SUMMARY
Patients with autosomal dominant vibratory urticaria have localized hives and systemic manifestations in response to dermal vibration, with coincident degranulation of mast cells and increased histamine levels in serum. We identified a previously unknown missense substitution in ADGRE2 (also known as EMR2), which was predicted to result in the replacement of cysteine with tyrosine at amino acid position 492 (p.C492Y), as the only nonsynonymous variant cosegregating with vibratory urticaria in two large kindreds. The ADGRE2 receptor undergoes autocatalytic cleavage, producing an extracellular subunit that noncovalently binds a transmembrane subunit. We showed that the variant probably destabilizes an autoinhibitory subunit interaction, sensitizing mast cells to IgE-independent vibration-induced degranulation. (Funded by the National Institutes of Health.)
Physical urticarias are disorders in which localized hives develop in response to any of various stimuli.1 The histamine release that is associated with urticarias has implicated aberrant degranulation of mast cells in their pathogenesis.2 Isolated or syndromic cold urticaria can be caused by variants in NLRP3,3 which encodes a component of the inflammasome signaling complex, or in PLCG2,4 which encodes a regulatory phospholipase. Otherwise, no pathogenic variants underlying physical urticarias have been identified.
ADGRE2 encodes a member of the epidermal growth factor (EGF)–seven transmembrane (TM7) subclass of adhesion G-protein–coupled receptors (GPCRs); the ADGRE2 protein has an N-terminal extracellular region that consists of five tandem EGF-like adhesion domains, an internal mucin-like stalk domain containing a short G-protein proteolytic site, and a C-terminal seven-pass transmembrane domain.5 Like many adhesion GPCRs, ADGRE2 undergoes autocatalytic cleavage within its G-protein proteolytic site motif6; ADGRE2 cleavage occurs between residues p.L517 and p.S518.7 The resulting extracellular α subunit is noncovalently bound to the transmembrane β subunit.8 A p.S518A mutant of ADGRE2 is resistant to cleavage6 and, unlike nonmutant ADGRE2, is unable to induce cellular migration.9 ADGRE2 is expressed predominantly in myeloid leukocytes, most strongly on neutrophils5 and macrophages8 but also on the surface of lung mast cells and the HMC1 human mast-cell line.10 The endogenous ligand of ADGRE2 is dermatan sulfate,11 which is the predominant glycosaminoglycan in skin.12 ADGRE2 is also ligated by the monoclonal antibody 2A1, which can increase migration, adhesion, degranulation, and cytokine production in myeloid cells.9,13,14
The most closely related paralogue of ADGRE2 is ADGRE5 (also called CD97).15 Under static conditions, ligand binding of ADGRE5 mediates cell–cell adhesion of leukocytes. However, shear stress destabilizes this adhesion, probably through disruption of the interaction between the α and β subunits.16 Furthermore, studies of N-terminal truncation mutants of the adhesion GPCRs ADGRE5,17 ADGRB2 (BAI2),18 and ADGRG1 (GPR56)19 have shown that their β subunits are constitutively active. Noncovalent binding of an autoinhibitory extracellular α subunit thus provides a plausible regulatory mechanism for signaling in response to physical forces. In this report, we describe the identification of a novel missense variant in ADGRE2 in families with a rare, hereditary, vibration-induced urticaria, implicating the encoded adhesion GPCR as a mechanosensor in mast cells.
