Abstract
Incorporation of selenocysteine (Sec) in bacteria requires a UGA codon that is reassigned to Sec by the Sec-specific elongation factor SelB and a conserved mRNA motif (SECIS element). These requirements severely restrict the engineering of selenoproteins. Earlier a synthetic tRNASec was reported that allowed canonical Sec incorporation by EF-Tu; however, serine misincorporation limited its scope. We report a superior tRNASec variant (tRNAUTuX) that facilitates EF-Tu dependent stoichiometric Sec insertion in response to UAG both in vivo in Escherichia coli and in vitro in a cellfree protein synthesis system. We also demonstrate recoding of several sense codons in a SelB supplemented cell-free system. These advances in Sec incorporation will aid rational design and directed evolution of selenoproteins.
1. Introduction
Organisms pay a high fitness cost for the benefit of endowing proteins with the unique properties of the 21st amino acid, selenocysteine (Sec) [1,2], and have evolved complex biosynthetic and translational mechanisms to incorporate Sec [3,4]. At the interface of Sec synthesis and insertion lies tRNASec. Initially acylated by seryl-tRNA synthetase (SerRS) to form Ser-tRNASec, the bacterial enzyme SelA catalyzes the conversion of Ser to Sec in a single step on the tRNA [3]. Once synthesized, selenocysteinyl-tRNA (Sec-tRNASec) is bound by the specialized Sec-specific elongation factor SelB, which subsequently binds to a highly conserved mRNA motif denoted as Selenocysteine Insertion Sequence (SECIS), facilitating insertion of Sec at a UGA codon [3]. In bacteria, the SECIS sequence is located directly after the suppressed UGA and is thus part of the coding sequence of bacterial genes, making engineering of newly designed selenoproteins very difficult [5–7]. Recently, we reported construction of a synthetic tRNA (tRNAUTu) that enabled SECIS-independent and EF-Tu-dependent insertion of Sec in Escherichia coli [8]. This tRNAUTu combines the aminoacyl acceptor helix of tRNASec with the backbone of tRNASer, and serves as a substrate for the essential proteins SerRS, SelA, and EF-Tu. By virtue of its interaction with EF-Tu, Sect-RNAUTu circumvents the need for the Sec-specific elongation factor SelB, and more importantly does not require the SECIS mRNA motif. Sec-tRNAUTu therefore participates in canonical translation, allowing versatile sequence-independent production of designed selenoproteins programmed by UAG.
While SelB recognizes only Sec-tRNASec [9,10], EF-Tu serves all other aminoacyl-tRNAs (aa-tRNAs). Therefore, if the SelA-dependent conversion of Ser-tRNAUTu to Sec- tRNAUTu is not complete, Ser will be incorporated instead of the desired Sec residue. This was an impediment in the earlier work in which ~30% misincorporation of Ser was observed [8]. We reasoned that by designing an improved tRNAUTu with better substrate properties for SelA, misincorporation could be prevented. Here we report such a tRNA (tRNAUTuX) that allows complete Sec incorporation in vivo and in vitro in response to UAG.
2. Materials and methods
2.1. In vitro Sec-tRNA formation
To characterize in vitro formation of Sec-tRNA, tRNA species were radiolabeled using [α-32P]ATP and the E. coli CCA editing enzyme [11]. Ser-tRNA formation by SerRS, selenophosphate production by SelD, and Ser to Sec conversion by SelA was carried out under anoxic conditions as previously described [8]. Conversion rates were determined by autoradiography and quantitation of aminoacyl-AMP after thin layer chromatography of nuclease P1 digests of aminoacyl-tRNAUTu [12]. For use in cell free protein synthesis experiments, Sec-tRNA was phenol-chloroform extracted, ethanol precipitated, and resuspended in RNase free H2O to desired concentration.
2.2. In vivo tRNAUTu utilization assay
E. coli ΔselA ΔselB ΔfdhF strain MH5 was co-transformed with plasmids pACYC-[E. coli selA+, M. jannaschii pstk] and pGFIB-[tRNAUTuam], or pGFIB-[tRNAUTuXam] variants as well as pRSF-[E. coli serS-fdhFam] and grown on LB medium supplemented with the corresponding antibiotics ampicillin, chloramphenicol, or kanamycin. As a control E. coli MH5 was co-transformed with the plasmids pACYC-[E. coli selA+, M. jannaschii pstk], pRSF-[E. coli serSfdhFop] and pET15b-[E. coli selB] to reconstitute the wild-type (WT) Sec formation apparatus using the genomically encoded tRNASec. E. coli MH5 carrying plasmids pACYC-[E. coli selA+M. jannaschii pstk], pRSF-[E. coli serS-fdhFam] and pET15b-[E. coli selB] served as a second control. Overnight cultures of these clones were plated on LB agar plates supplemented with 10 µM IPTG, 1 µM Na2MoO4, 1 µM Na2SeO3 and 50 mM sodium formate, as previously described [13,14], and were grown anaerobically at 37°C overnight. Plates were then overlaid with a top agar containing 1 mg/mL benzyl viologen (BV), 250 mM sodium formate, and 25 mM KH2PO4 pH 7.0. The appearance of a purple color indicates catalytically active FDHH, which depends on Sec insertion at position 140.
