Abstract
One of the most consistent neuropsychological findings in autism spectrum disorders (ASD) is a reduced interest in and impaired processing of human faces. We conducted an activation likelihood estimation meta-analysis on 14 functional imaging studies on neural correlates of face processing enrolling a total of 164 ASD patients. Subsequently, normative whole-brain functional connectivity maps for the identified regions of significant convergence were computed for the task-independent (resting-state) and task-dependent (co-activations) state in healthy subjects. Quantitative functional decoding was performed by reference to the BrainMap database. Finally, we examined the overlap of the delineated network with the results of a previous meta-analysis on structural abnormalities in ASD as well as with brain regions involved in human action observation/imitation. We found a single cluster in the left fusiform gyrus showing significantly reduced activation during face processing in ASD across all studies. Both task-dependent and task-independent analyses indicated significant functional connectivity of this region with the temporo-occipital and lateral occipital cortex, the inferior frontal and parietal cortices, the thalamus and the amygdala. Quantitative reverse inference then indicated an association of these regions mainly with face processing, affective processing, and language-related tasks. Moreover, we found that the cortex in the region of right area V5 displaying structural changes in ASD patients showed consistent connectivity with the region showing aberrant responses in the context of face processing. Finally, this network was also implicated in the human action observation/imitation network. In summary, our findings thus suggest a functionally and structurally disturbed network of occipital regions related primarily to face (but potentially also language) processing, which interact with inferior frontal as well as limbic regions and may be the core of aberrant face processing and reduced interest in faces in ASD.
Keywords: Autism, Autism spectrum disorders, Face processing, Fusiform face area, V5, Meta-analysis
Introduction
The term “autism spectrum disorders” (ASD) summarizes a group of pervasive developmental disorders characterized by a triad of core symptoms: (1) severe and sustained impairment in social interaction, (2) reduced and impaired communication, and (3) restricted and/or stereotyped patterns of behavior and interest. Reflecting these core symptoms, a reduced interest in faces and impaired face processing are among the most consistent behavioral findings in ASD. Face processing deficits ranging from impairments in face recognition, perception of emotional expressions, and production of facial expression for social communication to reduced direct gaze and failure to benefit from gaze cues have been shown in numerous studies (e.g., Campbell et al. 2006; Dawson et al. 2005). Inattention to faces is one of the earliest developmental signs of autism and may be detected within the first year of life (Osterling and Dawson 1994; Osterling et al. 2002; cf. Joseph and Tager-Flusberg 1997). Aberrant face processing may hence be a cause rather than a consequence of social impairments in ASD (Dalton et al. 2005). Another aspect of “social perception” that has repeatedly been hypothesized to be impaired in ASD (Williams et al. 2001; Iacoboni and Dapretto 2006) is the so-called mirror neuron system (MNS, Gallese et al. 1996; Rizzolatti and Craighero 2004). Mirror neurons were first described as cells in the premotor and parietal cortex of macaques that fire when the animal performs a goal-directed action and when it sees others performing the same action. This led to the idea that the MNS is linked to imitational learning but also social cognition in general (Iacoboni and Dapretto 2006). By now, numerous neuroimaging studies have provided information on brain regions in humans showing activity during action observation and imitation (Iacoboni 2005; Vogt et al. 2007). These were summarized by a recent meta-analysis, implicating not only “classical” fronto-parietal circuits but also occipito-temporal regions including the bilateral cortex in the region of visual motion sensitive area V5 in the human functional mirror neuron network (Caspers et al. 2010).
It is tempting to speculate about a potential link between the two aforementioned aspects of socially relevant perception that have both been reported to be aberrant in ASD, i.e., face processing and the MNS. Any such comparison, however, would be dependent on a more precise investigation of which, if any, modules of the human face-processing network (Sabatinelli et al. 2011) are disturbed in patients with ASD. While several studies have already reported aberrant neuronal correlates of face processing in these patients using functional magnetic resonance imaging (fMRI) studies, inference on general pathomechanisms from these findings is difficult given the inherent drawbacks of clinical neuroimaging studies: (1) The sample size of most neuroimaging studies is rather small, potentially as a reflection of the fact that ASD is a comparatively rare disorder. (2) The current neuroimaging literature on face processing in ASD shows an inconsistent terminology relating to diagnoses and a considerable variation in diagnostic tools used. (3) Given the notion of potentially disturbed brain trajectories in ASD, the heterogeneous age-ranges investigated in the different studies may further hinder generalization of the respective findings. (4) There is also a marked heterogeneity in the tasks used to assess face processing in ASD patients, which prompts the question as to whether the reported changes reflect common disturbances in neural mechanisms or paradigm-specific effects. In order to consolidate this diverse literature, we here report on a quantitative coordinate-based meta-analysis of fMRI findings on aberrant face processing in ASD using the activation likelihood estimation (ALE) approach (Turkeltaub et al. 2002, 2012; Eickhoff et al. 2009, 2012). The purpose of this approach is to identify brain regions that consistently show aberrant activity during face processing in ASD patients relative to healthy controls in spite of the aforementioned variability between studies. In a subsequent step, we then delineated the neuronal network interacting with the ensuing regions in healthy subjects using both task-dependent and task-independent connectivity analysis and performed quantitative functional decoding. This approach allows inference on brain systems that physiologically interact with the identified regions and may, therefore, be potentially perturbed in ASD. Finally, we compared the delineated network to the human action observation/imitation network (Caspers et al. 2010) and meta-analytic findings on structural aberrations in ASD (Nickl-Jockschat et al. 2012). In summary, this study thus identifies brain regions showing consistently aberrant activation during face processing in ASD as well as networks physiologically connected to these and compares them to regions implicated in the MNS and structural ASD pathology.