METHODS
We evaluated the participants’ clinical and family history, results of a forearm vortex challenge, serum histamine measurements, and findings on skin biopsy. We performed genetic analysis of the families with vibratory urticaria by means of linkage scans, exome sequencing, and Sanger sequencing. The effects of the p.C492Y substitution were investigated with the use of patient and control primary mast cells or transfected mast cells. Additional details regarding the methods are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
RESULTS
CASE REPORTS
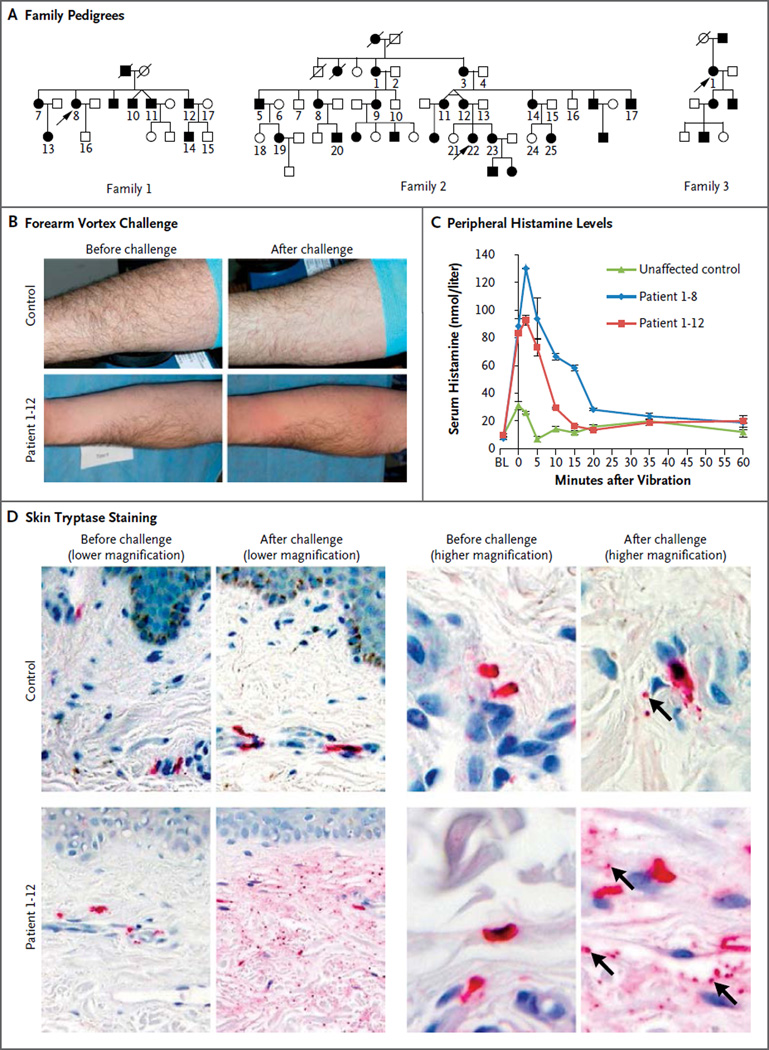
We ascertained a multiplex Lebanese family (Family 1) with vibratory urticaria that segregated as an autosomal dominant trait (Fig. 1A). In response to repetitive mechanical stimulation of the skin, such as clothing contact during exercise or towel drying, the affected family members had localized, erythematous, edematous, pruritic hives (Fig. 1B), sometimes accompanied by facial flushing, headache, or the sensation of a metallic taste. Identical symptoms could be provoked by vibration of the forearm on a laboratory vortex. Symptoms appeared within 5 minutes after stimulation and subsided within 60 minutes. The patients tested negative for dermatographism as assessed by means of a scratch test with a dermographometer set at four different pressures (20 to 144 g per square millimeter). Episodes of vibratory urticaria were marked by an increase in the histamine level in serum (Fig. 1C), which could account for the systemic manifestations. Tryptase levels in serum did not increase in response to vibration (Fig. S1 in the Supplementary Appendix), a finding consistent with our previous report on cold urticaria.20 Immunohistochemical staining of dermal mast-cell tryptase revealed degranulation resulting from vortex challenge that was more prevalent in patients than controls (Fig. 1D).
Figure 1. Inheritance and Clinical Features of Vibratory Urticaria.
Panel A shows the pedigrees of the three families originating from Lebanon that had autosomal dominant inheritance of vibratory urticaria. The numbered family members in the pedigrees were available for genotyping and sequencing. Squares denote male family members, circles female family members, solid symbols affected family members, and open symbols unaffected family members. Slashes indicate deceased family members, and arrows indicate the proband in each family. Panel B shows the results of the forearm vortex challenge. Pronounced hives developed in a patient after the challenge, with redness evident to either side of prominent swelling at the site of vortex contact. Panel C shows serial measurements of histamine in serum after forearm vortex challenge; substantially greater histamine release was seen in patients than in the control. Histamine levels peaked within 5 minutes after the challenge and subsided within 60 minutes. BL denotes baseline (prechallenge level). The data points indicate the means and I bars the standard error for two technical replicates. Panel D shows immunohistochemical staining of tryptase (red) in samples obtained by means of skin biopsy; this staining labels both intact and degranulated mast cells, as well as secreted granules (indicated by arrows in the postchallenge higher-magnification views) and extracellular tryptase (diffuse red staining). Release of the granular contents of mast cells after vibration was widespread in a patient sample and limited in a control sample.
