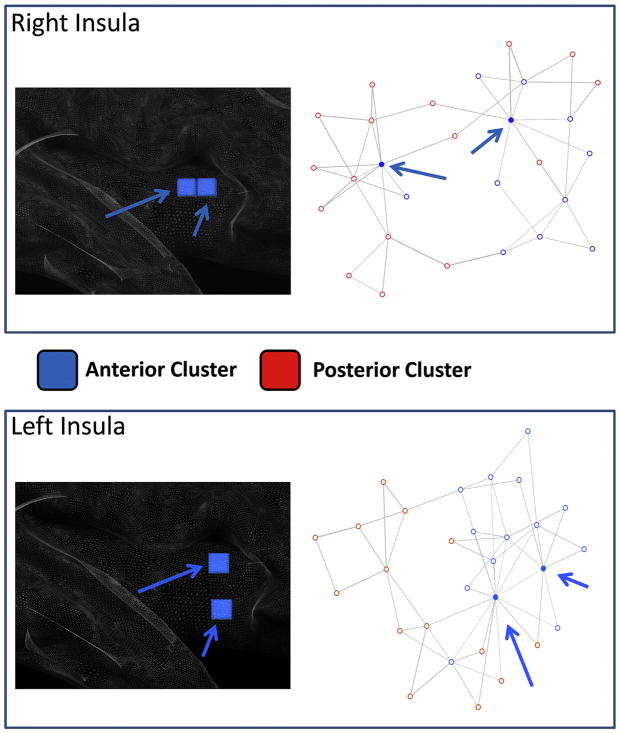
The network representation of the Euclidean distances between blocks is shown in Fig. 7, the graphical network representation is optimized with the Fruchterman–Reingold method. The blocks with the highest connectivity degree are evidenced (filled circles). This analysis evidenced that the anterior and posterior clusters form different networks that are connected by a few hub areas. These blocks belong to both sides of the anterior cluster and are visualized in the left panels of Fig. 7.
Fig. 7.
Network derived from the distance between blocks of the insular cortex. Right panel: points represent blocks and are color-coded indicating the cluster to which they belong. In this image the distance between two points represents the Euclidean distance between the MACM maps of each block. The network representation is optimized using a force-directed layout algorithm (Fruchterman–Reingold). Arrows indicate the two blocks with the highest number of connections. Left panel: the two blocks with the highest number of connections are graphically represented over a sliced standard brain surface.
Density of foci
Fig. 8 shows that the insular areas with the highest density of foci (i.e. the areas more often activated by fMRI active tasks) are placed in the anterior insula. These areas are super-imposable to the hub areas represented in Fig. 7 (blue squares).
Fig. 8.
Density of foci. Left panels: colors from red to green represent increased foci density. Hub areas are indicated by blue squares. Right panels: Voronoi tessellation of the insular cortex from which we derived the density maps; Colors from blue to red are inversely proportional to the Voronoi polygon area (i.e. proportional to the density). All values are normalized.
Behavioral results
Fig. 9 upper panels show which of the five behavioral classes that represent 95% of the total activations has a higher probability to generate activation in each of the MACM-based cluster.
Fig. 9.
Behavioral classes that activate MACM and behavioral domain-based clusters. Upper panels: the graphs show the number of papers (percent of difference from the mean) that statistically produced activations in each MACM-based cluster. All the behavioral classes (first term) that exceeded 5% of the mean are represented. Lower panel: the graphs represent the number of papers (percent of difference from the mean) that statistically produced activations in each behavioral domain-based cluster. All the behavioral classes (first term) that exceeded 5% of the mean are represented.
Fig. 9 lower panel shows which of the five behavioral classes that represented 95% of the total activations has a higher probability to generate activation in each of the Behavioral domain-based cluster.
In the MACM-based clusterization results, the anterior clusters are dominated by cognition, whereas action, perception, interoception and emotion contribute almost equally. Posterior clusters have a more distributed probability to be generated by one of the five behavioral classes whereas cognition is less represented.
In the behavioral domain-based clusters we can recognize a similar distribution but with a stronger predominance of cognition in anterior.
Cortical discriminative maps
Fig. 10 shows the cortical discriminative maps for anterior and posterior insular clusters.
Fig. 10.