2.3. Cell-free selenoprotein synthesis
Cell-free in vitro translation experiments were conducted using the PURExpress in vitro Protein Synthesis Kit (E6800S) or PURExpress ΔRF123 kit (E6850S, New England Biolabs Inc.), as noted. Reactions were prepared according to the manufacturer’s instructions inside an anaerobic chamber, and were supplemented with 1 µM sodium molybdate, 40U RNasin Plus RNase Inhibitor (Promega), and 250 ng of fdhF mutants at position 140 cloned into PURE vector. Reactions were normalized against expressed dihydrofolate reductase (DHFR) as a negative control, a protein that did not exhibit measureable reduction of BV.
For translation mediated by tRNASecam, tRNASecop, tRNASecCGU, tRNASecGCC, tRNASecCCU, and tRNASecUCG, PURExpress kit reactions were supplemented with 12 µM Sec-tRNASelCam, 12 µM SelB, and were allowed to proceed for two hr at 37°C. For expression mediated by tRNAUTuam, tRNAUTuXam, or tRNASecUXam, PURExpressΔRF123 kit reactions were prepared in the absence of RF1, supplemented with 70 µM Sec-tRNA, 67 µM EF-Tu, and were allowed to proceed for five hr at 37°C. Plasmid containing fdhF with corresponding cognate codon at position 140 were also added to each reaction. Following protein synthesis, 0.7 mg/ml BV and 7 mM sodium formate were added to the reaction mixture to a final volume of 30 µl. FDHH activity was monitored over time via absorbance at 578 nm measuring using a Nanodrop 2000 (Thermo-Scientific NanoDrop 2000 UV-Vis Spectrophotometer). As a negative control, a reaction was prepared with 12 µM Ser-tRNASecam instead of SectRNASecam; absence of BV reduction activity was confirmed following this reaction.
3. Results
The co-crystal structure of decameric Aquifex aeolicus SelA protein in complex with ten Thermus tengcongensis tRNASec molecules provided molecular insight into the enzyme’s substrate recognition and coordination, illustrating the mechanism by which SelA discriminates between tRNASer and tRNASec [15]. This information was used to rationally engineer additional tRNAUTu variants to act as ideal substrates for SelA that would increase the yield of Sec insertion.