This presentation closely matched a previous description of dermo-distortive urticaria, which also segregated in an autosomal dominant pattern in another Lebanese family (Family 2).21 We subsequently ascertained the proband from a third Lebanese family with dominant vibratory urticaria (Family 3) (Fig. 1A). None of these families were known to be related to one another, although the similarities in their phenotypes and origins strongly suggested a shared ancestor.
MISSENSE VARIANT IN ADGRE2
Linkage scans of DNA from members of Families 1 and 2 conclusively implicated a 2.2-Mb region on chromosome 19p13, with a combined logarithm of the odds ratio (LOD) score of 7.224 (Fig. S2 in the Supplementary Appendix). Exome sequencing of DNA samples from Family 1 revealed three variants within the linkage interval that met the filtering criteria; Sanger sequencing showed that one of these was a false positive call, and one was validated as a true call but was absent from affected members of Family 2. The sole remaining candidate variant was c.1475G→A in ADGRE2 (NCBI reference sequence NM_013447.3), which was predicted to result in a p.C492Y missense substitution (NP_038475.2). Amino acid position 492 lies within the G-protein proteolytic site motif of the α subunit, 26 amino acids upstream from the cleavage site (Fig. 2A). ADGRE2 is located within a 1.7-Mb haplotype shared by affected members of Families 1 and 2, and the c.1475G→A variant perfectly cosegregated with the vibratory urticaria phenotype in all 10 available members of Family 1 and all 25 available members of Family 2; it was also heterozygous in the sole available member of Family 3, the proband. The variant was absent from multiple databases (Table S1 in the Supplementary Appendix), as well as from 1105 unaffected Lebanese and 100 unaffected Israeli controls. None of the 7 affected members of Family 1 had a rare coding or splice-site variant in either NLRP3 or PLCG2, and these genes were also ruled out by linkage analysis.
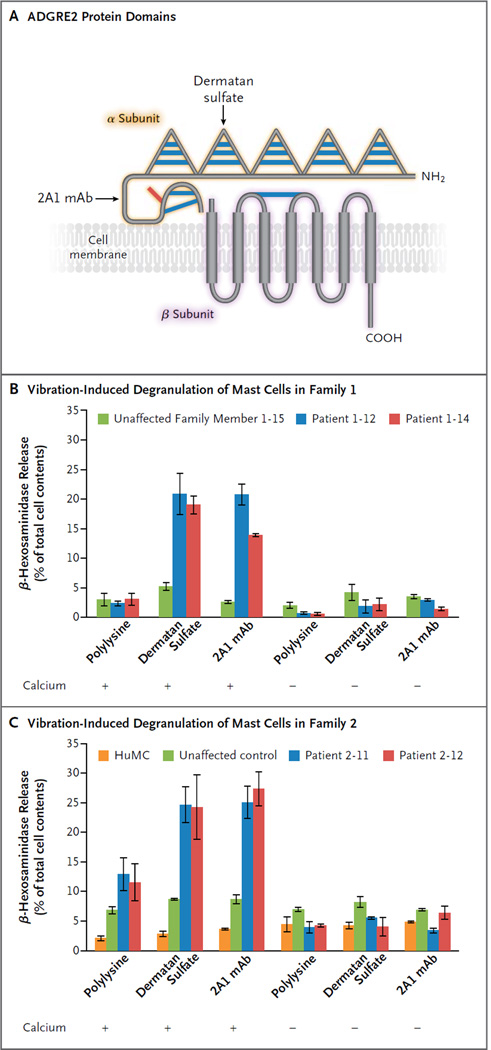
Figure 2. Vibration-Induced, ADGRE2-Enhanced Degranulation of Mast Cells from Patients.