Upper panel: cortical discriminative maps for anterior (red to yellow colors) and posterior (blue to green colors) insular clusters. Lower panel: generalization performance plot: the plot shows the classification accuracies for each feature elimination step (left) and discriminative maps (middle) for between-category comparisons. The box-and-whisker plot indicates the distribution of obtained accuracy values across all permutation tests. The lower boundary line of the green box indicates the 25% percentile and the upper line the 75% percentile; the red line indicates the 95% percentile that can be used to assess significance of accuracy values. The lower/upper end points of the white vertical line indicate the minimal/maximal accuracy value obtained during permutation testing. The yellow dot shows the classification accuracy of test dataset and the red dot indicates the classification accuracy.
We trained the algorithm in discriminating a first series of MACM patterns from anterior insula (learn set) or from posterior insula and tested the correctness of this discrimination in another series of patterns (test set). The test accuracies were significant (>95% in the permutation test, see Fig. 10 lower panel). The discriminative patterns for the anterior insular cluster involve the left dorsal anterior insula, inferior and middle frontal gyri, superior temporal gyrus, anterior and dorsal anterior cingulate cortex and angular gyrus. On the right: ventral anterior insula, SMA/preSMA, middle and superior frontal gyri, inferior temporal gyrus and sensorimotor cortex (see Fig. 10, middle panel, red to yellow colors).
The discriminative patterns for the posterior insular cluster involve on the left some areas of the sensorimotor cortex. On the right, the dorsal anterior insula, inferior frontal gyrus and the sensorimotor cortex (see Fig. 10 middle panels, blue to green colors).
Discussion
In this work, starting from a query of a large repository of data as the BrainMap database, and developing novel techniques of analysis such as MaC and Voronoi polygons, we confirmed and extended previous data obtained using resting state connectivity analysis, relative to the anterior–posterior and left–right specific characteristics of the insula. Moreover, our study, conducted on such a large number of studies allowed us to reveal for the first time that some area of the anterior insula play the role of hubs between anterior and posterior insulae.
The use of meta-analytic connectivity modeling and the development of a new technique, which we called meta-analytic clustering (MaC), aimed at discovering the MACM-based and behavioral domain-based parcellation of the insular cortex, allowed us to show that the insulae of both sides are characterized by a rostrocaudal subdivision in different functional areas. Indeed insular surface can be parcellated in two separate clusters, one anterior and one posterior, which are different for connectivity and function. Nevertheless, left and right insulae show different patterns of activation and connectivity.
Compared to resting state connectivity, the BrainMap database (www.brainmap.org) represents a new means for the functional interpretation of intrinsic connectivity networks. The BrainMap database is founded on a taxonomy which records functional neuroimaging experiments (Fox et al., 2005b) as metadata to link brain activations with their associated mental operations. BrainMap’s database structure, thus, allows the quantitative determination of how strongly each intrinsic connectivity network relates to a given task or mental process (Laird et al., 2011). Laird et al. found a good correlation between MACM and resting state networks (Laird et al., 2011). In fact, several brain networks have been described by both resting state network analysis (Biswal et al., 2010) and BrainMap data analysis (Laird et al., 2011). Behavior analysis performed by Laird et al. put in evidence several groupings of cognitive tasks and processes, two of which correspond to ours, relative to somesthesis and interoception/emotion. Therefore, spatial and behavioral analyses of metadata in Brain Map can provide a valuable tool for the study of brain activity related to human cognition and behavior.
Anterior and posterior clusters in the insula
Several data obtained with functional connectivity, resting state (Cauda et al., 2011) and probabilistic cartography (Cerliani et al., in press) have underscored the existence of a rostrocaudal organization of anatomical and functional connectivity in the insula. In addition, all studies put in evidence a functional lateralization in the two insulae. Our present results, together with those obtained with resting state analysis in humans (Cauda et al., 2011; Cauda et al., 2012) are supported by data of anatomical connectivity reported from the primate (Flynn et al., 1999). Using resting state analysis, we have recently shown that the ventral anterior insula in humans is functionally connected to the anterior cingulate (ACC) and frontal cortices, whereas the dorsal posterior insula is linked to motor, somatosensory, and temporal cortices (Cauda et al., 2011). In fact, the antero-inferior division of the insula is strongly connected to the rostral anterior cingulate cortex (Vogt and Pandya, 1987; Vogt and Vogt, 2003; Vogt et al., 1987, 1995). Tract tracing studies in primates further show that the insula is connected to the primary and secondary somatosensory areas, to orbitofrontal, prefrontal and motor cortex, superior temporal gyrus, temporal pole, frontal operculum, parietal operculum, primary auditory and auditory association cortices, visual association cortex, olfactory bulb, anterior cingulate cortex, amygdaloid body, hippocampus and entorhinal cortex (Flynn et al., 1999). The antero/posterior subdivisions of the insula also receive different patterns of thalamic projections in primates (Jones et al., 1976).