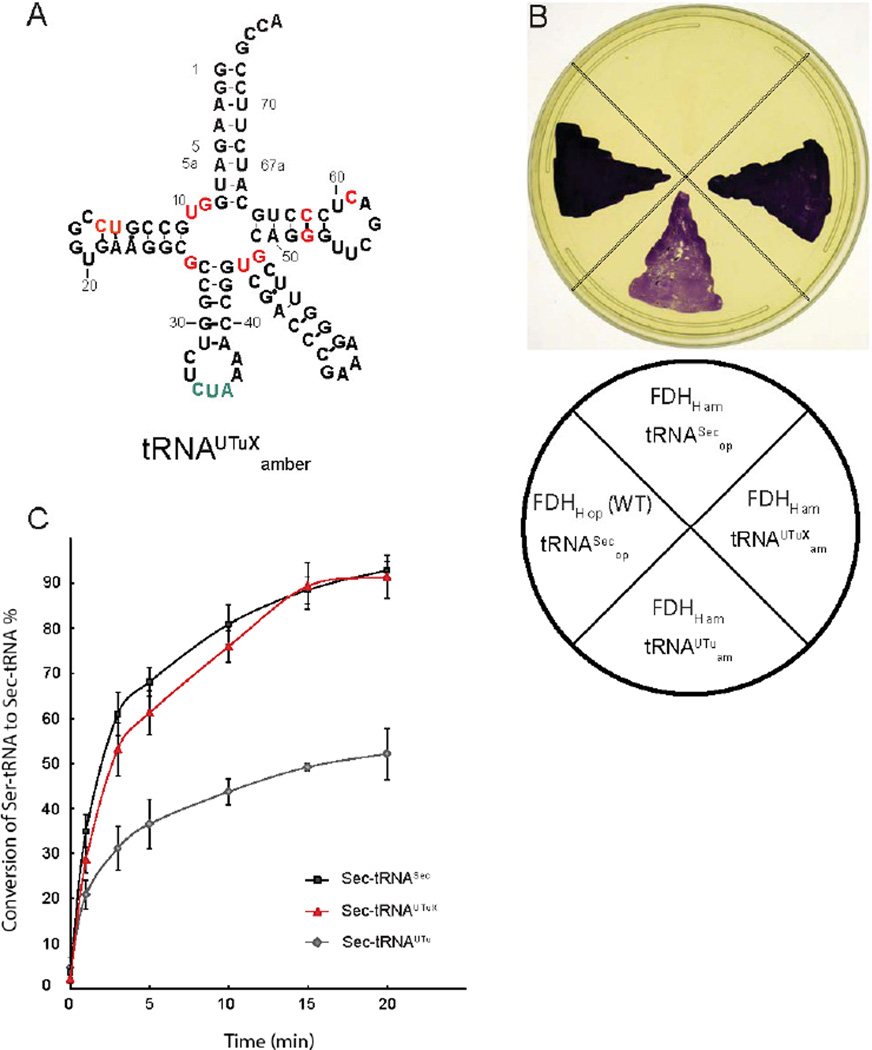
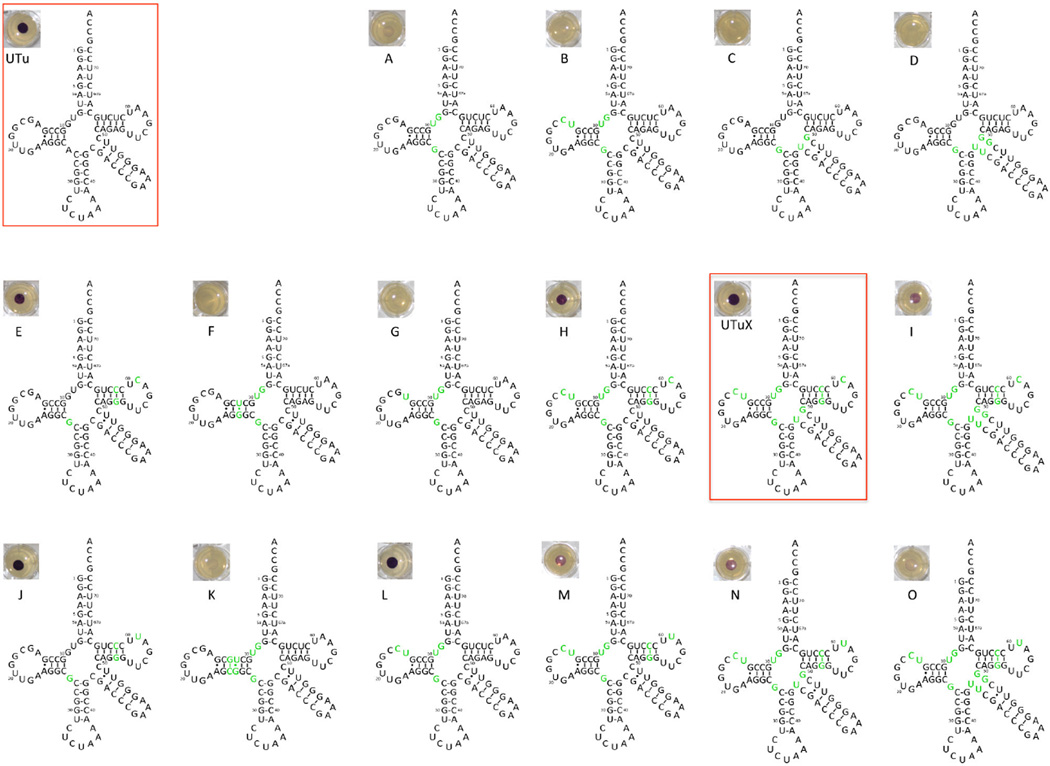
Using site-directed mutagenesis, we incrementally changed the sequence of the original tRNAUTu to more closely resemble the features of tRNASec that contribute to binding of SelA. These modifications comprised both single and combined exchanges as well as insertions and deletions of nucleotides in various regions of the tRNA. However, they left the critical tRNAUTu features that (i) provide thermodynamic binding specificity for EF-Tu [16] and (ii) contribute to the incompatibility between tRNASec and EF-Tu [17] (Fig. 1A). A total of 29 tRNA variants were produced and subsequently tested for their capacity to mediate Sec insertion, using E. coli FDHH as a reporter protein (Fig. 2). A natural selenoenzyme, FDHH contains an essential Sec residue at position 140, and catalyzes the electron transfer from formate onto the artificial electron acceptor benzyl viologen under anaerobic conditions. We used the FDHH-mediated BV reduction as a sensitive colorimetric in vivo reporter system for functional Sec insertion, as reduced BV adopts a dark purple color [18] (Fig. 1B). Consequently, the E. coli ΔselA ΔselB ΔfdhF triple deletion strain MH5 was complemented with a vector encoding SelA, PSTK, FDHH140am and each tRNAUTuam variant, subsequently grown in the presence of formate and BV [8]. To serve as a positive control, FDHH140op expressed with the genomic WT tRNASecop was also grown (Fig. 1B). Using this growth assay, a tRNA variant capable of producing active FDHH with the same apparent BV reduction activity as the WT was identified from among the 29 tRNAUTu variants (Fig. 1B, Fig. 2), notably producing visibly more reduced BV than the original synthetic variant. This improved tRNA was named tRNAUTuXam; it differs from the original tRNAUTu in 11 positions (Fig. 1A, Fig. 2, Fig. S1). To validate the observed BV color change, tRNAUTuXam was then characterized in a set of in vitro experiments.