Panel A shows a schematic diagram of ADGRE2 domain structure, with five epidermal growth factor (EGF)–like adhesion domains (triangles) and a central stalk in the extracellular N-terminal α subunit, the autocatalytic cleavage site between residues p.L517 and p.S518, and the seven-pass transmembrane domain in the C-terminal β subunit, depicted noncovalently bound to the α subunit. Blue bars represent disulfide bonds predicted on the basis of homology to other EGF domain–containing proteins and EGF–seven transmembrane (TM7) adhesion G-protein–coupled receptors (GPCRs). The red bar represents the location of the p.C492Y substitution. Dermatan sulfate is the endogenous ligand for ADGRE2 and binds its fourth EGF domain. The monoclonal antibody (mAb) 2A1 was raised against the stalk domain. Panels B and C show the results of assays for vibration-induced degranulation of mast cells (measured as the release of β-hexosaminidase) in Families 1 and 2. Primary mast cells (PMCs) were derived in parallel from two patients and one control member of Family 1 and allowed to adhere to plates coated with substrate as indicated, then vibrated in the presence or absence of calcium chloride, and finally assayed for degranulation (Panel B). PMCs from two patients in Family 2, PMCs from an unrelated control, and stock human mast cells (HuMCs) were also assayed (Panel C). PMCs from patients and controls were derived in parallel from CD34+ progenitors isolated from fresh whole blood, whereas HuMCs were derived from frozen CD34+ progenitors that were previously isolated from peripheral blood after granulocyte colony-stimulating factor (G-CSF) stimulation and apheresis. Data are the means and standard errors of two independent experiments, each assayed in duplicate, from cells derived from a single blood sample from either the patient or the control.
Within the linkage interval, there were 54 protein-coding exons in which all 7 exome-sequenced affected participants from Family 1 had at least one nucleotide position with a depth of coverage of less than 20×. We performed Sanger sequencing on these exons in DNA from an affected participant, but no other candidate variants were found, which left p.C492Y in ADGRE2 as the only rare, cosegregating nonsynonymous or splicing variant within known exons in the candidate interval. The coding exons of ADGRE2 were screened by means of Sanger sequencing in a panel of 60 patients with various sporadic physical urticarias (Table S2 in the Supplementary Appendix), and no additional rare coding or splice variants were identified.
EXPRESSION OF ADGRE2
ADGRE2 mRNA was expressed in multiple primary human mast-cell populations and mast-cell lines and was ubiquitously detectable in a panel of human tissues, although its expression appeared to be qualitatively higher in primary human mast cells and the LAD2 human mast-cell line than in other tissues. Flow cytometric assessment of both surface and total ADGRE2 protein levels revealed robust expression in multiple types of mast cells (Fig. S3 in the Supplementary Appendix).
VIBRATION-DEPENDENT DEGRANULATION OF MAST CELLS
Primary mast cells from two patients and one control in Family 1 were allowed to adhere to plates, which were then vibrated on an orbital shaker to mimic the stimuli that provoke symptoms in patients with vibratory urticaria. Cells that were adhered to a substrate of polylysine had minimal degranulation in response to vibration. However, when they were adhered to either dermatan sulfate (the endogenous ligand of ADGRE2) or the monoclonal anti-ADGRE2 antibody 2A1, cells from patients but not from controls had a significant increase in degranulation in response to vibration. This response was dependent on the presence of calcium in the buffer during vibration, a finding consistent with activation of an intracellular signaling mechanism (Fig. 2B). There were no significant differences between experiments performed with different plate coatings, buffers, or samples of cells in the percentage of detached cells after vibration (data not shown), so the absence of degranulation when calcium was not present was not a result of more cells being detached from the substrate. In a separate experiment, patient cells from Family 2 showed modest vibration-induced degranulation when adhered to polylysine, but they degranulated to a greater extent when ADGRE2 was ligated through adhesion to dermatan sulfate or to the 2A1 antibody (Fig. 2C).