Methods
Literature search and inclusion criteria
The PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) database was searched by using the following search strings: “autism + face + processing”, “autism + face + processing + fmri”, “asd + face + processing”, “asd + face + processing + fmri”, “autism + face + fmri”, “asd + face + fmri” to identify fMRI studies investigating face processing in ASD. Additional papers were then identified through reference tracing from the retrieved articles as well as from qualitative reviews of the literature. Inclusion criteria for this meta-analysis were (1) fMRI studies published between 2001 and 2012, (2) original peer-reviewed studies, (3) whole brain analysis that did not restrict analysis or inference to a priori specified regions of interest, (4) comparison of ASD patients as confirmed by a standard clinical protocol to a matched healthy control group, and (5) reporting of results as coordinates in stereotactic space (Talairach/MNI). In total, 14 studies published between 2001 and 2011 fulfilled all of these criteria and were included in our meta-analysis (see Table 1). In total, these studies enrolled 164 (158 male) ASD patients and 165 (158 male) healthy controls. The included papers reported a total of 14 experiments and a total of 151 activations (110 indicating decreased, 41 indicating increased activation in ASD patients). Importantly, for the subsequent analysis, we pooled across hypo- and hyper-activations as well as, most commonly, different kinds of group × task interactions. The rationale behind this approach is that we were interested in regions consistently show abnormal activity in ASD patients, while the specific pattern of the task × group interaction may be very heterogeneous depending on the exact nature of the employed task and stimuli.
Table 1.
Overview over the studies included in this metaanalysis
| Study | n (Patients) | n (Controls) | Mean age (patients) | Mean age (controls) | Diagnosis by |
|---|---|---|---|---|---|
| Ashwin et al. (2007) | 13 | 13 | 31.2 | 25.6 | DSM-IV/ADOS |
| Bird et al. (2006) | 16 | 16 | 33.3 | 35.3 | DSM-IV/ADOS |
| Bookheimer et al. (2008) | 12 | 12 | 11.3 | 11.9 | ADOS/ADI-R |
| Critchley et al. (2000) | 9 | 9 | 37 | 27 | ADI-R |
| Dalton et al. (2005) | 11 | 12 | 15.9 | 17.1 | ADI-R |
| Dapretto et al. (2006) | 10 | 10 | 12.05 | 12.38 | ADOS/ADI-R |
| Deeley et al. (2007) | 9 | 9 | 34 | 27 | ICD-10/DSM-IV/ADOS/ADI-R |
| Dichter and Belger (2007) | 17 | 15 | 22.9 | 24.6 | ADOS/ADI-R |
| Hubl et al. (2003) | 10 | 10 | 27.7 | 25.3 | ADOS/ADI-R |
| Koshino et al. (2008) | 11 | 11 | 24.5 | 28.7 | ADOS/ADI-R |
| Pelphrey et al. (2007) | 8 | 8 | 24.5 | 24.1 | ADOS/ADI-R |
| Pierce et al. (2004) | 8 | 10 | 27.1 | ADOS/ADI-R | |
| Schulte-Rüther et al. (2011) | 18 | 18 | 27.4 | 25.5 | ICD-10/DSM-IV/ADOS |
| Wang et al. (2004) | 12 | 12 | 12.2 | 11.8 | ADOS/ADI-R |
Activation likelihood estimation meta-analysis
The meta-analysis was carried out using a revised version (Eickhoff et al. 2009, 2012) of the ALE approach for coordinate-based meta-analysis of neuroimaging results (Laird et al. 2005; Turkeltaub et al. 2002, 2012). This algorithm aims at identifying areas showing a convergence of findings across studies, which is higher than expected under a spatially random spatial association. The key idea behind ALE is to treat the reported foci as centers of 3D Gaussian probability distributions reflecting the spatial uncertainty associated with each reported set of coordinates (Turkeltaub et al. 2002; Eickhoff et al. 2009). All activation foci for a given experiment were combined for each voxel to produce a modeled activation map (MA map; Turkeltaub et al. 2012). ALE scores describing the convergence of coordinates for each location were then calculated via the union of individual MA maps. To distinguish areas where the convergence between studies was greater than it would be expected by chance (i.e., to separate true convergence from noise), ALE scores were compared to a nonlinear histogram integration based on the frequency of distinct MA values (see Eickhoff et al. 2012). For statistical inference, the ensuing statistical parametric maps were then thresholded at p < 0.05 [cluster-level FWE, corrected for multiple comparisons, cluster-forming threshold at voxel level p < 0.001 (Eickhoff et al. 2012)].
Task-dependent functional connectivity: meta-analytic connectivity modeling (MACM)
To characterize the co-activation profile of the regions found to show consistently (across experiments) aberrant activation during face processing tasks in subjects with ASD, we used MACM. This approach to functional connectivity assesses which brain regions are co-activated above chance with a particular seed region across a large number of functional neuroimaging experiments. MACM thus takes advantage of the fact that functional imaging studies are normally presented in a highly standardized format using ubiquitously employed standard coordinate systems, and the emergence of large-scale databases such as BrainMap or Neurosynth, which store this information. The first step in a MACM analysis is to identify all experiments in a database that activate the seed region, i.e., that reported at least one focus within the seed volume. Subsequently, quantitative meta-analysis is employed to test for convergence across the foci reported in these experiments. As experiments are selected by activation in the seed, the highest convergence will be observed in the seed region. Significant convergence of reported foci in other brain regions, however, indicates consistent co-activation, i.e., functional connectivity with the seed (Eickhoff et al. 2010; Rottschy et al. 2013b).