Fig. 1.
(A) Secondary structure of tRNAUTuX. Nucleotides that were changed from the original tRNAUTu are highlighted in red, the amber anticodon is depicted in green. (B) tRNAUTuX mediates functional Sec insertion in FDHH. An E. coli ΔselA ΔselB Δfdhf triple deletion strain was separately complemented with E. coli SelA, M. jannaschii PSTK alongside tRNASecop, E. coli SelB, and WT FDHHop; tRNAUTuam and FDHHam; tRNAUTuXam and FDHHam; and a negative control with tRNASecop, E. coli SelB, and FDHH140am. FDHH activity was assessed by appearance of the purple colored reduced BV. (C) In vitro conversion of Ser-tRNASec, Sert-RNAUTu, and Ser-tRNAUTuX by SelA. 5 µM SelD, Reactions were pre-incubated with 1 mM Na2SeO3 and 5 mM ATP at pH 7.2 under anaerobic conditions at 37°C for 30 min and then supplemented with 1 µM SelA and 10 µM of [α32-P] radiolabeled Ser-tRNA species for up to 20 min. Aliquots of 1.5 µL were taken at different time points, digested with nuclease P1, and spotted onto cellulose thin layer chromatography plates. After developing, plates were analyzed by autoradiography. While approximately 50% of Ser-tRNAUTu was converted, both tRNASec and tRNAUTuX support nearly full conversion to Sec-tRNA over a course of 20 min.
Fig. 2.
Activity of rationally desgined tRNAUTu variants. For each of sixteen variants, mutations relative to tRNAUTu are depicted in green, with the original variant shown in the upper left. Each variant as well as E. coli SelB and SelA was used to complement FDHHam in E. coli ΔselA ΔselB Δfdhf,. FDHH activity was assessed by appearance of the purple colored reduced BV, with the activity mediated by each variant shown adjacent to it.
While the modifications introduced in tRNAUTuX focused on better interaction with SelA, it was a prerequisite to retain robust Ser-tRNAUTuX formation by SerRS. Serylation assays of tRNAUTuX, tRNASec, and the original tRNAUTu did not reveal any significant differences among the three tRNA species (Table S1), as the KM (3.5 µM), kcat (0.42min−1), and kcat/KM (0.12 µM−1min−1) of tRNAUTuX were found to be very close to those of tRNASec and tRNAUTu. Subsequently, conversion of Ser-tRNAUTuX to Sec-tRNA by SelA was examined (Fig. 1C). In contrast to the original tRNAUTu, which showed about ~50% SelA-dependent conversion to Sec-tRNA, Ser-tRNAUTuX and WT Ser-tRNASec were very similar and yielded ~90% Sec formation (after 20 min). Tighter binding of SelA was further confirmed by RNase protection in the presence of excess SelA protein, revealing that within 20 min twice the amount of Ser-tRNAUTu was digested by nuclease P1 than Ser-tRNAUTuX or Ser-tRNASec (Fig. S2).