EXPRESSION AND PROCESSING OF MUTANT ADGRE2
There was no apparent difference between patient and control primary mast cells in the basal expression of either the α or the β subunit of ADGRE2, which suggested that the mutant protein is normally expressed. Because p.C492Y is located within the G-protein proteolytic site motif, we assessed whether the substitution affected ADGRE2 cleavage. LAD2 mast cells were efficiently transfected with either control vector or one of five ADGRE2 expression constructs, with little effect on viability and with similar expression levels. Immunoblotting against both ADGRE2 subunits showed no difference between cells expressing nonmutated ADGRE2 and those expressing p.C492Y ADGRE2 in the abundance of full-length protein, α subunit, or β subunit. Likewise, both transfectants had lower levels of full-length protein and higher levels of cleavage products than were present in the cleavage-deficient p.S518A and p.[C492Y; S518A] mutants. Moreover, we observed no substantial difference between cells transfected with nonmutated ADGRE2 and those transfected with p.C492Y ADGRE2 in their ability to adhere to dermatan sulfate–coated plates, nor did we observe differences among any transfected cells in ADGRE2 trafficking to the cell surface (Fig. S4 in the Supplementary Appendix).
VIBRATION-INDUCED SUBUNIT DISSOCIATION
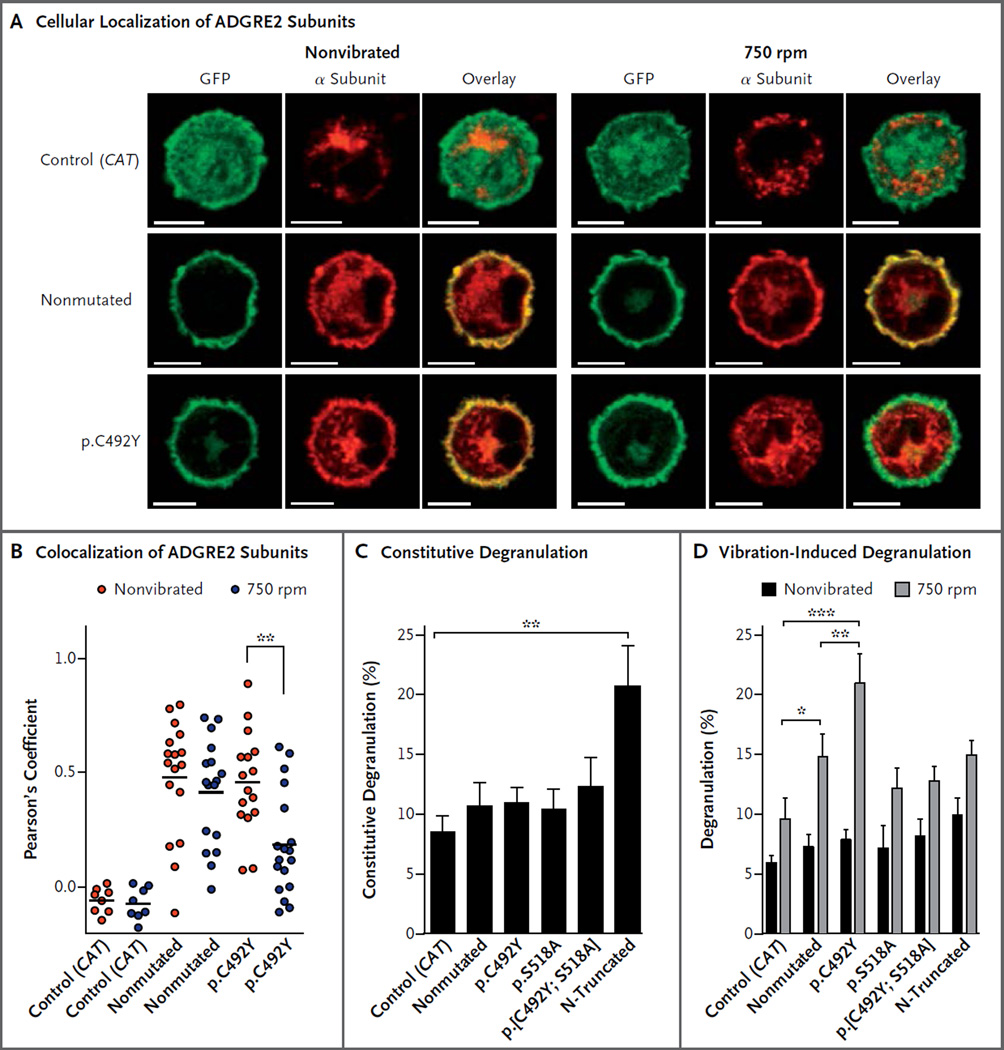
In transfected mast cells that were not subjected to vibration, both the nonmutant and p.C492Y α subunits localized to the plasma membrane with the transmembrane β subunit. After vibration, the p.C492Y α subunit was lost from the cell surface (Fig. 3A), which resulted in reduced co-localization of the subunits (Fig. 3B). This dissociation suggests that the p.C492Y substitution destabilizes the interaction between the α and β subunits, such that shear stress that is incurred during vibration disrupts their noncovalent attachment.