Thus, we first identified all experiments in the BrainMap database (Laird et al. 2009, 2011; http://www.brainmap.org), which featured at least one focus of activation in the seed region derived from the functional meta-analysis in ASD subjects. Only studies reporting group analyses of functional mapping experiments of healthy subjects were included, while studies dealing with disease or drug effects were excluded. This resulted in inclusion of 160 experiments with a total of 2,454 subjects and 2,335 foci. Subsequently, coordinate-based meta-analysis was performed to identify consistent co-activations across experiments by using the revised ALE algorithm (Eickhoff et al. 2009, 2012) as described above. The statistical threshold was again p < 0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p < 0.001).
Task-independent functional connectivity: resting-state
To further delineate the neuronal network interacting with the previously found region in the fusiform gyrus, we additionally analyzed its resting-state connectivity patterns in a sample of healthy subjects. More precisely, our aim was to cross-validate task-independent functional connectivity using an independent dataset, following previous reports that this combination allows the robust definition of consensus (across task and rest) functional connectivity patterns (Amft et al. 2014; Reetz et al. 2012; Jakobs et al. 2012; Rottschy et al. 2013a; Müller et al., 2013; Hoffstaedter et al. 2013; Bzdok et al. 2013; Cieslik et al. 2013). In turn, the knowledge on the regions robustly interacting with the observed region on the fusiform gyrus should provide a better characterization of the observed region of consistent hypo-activation in ASD.
Resting state images were obtained from the Nathan Kline Institute “Rockland” sample, which are available online as part of the International Neuroimaging Data-sharing Initiative (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). In total, the processed sample consisted of 132 healthy subjects between 18 and 85 years (mean age 42.3 ± 18.08 years; 78 males, 54 females) with 260 echo-planar imaging (EPI) images per subject. Images were acquired on a Siemens TrioTim 3T scanner using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, repetition time = 2.5 s, echo time = 30 ms, flip angle = 80°, in-plane resolution = 3.0 × 3.0 mm, 38 axial slices (3.0 mm thickness), covering the entire brain]. Data were processed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Prior to further analyses, the first four scans were discarded, which allowed for magnetic field saturation. The EPI images were then corrected for head movement by affine registration using a two-pass procedure in which in a first step, images were aligned to the initial volumes and then subsequently to the mean of all volumes. Next, for every subject, the mean EPI image was spatially normalized to the Montreal Neurological Institute (MNI) single-subject template (Holmes et al. 1998) using the “unified segmentation” approach (Ashburner and Friston 2005). Ensuing deformation was then applied to the individual EPI volumes and images smoothed by a 5-mm full-width at half-maximum Gaussian kernel to improve signal-to-noise ratio and to compensate for residual anatomical variations. For the analyses of the time-series of each voxel, spurious correlations were reduced by excluding variance, which can be explained by the following nuisance variables: (1) the six motion parameters derived from image realignment; (2) their first derivatives; (3) mean GM, WM, and CBF intensity. All nuisance variables entered the model as first and also as second-order terms (cf. Satterthwaite et al. 2013 for an evaluation of this framework). Finally, data were band-pass filtered with the cut-off frequencies of 0.01 and 0.08 Hz. Just as for the MACM analysis, the seed region was provided by significant results of the meta-analysis on aberrant face processing in patients with ASD.
The Global Signal regression method employed (as one aspect of the processing pipeline) by Satterthwaite et al. (2013), which we used here in this study, has been criticized for its potential influence on between-group comparisons and the ensuing difficulties in interpreting these (Saad et al. 2012; Gotts et al. 2013). Since this criticism focuses on the use of Global Signal regression related to group comparisons, we regard our approach as unchallenged by these results, given that we analyzed here within-group main effects of resting-state functional connectivity.
Time-courses of all voxel within that seed were then extracted and expressed as the first eigenvariate (cf. Zu Eulenburg et al. 2012; Reetz et al. 2012). To quantify resting-state functional connectivity linear (Pearson) correlation coefficients were computed between the ensuing characteristic time series of the seed region and the time-series of all other gray matter voxels of the brain. These voxel-wise correlation coefficients were then transformed into Fisher's Z-scores and then fed into a second-level analysis of variance including an appropriate non-sphericity correction as implemented in SPM8. As for the MACM analysis, the statistical threshold was p < 0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p < 0.001).
Conjunction analyses
Our aim was to identify regions that show functional connectivity with the seed across different mental states, i.e., an endogenously controlled resting- and an exogenously driven task-state (cf. Jakobs et al. 2012). Therefore, a conjunction analysis between MACM and resting-state functional connectivity results was performed using the minimum statistics (Nichols et al. 2005). That is, by computing the intersection of the thresholded connectivity maps derived from two different concepts of functional connectivity, we aimed to delineate consistent functional connectivity with the meta-analytically defined seed. We furthermore carried out conjunction analyses for the network obtained by this analysis and the action observation/imitation network (Caspers et al. 2010), and structural changes in ASD patients (Nickl-Jockschat et al. 2012) using the same approach. As reported in the corresponding publication, we used the Anatomy Toolbox (Eickhoff et al. 2005, 2006, 2007) to anatomically allocate the regions with convergent evidence for structural changes (for a more detailed description of the methods used cf. Nickl-Jockschat et al. 2012). For our conjunction analysis, we stick to these labels.