In the present study, we found that the meta-analytic connectivity during the execution of an active task is similar to that observed at rest (Cauda et al., 2011). As shown in Fig. 5 with MACM, both the right and the left insulae display two clusters, one anterior characterized by an attentional pattern of connectivity with frontal, cingulate, parietal, cerebellar and anterior insular highly connected areas, and one posterior characterized by a more local pattern with connection to sensorimotor, temporal, posterior cingulate and posterior insular areas. Connectivity-based clusterization gives very sharp results, with an anterior cluster occupying the 41% of the right insula and the 62% of the left insula. Differently, a behavioral domain-based clusterization gives a less sharp anteroposterior subdivision of the insula: in fact, in the left insula the anterior cluster results very small, since the insula appears mostly filled by the posterior cluster. Similarly, on the right, the posterior cluster extends to part of the dorsal anterior insula. It is very likely that both networks are partially, but differently activated by diverse paradigms. It must be considered however that, for the behavioral domain-based clusterization, we employed only the top tier behavioral classification. Indeed, the behavioral data are much deeper than this single tier and, potentially, could segregate more effectively/precisely, if done at deeper levels. Also, in their recent paper, Laird et al. (2011) showed that the behavioral data can be mixed with paradigm class information to improve clustering.
The fact that the connectivity and the behavioral domain-based clusterizations give similar results strengthens the insular subdivision evidenced in our paper and supports a convergence between two different domains as connectivity and behavior.
Recently, Kurth et al. (2010) in a ALE/behavioral meta-analysis divided the insula into sensorimotor, cognitive, social emotional and olfacto-gustatory domains. According to these authors, a conjunction analysis revealed that the anterior dorsal insula is involved in the processing of all the investigated tasks except of somatosensory and motor ones. This finding would suggest that the anterior dorsal insula may act as a region of multimodal integration. The anterior dorsal insula would represent the final stage of a hierarchical processing of information in the insular cortex. This processing would start with the elaboration of sensory information in the posterior insula and proceed with the integration of emotional and cognitive values in the anterior parts. The result would then be a full representation of the sentient self. For these reasons it has been proposed that the anterior-dorsal insula may be a potential neural correlate of awareness (Craig, 2009). In contrast, pure somatosensory and motor tasks do not need cognitive or social–emotional evaluations, i.e. they do not require additional integrations. Thus, they would not elicit activation in the anterior-dorsal multimodal integration region, but rather in the posterior part of the insula. An alternative interpretation of the role of the anterior-dorsal insula would posit that the insula represents a task-set region responsible for the maintenance of a ‘task-set’ necessary to perform any cognitive/perceptual task.
The circuits belonging to the anterior insula are very similar to each other, and their blocks in multidimensional scaling of MACM-based profiles are close to each other and in central position. In contrast, those belonging to the posterior insula, especially on the left, are located at the periphery and sparse. This finding supports the idea that posterior circuits bear a more heterogeneous connectivity, in line with Craig’s hypothesis that the posterior insula can serve as a data collector from many different networks (Craig, 2005, 2009). Moreover, as shown in Fig. 5, the connectivity of the anterior cluster is in the long range, i.e. it bears frontoparietal connections, whereas those of the posterior cluster is shorter, due to the connectivity with the sensorimotor cortex, the parietal operculum and the midcingulate cortex, as shown also recently with probabilistic tractography (Cerliani et al., in press).