Figure 3. Cleavage-Dependent Degranulation Elicited by p.C492Y Mutant ADGRE2 Associated with a Loss of Surface α Subunit.
Panel A shows confocal slice micrographs of transfected murine bone marrow–derived mast cells. As detected by immunostaining with the 2A1 antibody (red), the p.C492Y mutant α subunit, but not the nonmutant α subunit, is lost from the plasma membrane in response to vibration. In contrast, as determined by fluorescent detection of GFP (green), the GFP-tagged β subunit is retained in the membrane. Yellow indicates an overlap of the red and green signal. The scale bars indicate 5 µm. Panel B shows the significance of the decrease in colocalization. The α subunit staining evident in cells expressing CAT–GFP fusion control vector is nonspecific; however, it was confined to the cytoplasm, whereas the β subunit of ADGRE2–GFP fusion clones was located primarily in the plasma membrane. Therefore, cytoplasmic α subunit staining was masked out for the colocalization analysis (see the Methods section in the Supplementary Appendix). Each data point is the Pearson’s coefficient, measuring the correlation between the location and intensity of the α and β subunits, in the colocalized volume of a stack of high-power-field images through the depth of the cells. Data are combined from two independent experiments. There is no mouse orthologue for ADGRE2, so only transfected human ADGRE2 is detected. Panels C and D show the measurement of constitutive (Panel C) and vibration-induced (Panel D) degranulation. To measure constitutive degranulation, transfected LAD2 cells were incubated for 5 hours to allow protein expression, and nonvibrated cells were assayed. To measure vibration-induced degranulation, transfected LAD2 cells were incubated for 5 hours to allow protein expression and were adhered to dermatan sulfate–coated plates for 3 hours; parallel cultures were then either vibrated at 750 rpm for 20 minutes or not vibrated and then were assayed. In Panels C and D, the data are means and standard errors for four independent experiments, each performed in duplicate (Panel C) or triplicate (Panel D). A single asterisk denotes P<0.05, a double asterisk P<0.01, and a triple asterisk P<0.001.
CONSTITUTIVE DEGRANULATION ELICITED BY THE β SUBUNIT
In nonvibrated transfected LAD2 cells, an N-terminal truncation mutant expressing only the transmembrane β subunit of ADGRE2 elicited greater constitutive degranulation than did all full-length clones (Fig. 3C). This observation is consistent with studies of other adhesion GPCRs, which have shown that the β subunit, on its own, is constitutively active17–19; this suggests that the extracellular α subunit inhibits signaling by the β subunit.
CLEAVAGE-DEPENDENT, VIBRATION-INDUCED DEGRANULATION
In response to vibration, nonmutated ADGRE2 induced greater degranulation of transfected LAD2 cells than did the control vector, and p.C492Y ADGRE2 induced greater degranulation than did nonmutated ADGRE2 (Fig. 3D). There were no significant differences among any of the non-vibrated cell populations (Tables S3 and S4 in the Supplementary Appendix). These data suggest that nonmutant ADGRE2 is somewhat sensitive to vibration and corroborate the hypothesis that the p.C492Y variant is hypermorphic. Neither the p.S518A nor the p.[C492Y; S518A] cleavage-deficient mutant elicited significantly greater degranulation than did the control vector, and the p.[C492Y; S518A] mutant did not induce greater degranulation than did the p.S518A mutant; this indicates that cleavage is required for degranulation that is induced by either nonmutated ADGRE2 or p.C492Y ADGRE2. This result is also consistent with a model in which liberation of the inhibitory α subunit from the transmembrane β subunit is responsible for ADGRE2-mediated degranulation, since covalent attachment of the subunits precludes the response. Vibration 8 hours after transfection did not elicit a greater response from N-truncated ADGRE2 than from the other clones, probably because constitutive degranulation of these cells during incubation had depleted their secretory granules.