Functional decoding
Based on the conjunction of MACM and resting-state, ensuing regions, showing consensus functional connectivity with the seed, were further investigated. That is, all regions that showed functional connectivity across the task-dependent and task-independent analysis were further assessed in terms of their functional properties (Rottschy et al. 2013b; Cieslik et al. 2013). More precisely, functional characterization of the derived network was performed using the behavioral domain and paradigm class meta-data categories from the BrainMap database (Laird et al. 2009, 2011; http://www.brainmap.org), describing the classes of mental processes isolated by the archived experiments’ statistical contrasts. In this context, behavioral domains denote the mental processes isolated by the respective contrast, whereas paradigm classes categorize the specific task employed (see http://brainmap.org/scribe/ for the complete BrainMap taxonomy). In the current study, we were mainly interested in the functional role of the seed (obtained from the meta-analysis on aberrant face processing in ASD) in co-activation with the regions showing functional connectivity with it in both the MACM and resting-state analysis. Therefore, functional decoding was performed for the pairwise combination of the seed with those regions obtained through the conjunction of its resting-state and task-based connectivity as follows: first, we identified all experiments in the BrainMap database, which featured at least one focus of activation within the seed from the meta-analysis on aberrant face-processing in ASD cortex and each region (individually) that was consistently connected to it. Forward inference and reverse inference were calculated for each set of the retrieved experiments (featuring co-activation of the seed and one of the consistently connected regions) to characterize the functional profiles of the respective (sub-) network. In this context, forward inference is based on the probability of observing activity in a brain region (or network) given knowledge of a psychological process, whereas reverse inference tests the probability of a psychological process being present given knowledge of activation in a particular brain region (or network).
In particular, in the forward inference approach, we determined a network's functional profile by identifying taxonomic labels for which the probability of finding activation in the respective network is significantly higher than the overall chance (across the entire database) of finding activation in that particular network. Significance was established using a binomial test (p < 0.001). That is, we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was higher than the baseline probability of activating the network in question per se [P(Activation)]. This base rate thus denotes the probability of finding a (random) activation from BrainMap in the seed and its connected region(s). In the reverse inference approach, a network's functional profile was determined by identifying the most likely behavioral domains and paradigm classes given activation in a particular network. This likelihood P(Task|Activation) can be derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes’ rule. Significance was then assessed by means of a Chi-square test (p < 0.001). In sum, forward inference assesses the probability of activation given a term (i.e., domain or paradigm) in studies from BrainMap, while reverse inference assesses the probability of a term given activation.
Results
Convergent evidence for altered neural activity during face processing in ASD patients
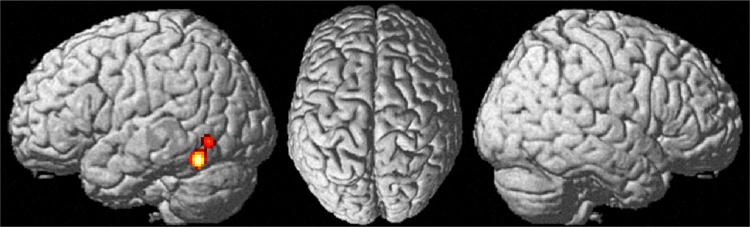
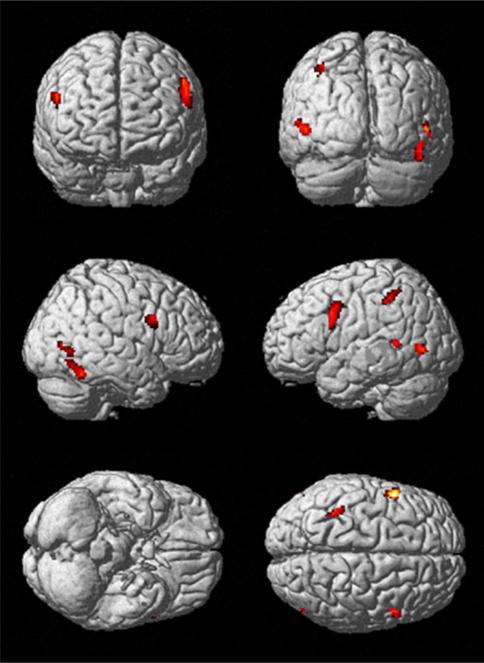
Across studies, we found one cluster indicating convergent evidence for aberrant BOLD responses in ASD patients. The cluster was located in the left lateral temporal lobe, in particular the fusiform gyrus (−43, −61, −10, k = 172) (Fig. 1). This cluster was used as seed region for our further analyses on task-independent and task-dependent functional connectivity. Interestingly, we also performed an additional analysis using only foci reported as de-activated in ASD by the authors of the original papers and found the very same cluster of convergence. In turn, we did not find any clusters indicating convergent evidence for increased BOLD responses in ASD patients. In summary, our quantitative meta-analyses which were performed initially without an a priori constraint on the nature (hypo-/hyper-activation) of aberrant responses thus indicated that patients with ASD show a spatially specific and across studies consistent decrease of activity in the left fusiform gyrus during face processing tasks.
Fig. 1.
A single cluster indicating convergent evidence for hypoactivation in ASD patients compared to healthy controls during face processing was located in the left lateral temporal lobe, in particular the fusiform gyrus (−43, −61, −10, k = 172) [p < 0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p < 0.001 at voxel level)]. There were no clusters indicating increased activation in ASD patients compared to healthy controls
Task-dependent functional connectivity
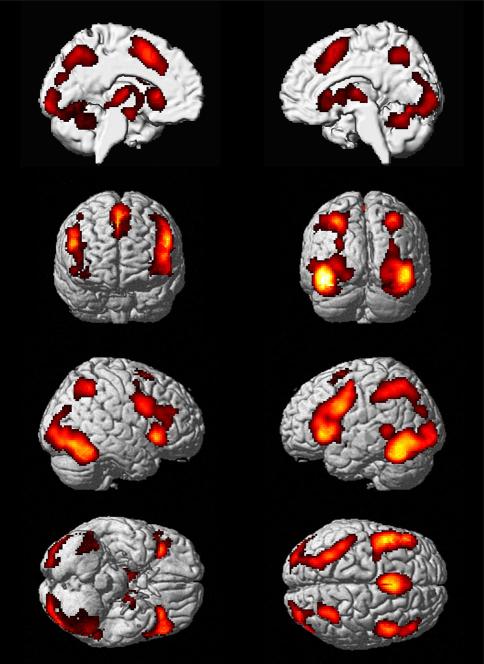
Task-dependent functional connectivity determined by MACM revealed significant co-activations of the seed identified in the meta-analysis, i.e., the region in the left fusiform gyrus showing consistent hypo-activation in patients with ASD during face processing in the inferior frontal gyrus (IFG) bilaterally, the temporooccipital cortex (TOC) bilaterally (left cluster extending to the left middle temporal gyrus), the supplementary motor area bilaterally, the superior parietal lobes (SPL) bilaterally, the thalamus bilaterally, and the left medial temporal lobe (MTL) (Fig. 2).