Left/right differences in the insula
The analysis of the distance matrix (Fig. 4) shows clear differences in the insulae of the two sides: whereas in the right one two clusters can be easily identified, in the left the two clusters are more difficult to be differentiated from each other. This lateralization could be related to the hypothesis that the two sides of the insula subserve different functions and are linked to different circuits, sympathetic energy consuming emotions on the right versus parasympathetic energy enriching emotions (Craig, 2003, 2009). In addition, the right insula has been proposed as a key node between the default mode network (DMN) and the central executive/attentional network (Sridharan et al., 2008). According to Vincent et al. (2008) and Spreng et al. (2009), this key role is played by the whole frontoparietal control system, which includes the anterior prefrontal, dorsolateral prefrontal, dorsomedial superior frontal/anterior cingulate, anterior inferior parietal lobule, and anterior insular cortex.
Our current results are in agreement with those obtained studying functional connectivity with resting state analysis (Cauda et al., 2011; Cauda et al., 2012): the anterior cluster related to salience network, was found to be frankly lateralized on the right and the visuomotor integration network (posterior cluster) found to have a mild right lateralization. These findings support the role of the right insular cortex as a pivotal region in the attentional systems of the brain (Nelson et al., 2010; Sridharan et al., 2008).
Role of hub for the anterior insula
Some blocks of the anterior insula play the role of hubs, bridging anterior and posterior circuits of the insula, as shown in Fig. 7. This is confirmed by their frequent activation in several different paradigms: in fact, density analysis puts in evidence that in the hub areas the density of foci is higher, i.e. there is the maximal superimposition of activations in the different studies. This is in agreement with the role of salience detection of the network to which the anterior insula belongs: in fact, the right frontoinsular cortex would play a critical and causal role in switching from a central executive network to a default mode network both during task performance and resting state, from endogenous to exogenous attentional systems (Sridharan et al., 2008): the right frontoinsular cortex would be responsible for redirecting endogenous attention in response to salient environmental stimuli. In addition, Dosenbach and colleagues (Dosenbach et al., 2006, 2008; Nelson et al., 2010) describe the anterior insula as a key component in a circuit involved in sustained task, which is always activated in active fMRI paradigms as observed in a published dataset of foci (Nelson et al., 2010). These meta-analytical studies suggest that the anterior insula may have a general role in attention and task-level control. The same pattern of activation in different tasks is observed in the dorsal anterior cingulate cortex, thus suggesting that these two areas together form a core system for the implementation of data sets (Dosenbach et al., 2006, 2008). The analysis of the behavioral classes that activate MACM and behavioral domain-based clusters shows that the anterior cluster is mostly activated by cognition, whereas the posterior is mostly activated by interoception, perception and emotion (Fig. 9). This is in agreement with the hypothesis that the posterior insula collects interoceptive, emotional and environmental data, which are in turn integrated in the anterior insula which evaluates their salience.
Areas which better discriminate for the connectivity patterns of the blocks belonging to the anterior cluster (Fig. 10) were located in the prefrontal, temporal and temporo-parietal cortex, which are part of the frontoparietal control network (Greenberg et al., 2010; Spreng et al., 2009; Vincent et al., 2008). In contrast, areas which better discriminate for the posterior cluster belong to the sensorimotor integration network (Cauda et al., 2011). The voxels, located in the right anterior insula, which discriminate for the posterior cluster remind of the behavioral clustering, which also includes a “posterior” area in the right dorsal anterior insula. Therefore, this finding confirms and extends the localization of the areas which, in the insula engaged in an active task, have the most discriminative anterior or posterior patterns of connectivity.
Limitations of the study
The major limitation of our study consists in the nature of the data retrieved from the database. First, BrainMap is not an exhaustive sample of the literature; different categories of studies may be differently represented and this may lead to a bias, especially in the behavioral statistics (Costafreda et al., 2008). Second, we employed only the top-tier level of behavioral information present in BrainMap to obtain our behavioral-based clusterizations. This may lead to some inaccuracies that may explain the relative differences between behavioral domain-based and MACM-based clusterizations. Third, with the exception of behavioral category, paradigm type and subject numerosity, the only other information available was peak location. We admit that including other important data such as statistical threshold, statistical significance of foci, area dimension and shape may lead to a more accurate statistical estimation (Costafreda, 2009; Costafreda et al., 2009). However, at now all the databases that permit an anatomical-based query do not store this type of information.