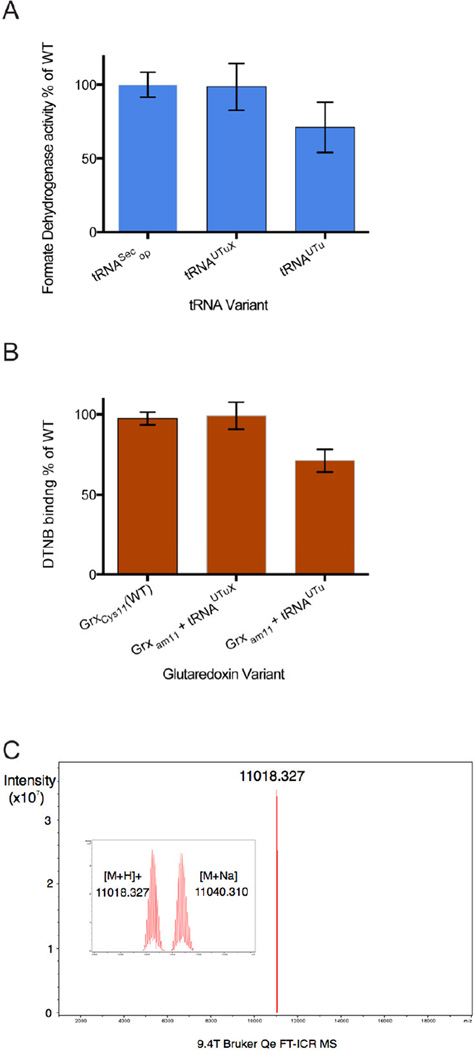
To determine the yield of Sec insertion by tRNAUTuX we next measured the specific activity of purified FDHH. Using FDHH140op produced by WT tRNASec as a standard, the specific activity of FDHH140am synthesized in the presence of either tRNAUTuXam or tRNAUTuam was measured for comparison. FDHH made with tRNAUTuam had ~70% of the specific activity of the enzyme made with WT tRNASec, and tRNAUTuXam produced an enzyme with activity equivalent to the WT (Fig. 3A).
Fig. 3.
Assessment of tRNAUTuX-mediated Sec insertion fidelity. (A) Sec-dependent in vitro BV reduction of recombinant purified FDHH variants. 100nM each of WT FDHH140op and FDHH140am produced with tRNAUTu and tRNAUTuX were assayed in the linear range of the reaction for 5 min. Relative specific activity of FDHH variants expressing using tRNASecop, tRNAUTu, and tRNAUTuX were determined. Compared to WT tRNASecop, synthetic variants tRNAUTuX and tRNAUTu mediated Sec insertion into FDHH140am allowed 98.5 ± 15.8% and 71.1 ± 17.0% BV reduction activity, respectively. (B) Sec incorporation into recombinant E. coli Grx1 variants as determined spectroscopically by assaying DTNB (Ellman’s reagent) binding. Relative to WT tRNASecop, expression of Grx1C11am with tRNAUTuX and tRNAUTu resulted in 99.2 ± 8.4% and 71.2 ± 7.1% DTNB coupling, respectively. (C) Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometry of purified Grx1C11am. Mass peaks of 11,018.33 and 11,040,31 m/z were observed, corresponding to the masses of a Grx1C11U glutathione adduct (11,019.38), and a glutathione plus Na+ adduct (11,041.34). The unit m/z describes the mass-to-charge ratio.
A similar analysis was then performed using the small E. coli redox protein glutaredoxin, Grx1, which contains two Cys residues (positions 11 and 14). These residues were mutated to amber11/Ser14 and Cys11/Ser14 to generate Grx1 variants containing a single Cys or Sec residue at position 11 [8]. Grx1amber11/Ser14 was then expressed in the presence of either tRNAUTuXam or tRNAUTuam and purified while Grx1Cys11/Ser14 served as a control. DTNB [5,5'-dithiobis-(2-nitrobenzoic acid)] reacts with Cys and Sec residues, while it has no affinity for Ser [19]. This results in a visible color change, which can be quantified spectroscopically. This reaction was used to determine the Sec insertion ratio in correlation to Grx1Cys11/Ser14. In agreement with the results obtained FDHH (Fig. 3A), DTNB treatment of the Grx1amber11/Ser14 gene product synthesized with tRNAUTuXam gave the same colorimetric signal intensity as Grx1Cys11/Ser14; this indicated stoichiometric Sec insertion. In contrast, the Grx1amber11/Ser14 gene product made by the original tRNAUTuam [8] had a weaker signal, exhibiting only 70% of the WT intensity. This is in line with the earlier finding of 30% misincorporation of Ser [8] (Fig. 3B). These results were confirmed by intact mass FT-ICR mass spectrometry (Fig. 3C). Peaks at masses of 11,018.33 and 11,040.31 Da were observed, which correspond to the calculated masses for a Grx1-Sec11-GSH (11,019.43) and a Grx1-Sec11-GSH/Na+ adduct (11,040.39), respectively. No mass peaks that coincide with a Grx1-Ser11 species were detected.
In vitro protein synthesis has previously been very successful in synthesizing proteins containing nonstandard amino acids [20,21]. Sec insertion has also been observed in eukaryotic cell free systems [22,23], detected as read-though products of a luciferase reporter protein. We sought to test the capacity of tRNASec and the synthetic tRNAUTu to synthesize a natural selenoenzyme by in vitro translation. For this we used the PURExpress in vitro translation system (NEB). To achieve Sec insertion, Sec-tRNASecam was prepared biochemically and added to the reaction. However, as Sec-tRNASec must compete with canonical tRNA for EF-Tu binding, we anticipated a large quantity would be required to mediate translation. Additionally, while translation of E. coli FDHH enabled sensitive colorimetric detection of Sec insertion, the large size of the protein and presence of both molybdenum and iron-sulfur cofactors meant an extended elongation time would likely be required.