Fig. 2.
Task-dependent functional connectivity determined by MACM revealed significant co-activations of seed identified in the meta-analysis, i.e., the left fusiform gyrus regions showing consistent hypo-activation in patients with ASD during face processing [p < 0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p < 0.001 at voxel level)]
Task-independent functional connectivity
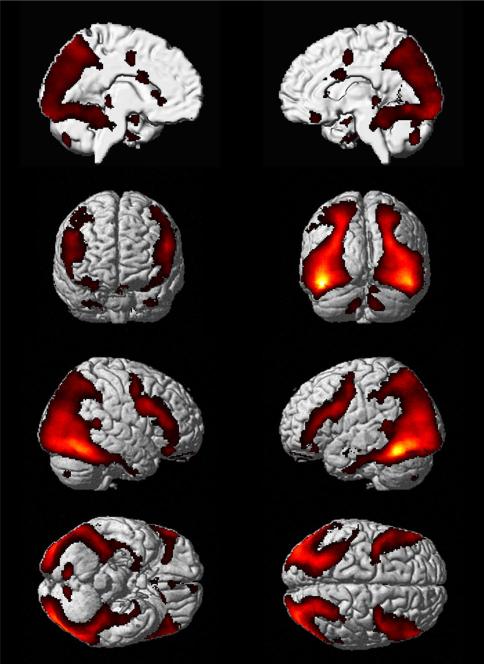
Resting-state functional connectivity analysis revealed a distributed network of areas showing correlated BOLD signal changes with the seed identified in the meta-analysis, i.e., the region in the left fusiform gyrus showing consistent hypo-activation in patients with ASD during face processing. The network consisted mainly of the ventral and lateral TOC bilaterally (left cluster extending to the left middle temporal gyrus), the IFG bilaterally, the SPL/inferior parietal sulcus (IPS) bilaterally, the MTL bilaterally, the thalamus bilaterally, and the cerebellum (Fig. 3).
Fig. 3.
Resting-state functional connectivity analysis revealed a distributed network of areas showing correlated BOLD signal changes with the seed identified in the meta-analysis, i.e., the left fusiform gyrus regions showing consistent hypo-activation in patients with ASD during face processing [p < 0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p < 0.001 at voxel level)]
Conjunction of task-dependent and task-independent functional connectivity
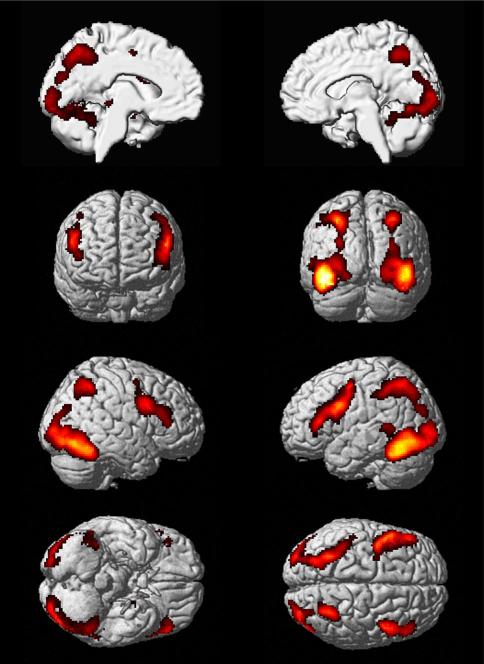
Based on the two analyses described above, we then delineated regions that showed consensus functional connectivity, i.e., significant resting-state correlations as well as significant task-based co-activations with the seed region in the left fusiform gyrus that was hypo-activated during face processing in ASD. Regions consistently observed to be coupled with that seed in both approaches were the bilateral TOC, the IFG, and the SPL/IPS, as well as the left middle temporal gyrus, the left thalamus, and the left MTL (Fig. 4).
Fig. 4.
Based on the two analyses described above, we then delineated regions that showed consensus functional connectivity, i.e., significant resting-state correlations as well as significant task-based co-activations with the seed region in the left fusiform gyrus that was hypo-activated during face processing in ASD
Functional decoding
Functional decoding was carried out for the seed region and in a pairwise fashion assessing the behavioral domains and paradigm classes significantly associated with the seed and each of the regions obtained in the conjunction of its resting-state and co-activation functional connectivity analysis. Behavioral domains and paradigms that were significantly (p < 0.05, FDR-corrected for multiple comparisons) over-represented among experiments in BrainMap activating the seed region were related to language and face processing.