Conclusion
After retrieving a large amount of data from BrainMap relative to studies in which the insular cortex was activated in different tasks, using different methodologies, some of which specifically designed for this study, we found a clear antero-posterior subdivision of the insula from the connectivity, functional and behavioral points of view. Some areas of the anterior subdivision play the role of hubs between the anterior and the posterior subdivisions. In addition, there is a clear lateralization of function, thus supporting the idea that the insulae of the two sides subserve different functions.
Supplementary Material
Acknowledgments
This research was supported by the RegionePiemonte, bando-ScienzeUmane e Sociali 2008, L.R. n. 4/2006.
Abbreviations
- MaC
Meta-analytic clustering
- fMRI
Functional magnetic resonance imaging
- ROI
Region of interest
- MACM
Meta-analytic connectivity modeling
- VOI
Volume of interest
- ALE
Activation likelihood estimation
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.04.012.
References
- Augustine JR. The insular lobe in primates including humans. Neurol Res. 1985;7:2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Ballard DH, Brown CM. Computer Vision. Prentice Hall; Englewood Cliffs, New Jersey: 1982. [Google Scholar]
- Bishop CM. Pattern Recognition and Machine Learning. Springer Link; New York: 2006. [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Solodkin A, Van Hoesen GW. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J Neuropathol Exp Neurol. 2005;64:910–922. doi: 10.1097/01.jnen.0000182983.87106.d1. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional connectivity of the posteromedial cortex. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the insular cortex: Characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.04.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D’Arceuil H, Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. 2011 Jul 14; doi: 10.1002/hbm.21338. in press. Epub ahead of print http://dx.doi.org/10.1002/hbm.21338. [DOI] [PMC free article] [PubMed]
- Costafreda SG. Pooling FMRI data: meta-analysis, mega-analysis and multi-center studies. Front Neuroinform. 2009;3:33. doi: 10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, David AS, Brammer MJ. A parametric approach to voxel-based meta-analysis. Neuroimage. 2009;46:115–122. doi: 10.1016/j.neuroimage.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- De Martino F, Valente G, Staeren N, Ashburner J, Goebel R, Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage. 2008;43:44–58. doi: 10.1016/j.neuroimage.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filzmoser P, Baumgartner R, Moser E. A hierarchical clustering method for analyzing functional MR images. Magn Reson Imaging. 1999;17:817–826. doi: 10.1016/s0730-725x(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of insula — functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005a;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005b;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frades I, Matthiesen R. Overview on techniques in cluster analysis. Methods Mol Biol. 2010;593:81–107. doi: 10.1007/978-1-60327-194-3_5. [DOI] [PubMed] [Google Scholar]
- Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Softw: Pract Exper. 1991;21:1129–1164. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Burton H, Saper CB, Swanson LW. Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. J Comp Neurol. 1976;167:385–419. doi: 10.1002/cne.901670402. [DOI] [PubMed] [Google Scholar]
- Klein R. Concrete and Abstract Voronoi Diagrams. Springer Verlag; 1989. [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005a;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005b;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. Lost in localization? The focus is meta-analysis Neuroimage. 2009;48:18–20. doi: 10.1016/j.neuroimage.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Laird AR, Fox PM, Glahn DE, Fox PT. Automated analysis of meta-analysis networks. Hum Brain Mapp. 2005;25:174–184. doi: 10.1002/hbm.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 2009;47:1666–1677. doi: 10.1016/j.neuroimage.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FA. The Brede database: a small database for functional neuroimaging. Presented at the at the 9th International Conference on Functional Mapping of the Human Brain; June 19–22, 2003; New York, NY. New York: 2003. http://208.164.121.255/hbm2003/abstract/abstract2906.htm. [Google Scholar]
- Nielsen FA. Visualizing data mining results with the brede tools. Front Neuroinform. 2009;3:26. doi: 10.3389/neuro.11.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK. Experiences with Matlab and VRML in functional neuroimaging visualizations. 2000. [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multivoxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rivier F, Clarke S. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: evidence for multiple auditory areas. Neuroimage. 1997;6:288–304. doi: 10.1006/nimg.1997.0304. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. The comparison of dendrograms by objective methods. Taxon. 1962;11:33–40. [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil DC, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J Chem Neuroanat. 2003;26:301–309. doi: 10.1016/j.jchemneu.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex —surface-features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Ward JH., Jr Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963:236–244. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.