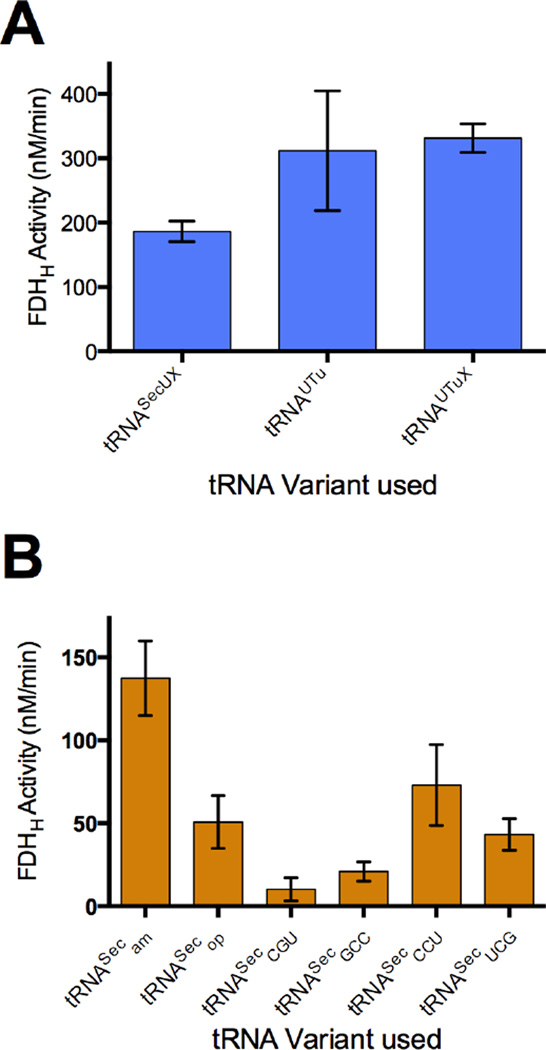
Translation reactions were run under anoxic conditions, using a plasmid containing the fdhF140am gene under a T7 promoter to facilitate transcription to FDHH140am mRNA. To eliminate competition for the UAG140 codon, the translation system lacked release factor 1 (PURExpressΔRF123 kit, NEB) while including RF2 and RF3, and was supplemented with sodium molybdate, RNase inhibitor, and elongation factor SelB. Using Sec-tRNASec, active FDHH was successfully produced after the standard 2 hr reaction time, yet these conditions produced active selenoenzyme only with wild-type Sec-tRNASec. However, with the addition of an excess of EF-Tu, elevated levels of Sec-tRNA, and a long (5 hr) incubation time, all three synthetic tRNA variants (tRNAUTuam, tRNAUTuXam, and the recently reported tRNASecUXam [24]) were found to give active FDHH protein (Fig. 4A). Using the specific activity calculated for WT FDHH, the in vitro yield of active protein under the respective optimal conditions for each tRNA was roughly 34.7 ng using tRNASecam, 47.1 ng using tRNASecUX, 78.7 ng using tRNAUTu, and 83.6 ng using tRNAUTuX. Thus, each synthetic tRNA produced FDHH activity similar to both one another and WT tRNASec.
Fig. 4.
Activity of FDHH produced through cell-free protein synthesis. Cell-free translation of selenoprotein FDHH was mediated by different Sec-tRNA variants and cognate fdhF140 mutants under optimized conditions (see Materials and Methods), and FDHH140am activity was monitored through reduction of BV at 578 nm. (A) Activity of tRNAUtuam, tRNAUTuXam, and tRNASecUXam-mediated FDHH140am translation in the absence of release factor 1 (while in the presence of RF2 and RF3) was found to be comparable for each variant. (B) Translation of FDHH mutants with cognate tRNA variants tRNASecam, tRNASecop, tRNASecCGU, tRNASecGCC, tRNASecCCU, and tRNASecUCG in the presence of SelB demonstrates that each sense codon is capable of recoding with selenocysteine in vitro. Absence of activity was confirmed in a negative control reaction prepared with Ser-tRNASecam in place of Sec-tRNASecam, and in a separate negative control prepared with plasmid encoding dihydrofolate reductase (DHFR) in place of fdhFam.