Experiments activating the left TOC were significantly associated with shape perception, face monitor/discrimination, language, and reading. The right TOC was mainly activated not only by experiments associated with shape perception, but also cognitive processes and fear-related processing. Experiments featuring activation in the left IFG were all significantly associated with language functions such as semantics, phonology, and word generation, while the right IFG was shown to be activated during experiments related to attentional and working memory processes. Both left and right SPL were shown to be activated by experiments associated with spatial cognition, working memory, shape and motion perception. The left middle temporal gyrus was activated mainly by experiments associated with language-related functions such as semantics and speech. Functional associations for the left thalamus were comparatively weak. We found an association with experiments inducing action execution. The left MTL was functionally associated mainly not only with emotional domains such as fear, disgust, happiness and sadness, but also olfaction. Remarkably, also paradigms associated with face discrimination activated that region.
Conjunction of the functional connectivity network of the hypoactivated fusiform and the action observation/imitation network
We then investigated whether the network showing both task-dependent and task-independent functional connectivity with the seed region overlapped with the action observation/imitation network (MNS; Caspers et al. 2010). Indeed, we found several regions that were implicated in the putative MNS and at the same time showed consensus functional connectivity with the seed identified in the meta-analysis on face processing in ASD: the IFG bilaterally, the right lateral temporal lobe, the left SPL, the TOC bilaterally, and the medial temporal gyrus (Fig. 5).
Fig. 5.
Conjunction analysis between the network showing both task-dependent and task-independent functional connectivity with the seed region overlapped with the action observation/imitation network (Caspers et al. 2010)
Conjunction of the fusiform network and structural anomalies in ASD patients
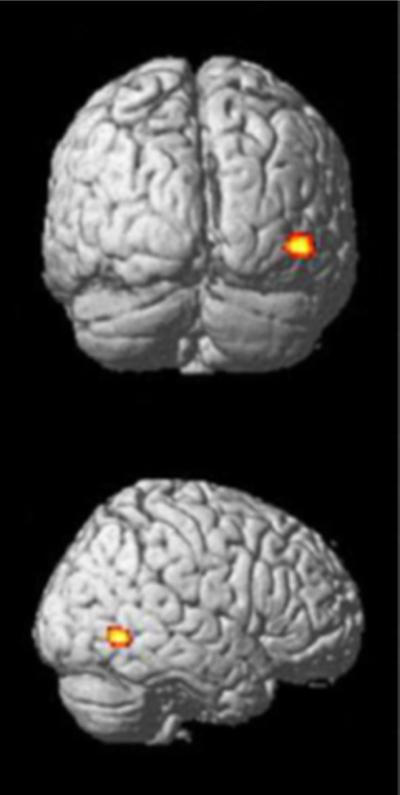
Finally, we aimed to investigate whether there was an overlap between the network showing both task-dependent and task-independent functional connectivity with the seed region and regions showing structural anomalies in ASD patients that have been previously identified by a quantitative meta-analysis on VBM studies (Nickl-Jockschat et al. 2012). We indeed found one cluster that was located in the right TOC (47, −65, 2, k = 178, V5) (Fig. 6).
Fig. 6.
Overlap between the network showing both task-dependent and task-independent functional connectivity to the seed region and regions showing structural anomalies in ASD patients that have been previously identified by a quantitative meta-analysis on VBM studies (Nickl-Jockschat et al. 2012)
Discussion
Deficits of face processing are a hallmark of ASD pathophysiology and may substantially contribute to the core triad of ASD symptoms (Campbell et al. 2006; Dawson et al. 2005; Osterling and Dawson 1994; Osterling et al. 2002). We here characterized the neural underpinnings of these face processing deficits by means of quantitative meta-analysis and revealed a single cluster in the left fusiform gyrus that was consistently hypo-activated during face processing across studies in ASD patients. The identified region on the fusiform gyrus has consistently been shown to be selectively activated during the perception of human faces (Puce et al. 1995; Kanwisher et al. 1997, 1999) and hence has been commonly referred to as the “fusiform face area” (FFA). The consistent recruitment this region in face processing was corroborated in a recent meta-analysis enrolling 100 fMRI studies on emotional face processing in healthy subjects (Sabatinelli et al. 2011). Moreover, this study found a clear distinction between fusiform regions responsible for emotional face processing and those processing emotional natural scenes, further corroborating evidence for their face-specificity.
There is, however, a growing body of evidence that challenges the notion of the FFA as a functionally and anatomically homogeneous module dedicated to processing a single perceptual category. High-resolution fMRI experiments point to three distinct face-selective regions located on the midfusiform sulcus, the posterior fusiform gyrus, and the inferior occipital gyrus (Weiner and Grill-Spector 2010). Also face-specificity of the FFA has been questioned by recent experiments, suggesting selective enhancement or suppression of activity in three spatially segregated clusters of voxels by a wide variety of categories in addition to faces (Çukur et al. 2013). In summary, rather than one single, face-specific FFA, functionally—and probably also anatomically (Caspers et al. 2013)—distinct parts of the fusiform gyrus seem to be involved in face processing. In other words, while there is little doubt that the fusiform gyrus is a highly relevant hub for face processing, this specialization may not be reduced to the term FFA, which denotes a single area within this (larger) region.
Given the rather coarse level of our meta-analytic approach, a precise allocation to one of these anatomically or functionally defined face-selective regions on the fusiform gyrus seems unwarranted. Rather, the activation and the resulting networks are best described as ‘fusiform’. This difference in resolution is especially crucial, when it comes to comparing meta-analytical data with results derived from high-resolution fMR imaging. However, despite these obvious problems concerning different levels of resolution, we would like to stress that our meta-analytic results resonate well with the functional specialization of the fusiform gyrus for face processing and should thus represent neural correlate for face processing deficits in ASD patients.