DISCUSSION
We identified a novel missense variant in ADGRE2 as the basis of autosomal dominant vibratory urticaria, a clinical and pathophysiological entity that is distinct from dermatographism and other physical urticarias. Moreover, ADGRE2 encodes a cell-surface receptor, in contrast to the mutant cytoplasmic proteins underlying cold urticarias.3,4 Our data suggest that the p.C492Y substitution effects a pathogenic gain of function in ADGRE2 by destabilizing the inhibitory interaction between the α and β subunits, thereby sensitizing mast cells to vibration-induced degranulation. Consistent with this mechanism, the hereditary vibratory urticaria phenotype probably reflects an exaggeration of a normal cellular response to dermal vibration that remains asymptomatic in unaffected persons. We observed a modest increase in systemic histamine in a control participant after vortex challenge, and LAD2 cells transfected with nonmutated ADGRE2 showed greater degranulation after vibration than did cells transfected with control vector, although the response was not as great as that in cells transfected with p.C492Y ADGRE2. In addition, the vortex challenge that is sometimes used to ascertain vibratory urticaria can be nonspecific.22 The evolution of an autocatalytic cleavage mechanism that permits even a nonmutant α subunit to dissociate from the β subunit under certain conditions further supports the hypothesis that the noncovalent subunit interaction has a regulatory function.
The physiological role of ADGRE2 in mast cells and other myeloid cell types remains to be determined. The orthologue of ADGRE2 has been lost in the murid lineage,15 which suggests either that it is not essential or that another gene compensates for its absence in mice and rats. However, the selective expression of ADGRE2 in myeloid cells and the previously described ability of antibody-mediated ADGRE2 ligation to alter neutrophil and macrophage behaviors9,13,14 generally suggest a role for ADGRE2 in innate immunity, possibly in the host response to an as yet unidentified pathogen. Collectively, the predominance of its ligand dermatan sulfate in the skin,12 the sensitivity of its paralogue ADGRE5 to shear stress,16 and the response of ADGRE2 to vibration shown here support a role for ADGRE2 in the detection of and response to physical stimuli.
Our data, together with those of previous studies,16,23 establish the sensitivity of EGF-TM7 adhesion GPCRs to shear stress, a finding that is consistent with their cleavage and retention of a noncovalently bound extracellular subunit. Vibration alone was sufficient to cause significant degranulation in primary patient-derived mast cells, indicating a purely mechanical means of inducing an allergy-like response. IgE-independent degranulation of mast cells can also be induced through other GPCRs, by stimuli such as complement C5a,24 which suggests that GPCR signaling may have a general role in the modulation of mast-cell responses. The aberrant degranulation associated with vibratory urticaria suggests that dermal mast cells may respond to physical forces through ADGRE2, in addition to their known response to allergenic stimuli through the IgE receptor FcεRI. This dual role defines a novel facet of the biology of mast cells that may also be important in other urticarias.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Programs of the National Human Genome Research Institute, National Institute of Allergy and Infectious Diseases, and National Cancer Institute at the National Institutes of Health, and by a contract from the National Cancer Institute (HHSN261200800001E, to Mr. Young).
We thank the families for their participation in this study; Elon Pras for providing control DNA samples; Rebecca Rosenke and Eva Haigh for technical assistance; Alasdair Gilfillan, Keith Choate, and Lynn Boyden for helpful discussions; and the staff of the NIH Intramural Sequencing Center for performing exome sequencing.