Although in nature selenocysteine is encoded by UGA, we have previously shown that many E. coli codons can be reassigned to Sec if the WT UGA codon preceding the SECIS element is replaced by a sense codon [10]. Using our cell-free selenoprotein synthesis system, we investigated the capacity to in vitro recode sense codons in FDHH to Sec. Mutant genes encoding fdhF140GGC, fdhF140CGA, fdhF140AGG, fdhF140ACG, and WT fdhF140op were paired with their respective cognate tRNASec mutants, and expressed in vitro using in the presence of SelB. While several of these codons did not produce active FDHH in vivo, all variants tested were found to yield active enzyme in vitro (Fig. 4B) with estimated yields of 5.3 ng using tRNASecGCC (fdhF140GGC), 10.9 ng using tRNASecUCG (fdhF140CGA), 18.4 ng using tRNASecCCU (fdhF140AGG), 2.6 ng using tRNASecCGU (fdhF140ACG) and 12.8 ng using WT tRNASecop (fdhF140op).
In light of the in vitro recoding capacity of codons AGG and CGA, we reinvestigated the activity of these fdhF variants in vivo. Translation of fdhF140AGG and fdhF140CGA mRNA, isolation of FDHH, and determination of specific activity showed recoding levels of AGG and CGA to be 65% and 46%, respectively. These results are incorporated in the data shown in Fig. S3.
4. Discussion
The major improvement of tRNAUTuX over tRNAUTu is seen in its ability to be an almost WT tRNASec-like substrate for SelA ensuring optimal Ser to Sec conversion (Fig. S2). At the same time, tRNAUTuX is a better SerRS substrate than tRNASec (Table S1). These properties make tRNAUTuX our best tRNAUTu molecule for selenoprotein production.
Based on similar criteria for elongation and release factor recognition/rejection a different tRNA molecule for canonical Sec insertion was recently selected [24]. As its design began with the WT tRNASec scaffold, tRNASecUX and tRNAUTuX are of different sequence in the D-arm, anticodon stem, variable arm, and T-arm; however, the two tRNAs contain the same critical structural parameters for EF-Tu binding [17]. This demonstrates that similar recognition and biological properties can be achieved by different nucleic acid landscapes.
While cell free synthesis of selenoproteins has previously been reported in partially purified components of the eukaryotic pathway (including purified Sec-tRNASec) [22], we established an in vitro translation system for selenoproteins using commercially available bacterial components. Since protein synthesis quality controls for EF-Tu and SelB are relaxed in this PURExpress translation system, it was foreseen that protein yields would be low. Yet the availability of a sensitive color assay (benzyl-viologen reduction [18]) encouraged us to synthesize FDHH, a selenoprotein whose enzyme activity depends on the presence of Sec, an iron sulfur cluster, and molybdenum as cofactor. Our strategy was successful; now the task at hand is to optimize protein production. Addition or co-expression of SelA and SelD (and selenite) may increase Sec-tRNAUTuX yield leading to additional rounds of protein synthesis. In view of the extensive efforts to optimize bacterial cell-free expression systems [25] and the success of generating sufficient Sec-tRNA in vitro, we anticipate this approach to lead to the in vitro generation of additional selenoproteins in the future.
Supplementary Material
Highlights.
A chimaera of tRNASer and tRNASec, tRNAUTuX, binds EF-Tu to insert Sec at UAG codons
tRNAUTuX was used for complete, high fidelity Sec insertion
We show in vitro selenoprotein synthesis, compatible with wild-type and synthetic tRNA
Sense codons were recoded in vitro in the presence of SelB
Formate dehydrogenase activity demonstrated in vitro selenoenzyme synthesis
Acknowledgments
We thank Chenguang Fan, Noah Reynolds and Joanne Ho for critical discussion and helpful insights. This work was supported by grants from the National Institute for General Medical Sciences (GM22854), the Defense Advanced Research Projects Agency (contracts N66001-12-C-4020 and N66001-12-C-4211), and from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the Department of Energy (DE-FG02-98ER20311; for funding the genetic experiments). MJB was supported by a Feodor Lynen Postdoctoral Fellowship from the Alexander von Humboldt Foundation (Bonn, Germany).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary Data
Supplementary data associated with this article can be found in the online version.