On a microstructural level, two different cytoarchitectonic areas, termed FG1 and FG2, have been described in the posterior fusiform gyrus (Caspers et al. 2013). Remarkably, the mid-fusiform sulcus appears as a fairly stable macroanatomical landmark that coincides with the boundary between FG1 and FG2. Whereas the former is located medial to it, the latter area is situated on the lateral fusiform gyrus. Moreover, the anterior tip of the midfusiform sulcus shows a consistent spatial relationship to mFus, one of the face-selective fusiform regions identified by high-resolution fMRI, while the relation between the posterior tip and pFus, another face-selective fusiform region, was less consistent. However, the correspondence between functional and cytoarchitectonic divisions is not likely one-to-one (Weiner et al. 2014). Given the lack of face-specificity for face-selective regions of the fusiform (Çukur et al. 2013), it seems highly probable that both FG1 and FG2 contain several fine-scale function regions (Weiner et al. 2014). Moreover, it must be noted that FG1 and FG2 only cover the posterior aspect of the fusiform gyrus, while face-sensitive regions have been found more anterior as well, leaving the possibility that additional cytoarchitectonic regions anterior to these are also involved in face processing.
Despite the fact that the regional functional–anatomical organization of the fusiform gyrus is still incompletely understood, it is tempting to speculate about the structural underpinnings of the fusiform hypoactivation found in this study. Several studies report findings that hint at subtle alterations in this brain region in patients with ASD. For example, the left fusiform gyrus has been reported as a brain region with pronounced reduction of cortical thickness in young and adolescent ASD patients (Wallace et al. 2010). Moreover, for ASD patients, a negative significant correlation between age and cortical thickness was found, which was not present in healthy subjects.
Correspondingly, a study utilizing a design-based stereotactic approach reported that ASD patients featured reduced neuron densities in layer III, total neuron numbers in layers III, V, and VI, and mean perikaryal volumes of neurons in layers V and VI of the fusiform gyrus (van Kooten et al. 2008). Since cortical layer III is the main source of corticocortical connections and layer V is the principal source of efferent fibers to sub-cortical regions (Jones 1986), these results suggest a disconnection of the fusiform in face-processing networks (van Kooten et al. 2008). However, the precise spatial relationship between these findings, the functional–anatomical subdivision of the fusiform gyrus as noted above, and our own results remains elusive.
Given these hints at microstructural changes, it needs to be emphasized at this point that a previous meta-analysis on VBM findings in ASD patients, i.e., macrostructural deficits, did not find convergent evidence for changes in the left fusiform region (Nickl-Jockschat et al. 2012). However, the use of different methodical approaches might explain the discrepant results. Gray matter volume—the mainly used parameter in VBM—represents the product of cortical thickness and surface area. Contrariwise, Wallace and colleagues (Wallace et al. 2010) relied on cortical thickness measurements. Consequently, the latter approach might find more subtle structural changes that are too discrete to be detectable by VBM. This argument is even more striking with regard to the findings of the stereotactic study reported by van Kooten et al. (2008). The idea of subtle neuroanatomic alterations underlying the fusiform hypoactivation found in our meta-analysis, therefore, seems plausible. Given the relatively high variability of structural findings in ASD patients, however, more studies are needed to corroborate these results. It will be an important goal of future studies to further elucidate the relationship between the organization of the fusiform gyrus and functional or neuroanatomic changes in ASD patients.
Functional connectivity network of the hypoactivated fusiform
Functional connectivity analysis revealed a distributed network comprising the bilateral TOC, area 44/45, thalamus and SPL as well as the left middle temporal gyrus and amygdala that was consistently coupled with the hypoactivated fusiform during both task- and during resting-state. Functionally, the ensuing network was related to visual and in particular face processing as well as language and affective processes. Functional profiling, however, also suggests three partially overlapping sub-networks connected to the fusiform: a visual network consisting of the bilateral TOC and SPL, an affective network encompassing the left amygdala and right TOC, and a language-related network centerd on the bilateral IFG (BA 44/45) and left middle temporal gyrus. These functionally defined sub-networks are now discussed in more detail.
One of the prominent findings in the functional connectivity analysis is the extensive coupling of the fusiform showing aberrant face processing in ASD with large parts of the visual system, which we summarized as TOC. Importantly, the functionally connected region comprises areas of both major branches of the visual cortex, the dorsal and ventral visual stream (Dakin and Frith 2005; Goodale and Milner 1992; Mishkin and Ungerleider 1982). This is noteworthy, given that the hypoactivated fusiform and surrounding regions on the fusiform gyrus/inferior temporal cortex are considered part of the ventral “what” processing stream (Macko et al. 1982), involved in the recognition of faces and objects (Goodale and Milner 1992; Ungerleider and Haxby 1994) as well as the encoding of spatial relationships between the subparts of scenes, potentially for manipulation (Rottschy et al. 2013b). The dorsal stream in turn is thought to play a major role in the localization of visual objects, the planning of reaching movements towards them as well as the processing of movement (Mishkin and Ungerleider 1982; Merigan et al. 1991). The latter function is particularly attributed to area V5, which then projects to parietal regions which are involved in spatial cognition, shape and motion perception (Goebel et al. 1998; Wang et al. 1999; Kansaku et al. 2001; Dakin and Frith 2005). Anterior to the dorsal aspects of the occipital visual cortex, the dorsal stream leads to the posterior superior parietal cortex, which we found likewise functionally connected to the seed. Our findings highlight a broad functional connectivity network for the fusiform, which in turn implicates a relevant interaction of the ventral fusiform gyrus with dorsal stream areas.