Footnotes
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Abajian M, Schoepke N, Altrichter S, Zuberbier T, Maurer M. Physical urticarias and cholinergic urticaria. Immunol Allergy Clin North Am. 2014;34:73–88. doi: 10.1016/j.iac.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RW, Rosenstreich DL. Discrimination between urticaria-prone and other allergic patients by intradermal skin testing with codeine. J Allergy Clin Immunol. 1986;77:802–807. doi: 10.1016/0091-6749(86)90377-5. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ombrello MJ, Remmers EF, Sun G, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HH, Stacey M, Hamann J, Gordon S, McKnight AJ. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics. 2000;67:188–200. doi: 10.1006/geno.2000.6238. [DOI] [PubMed] [Google Scholar]
- 6.Lin HH, Chang GW, Davies JQ, Stacey M, Harris J, Gordon S. Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J Biol Chem. 2004;279:31823–31832. doi: 10.1074/jbc.M402974200. [DOI] [PubMed] [Google Scholar]
- 7.Chang GW, Stacey M, Kwakkenbos MJ, Hamann J, Gordon S, Lin HH. Proteolytic cleavage of the EMR2 receptor requires both the extracellular stalk and the GPS motif. FEBS Lett. 2003;547:145–150. doi: 10.1016/s0014-5793(03)00695-1. [DOI] [PubMed] [Google Scholar]
- 8.Kwakkenbos MJ, Chang GW, Lin HH, et al. The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. J Leukoc Biol. 2002;71:854–862. [PubMed] [Google Scholar]
- 9.Huang YS, Chiang NY, Hu CH, et al. Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol. 2012;32:1408–1420. doi: 10.1128/MCB.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florian S, Sonneck K, Czerny M, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–1062. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 11.Stacey M, Chang GW, Davies JQ, et al. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood. 2003;102:2916–2924. doi: 10.1182/blood-2002-11-3540. [DOI] [PubMed] [Google Scholar]
- 12.Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12(9):117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 13.Yona S, Lin HH, Dri P, et al. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J. 2008;22:741–751. doi: 10.1096/fj.07-9435com. [DOI] [PubMed] [Google Scholar]
- 14.Chen TY, Hwang TL, Lin CY, et al. EMR2 receptor ligation modulates cytokine secretion profiles and cell survival of lipopolysaccharide-treated neutrophils. Chang Gung Med J. 2011;34:468–477. [PubMed] [Google Scholar]
- 15.Kwakkenbos MJ, Matmati M, Madsen O, et al. An unusual mode of concerted evolution of the EGF-TM7 receptor chimera EMR2. FASEB J. 2006;20:2582–2584. doi: 10.1096/fj.06-6500fje. [DOI] [PubMed] [Google Scholar]
- 16.Karpus ON, Veninga H, Hoek RM, et al. Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J Immunol. 2013;190:3740–3748. doi: 10.4049/jimmunol.1202192. [DOI] [PubMed] [Google Scholar]
- 17.Ward Y, Lake R, Yin JJ, et al. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 2011;71:7301–7311. doi: 10.1158/0008-5472.CAN-11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okajima D, Kudo G, Yokota H. Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J Recept Signal Transduct Res. 2010;30:143–153. doi: 10.3109/10799891003671139. [DOI] [PubMed] [Google Scholar]
- 19.Paavola KJ, Stephenson JR, Ritter SL, Alter SP, Hall RA. The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J Biol Chem. 2011;286:28914–28921. doi: 10.1074/jbc.M111.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer J, Gorbach AM, Liu WM, et al. Mast cell dependent vascular changes associated with an acute response to cold immersion in primary contact urticaria. PLoS One. 2013;8(2):e56773. doi: 10.1371/journal.pone.0056773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein PA, Kidd KK. Dermo-distortive urticaria: an autosomal dominant dermatologic disorder. Am J Med Genet. 1981;9:307–315. doi: 10.1002/ajmg.1320090407. [DOI] [PubMed] [Google Scholar]
- 22.Mathelier-Fusade P, Vermeulen C, Leynadier F. Vibratory angioedema. Ann Dermatol Venereol. 2001;128:750–752. (In French.) [PubMed] [Google Scholar]
- 23.Scholz N, Gehring J, Guan C, et al. The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep. 2015;11:866–874. doi: 10.1016/j.celrep.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Blokhuis BR, Garssen J, Redegeld FA. Non-IgE mediated mast cell activation. Eur J Pharmacol. 2015 Jul 8; doi: 10.1016/j.ejphar.2015.07.017. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.