References
- 1.Arner ES. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Beld J, Woycechowsky KJ, Hilvert D. Selenoglutathione: efficient oxidative protein folding by a diselenide. Biochemistry. 2007;46:5382–5390. doi: 10.1021/bi700124p. [DOI] [PubMed] [Google Scholar]
- 3.Böck A, Thanbichler M, Rother M, Resch A. Selenocysteine. In: Ibba M, Francklyn CS, Cusack S, editors. Aminoacyl-tRNA Synthetases. Georgetown, TX: Landes Bioscience; 2005. pp. 320–327. [Google Scholar]
- 4.Yoshizawa S, Böck A. The many levels of control on bacterial selenoprotein synthesis. Biochim. Biophys. Acta. 2009;1790:1404–1414. doi: 10.1016/j.bbagen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Aldag C, Gromov IA, Garcia-Rubio I, von Koenig K, Schlichting I, Jaun B, Hilvert D. Probing the role of the proximal heme ligand in cytochrome P450cam by recombinant incorporation of selenocysteine. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5481–5486. doi: 10.1073/pnas.0810503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arner ES. Recombinant expression of mammalian selenocysteine-containing thioredoxin reductase and other selenoproteins in Escherichia coli. Methods Enzymol. 2002;347:226–235. doi: 10.1016/s0076-6879(02)47022-x. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Croitoru V, Rutishauser D, Cheng Q, Arner ES. Wobble decoding by the Escherichia coli selenocysteine insertion machinery. Nucleic Acids Res. 2013;41:9800–9811. doi: 10.1093/nar/gkt764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldag C, Bröcker MJ, Hohn MJ, Prat L, Hammond G, Plummer A, Söll D. Rewiring translation for elongation factor Tu-dependent selenocysteine incorporation. Angew. Chem. Int. Ed. Engl. 2013;52:1441–1445. doi: 10.1002/anie.201207567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paleskava A, Konevega AL, Rodnina MV. Thermodynamic and kinetic framework of selenocysteyl-tRNASec recognition by elongation factor SelB. J. Biol. Chem. 2010;285:3014–3020. doi: 10.1074/jbc.M109.081380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bröcker MJ, Ho JM, Church GM, Söll D, O'Donoghue P. Recoding the genetic code with selenocysteine. Angew. Chem. Int. Ed. Engl. 2014;53:319–323. doi: 10.1002/anie.201308584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherrer RL, Ho JM, Söll D. Divergence of selenocysteine tRNA recognition by archaeal and eukaryotic O-phosphoseryl-tRNASec kinase. Nucleic Acids Res. 2008;36:1871–1880. doi: 10.1093/nar/gkn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard K, Akochy PM, Söll D. Assays for transfer RNA-dependent amino acid biosynthesis. Methods. 2008;44:139–145. doi: 10.1016/j.ymeth.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Palioura S, Salazar JC, Su D, O'Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araiso Y, Palioura S, Ishitani R, Sherrer RL, O'Donoghue P, Yuan J, Oshikane H, Domae N, Defranco J, Söll D, Nureki O. Structural insights into RNA-dependent eukaryal and archaeal selenocysteine formation. Nucleic Acids Res. 2008;36:1187–1199. doi: 10.1093/nar/gkm1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh Y, Bröcker MJ, Sekine S, Hammond G, Suetsugu S, Söll D, Yokoyama S. Decameric SelA:tRNASec ring structure reveals mechanism of bacterial selenocysteine formation. Science. 2013;340:75–78. doi: 10.1126/science.1229521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrader JM, Chapman SJ, Uhlenbeck OC. Understanding the sequence specificity of tRNA binding to elongation factor Tu using tRNA mutagenesis. J. Mol. Biol. 2009;386:1255–1264. doi: 10.1016/j.jmb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudinger J, Hillenbrandt R, Sprinzl M, Giege R. Antideterminants present in minihelixSec hinder its recognition by prokaryotic elongation factor Tu. EMBO J. 1996;15:650–657. [PMC free article] [PubMed] [Google Scholar]
- 18.Lacourciere GM, Levine RL, Stadtman TC. Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9150–9153. doi: 10.1073/pnas.142291199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Kang TJ, Suga H. Ribosomal synthesis of nonstandard peptides. Biochem. Cell. Biol. 2008;86:92–99. doi: 10.1139/O08-009. [DOI] [PubMed] [Google Scholar]
- 21.Seebeck FP, Ricardo A, Szostak JW. Artificial lantipeptides from in vitro translations. Chem. Commun. (Camb.) 2011;47:6141–6143. doi: 10.1039/c0cc05663d. [DOI] [PubMed] [Google Scholar]
- 22.Gupta N, DeMong LW, Banda S, Copeland PR. Reconstitution of selenocysteine incorporation reveals intrinsic regulation by SECIS elements. J. Mol. Biol. 2013;425:2415–2422. doi: 10.1016/j.jmb.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta A, Rebsch CM, Kinzy SA, Fletcher JE, Copeland PR. Efficiency of mammalian selenocysteine incorporation. J. Biol. Chem. 2004;279:37852–37859. doi: 10.1074/jbc.M404639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thyer R, Robotham SA, Brodbelt JS, Ellington AD. Evolving tRNASec for efficient canonical incorporation of selenocysteine. J. Am. Chem. Soc. 2015;137:46–49. doi: 10.1021/ja510695g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jewett MC, Calhoun KA, Voloshin A, Wuu JJ, Swartz JR. An integrated cell-free metabolic platform for protein production and synthetic biology. Mol. Syst. Biol. 2008;4:220. doi: 10.1038/msb.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.