Physiologically, such interaction makes sense when considering that object recognition (ventral stream) and motion processing (dorsal stream) need to interact closely when dealing with face stimuli in a naturalistic, real live environment. Moreover, the current observations also fit well into a growing literature reporting deficits in ASD patients in tasks attributed to dorsal stream functioning. For example, ASD patients have been shown to display superior performance on tasks necessitating the detection of a static visual target embedded in a larger field (Plaisted et al. 1999; Caron et al. 2004; Pellicano et al. 2005) and are more detail-oriented (Shah and Frith 1983, 1993; Jolliffe and Baron-Cohen 1997; Mottron et al. 1999; Lahaie et al. 2006). Moreover, they are less sensitive to a variety of complex motion stimuli (Gepner et al. 1995; Spencer et al. 2000; Milne et al. 2002; Bertone et al. 2003; Blake et al. 2003) and feature increased motion coherence thresholds (Spencer et al. 2000; Milne et al. 2002), findings that may be deemed to reflect structural alterations in the region of V5 (Nickl-Jockschat et al. 2012). These findings together with our current data on the functional connectivity of a ventral visual region showing reduced activation during face processing highlight that both visual streams may play a crucial role in ASD pathophysiology. In summary, global dysfunctions of the visual system might be a yet underappreciated core deficit in ASD patients (Dakin and Frith 2005).
Outside the visual system, the fusiform also featured significant task- and resting-state connectivity with the left amygdala. Classically, the amygdala has been associated with the processing of unpleasant and fearful stimuli (Morris et al. 1996), while newer evidence supports an extended role of this brain region as a hub for the extraction of biological significance from the environment (Sander et al. 2003) and the shaping of behavioral responses (Ousdal et al. 2008). Observer-independent histological parcellation of post-mortem human brains has led to the reliable definition of three major sets of nuclei, the laterobasal, superficial, and centromedial group (Amunts et al. 2005). Remarkably, this histological subdivision is largely paralleled by a functional subspecialization. A study on the human amygdala utilizing connectivity-based parcellation retrieved three distinct clusters that largely overlapped with the anatomically defined nuclei of the amygdala as determined by cytoarchitectonic probabilistic maps. Functional characterization consistently found an association of all three nuclei with observing and discriminating faces (Bzdok et al. 2013). Our findings in this study of an amygdala involvement in the fusiform network fit these findings well.
Functional decoding linked the cluster that we found in the left amygdala to emotional domains such as fear, disgust, happiness, and sadness. A multitude of functional imaging studies have demonstrated a close link between face and affective processing (Gur et al. 2002). Vice versa, the display of facial expressions has been used as a method to induce emotions in subjects in the fields of neurosciences, functional neuroimaging, and psychology (Gur et al. 2002; Schneider et al. 2006). Consequently, the amygdala has been interpreted as a crucial link between face processing mediated by the fusiform region and affective processing (Breiter et al. 1996; Davis and Whalen 2001; Vuilleumier et al. 2001; Ishai 2008; Herrington et al. 2011; Müller et al. 2011). Aberrant amygdala activations during various tasks involving social cognition such as inferring mental states from pictures of eyes (Baron-Cohen et al. 1999, 2000) and judging facial expressions (Critchley et al. 2000) have led to the hypothesis that the amygdala may fail to assign emotional relevance to social stimuli in ASD patients (Dichter 2012). Thus, our findings of an amygdala involvement in the fusiform network might point to a more global pathomechanism of disturbed social cognition in ASD.
Finally, we found that the hypo-activated fusiform also shows robust connectivity with the bilateral BA 44/45 and the left middle temporal gyrus, which were characterized by the performed functional decoding as a subnetwork related to language processing. An involvement of the fusiform in semantic processing has been repeatedly discussed (Martin and Chao 2001; Turk et al. 2005). A recent study supports the notion of a close neuroanatomical relationship between the fusiform and language processing networks. Functional connectivity analyses suggest the role of the FG2 area, a cytoarchitectonically defined subdivision of the posterior fusiform gyrus, as an important hub of these two distinct subsystems. Remarkably, in healthy individuals, FG2 functionality appears to be lateralized, with the left FG2 area more involved in language processing, while the right FG2 area is more involved in face processing (Caspers et al. 2013).
Impaired communication is a core symptom of ASD that might be caused by underlying changes in language processing. Functional imaging studies indicate a variety of evidence for abnormalities in the neural underpinnings of language processing, such as reduced brain lateralization in ASD patients (Kleinhans et al. 2008; Knaus et al. 2008; Müller et al. 1999; Redcay and Courchesne 2008; Tesink et al. 2009), decreased synchrony of brain regions processing language (Kana et al. 2006; Catarino et al. 2011), decreased automaticity of language processing (Eigsti et al. 2011), and recruitment of brain regions that do not typically process language (Eyler et al. 2012; Gervais et al. 2004; Groen et al. 2010; Kana et al. 2006; Kana and Wadsworth 2012; Mizuno et al. 2011). Our findings of hypoactivation of the left, not the right, fusiform gyrus during face processing and a potential involvement of language processing networks is a further hint at changes in brain lateralization in ASD patients. Moreover, it may be speculated that the delineated functional interactions between the fusiform gyrus and language-related regions in the frontal and temporal lobes may underlie deficits in the processing of semantic knowledge and/or covert vocalization in the context of social interaction that are implicitly triggered by face stimuli.
With regard to our findings of frontal and parietal regions as part of the robust fusiform network, it deserves to be pointed out that recent studies have also identified face-sensitive frontal and parietal regions in humans (Rajimehr et al. 2009; Chan and Downing 2011). These findings led to the idea of a core network for face processing that consists mainly of “ventral stream” (i.e., occipito-temporal structures) and an extended network that also includes parietal and frontal structures (Avidan and Behrmann 2009). Especially frontal regions in this network seem to be especially driven primarily by the eyes, not the face, as a whole (Chan and Downing 2011). On the other hand, we must note that the quantitative functional decoding of our frontal and parietal regions did not point towards an association of these with face processing. However, given the comparatively few studies on face-selective regions outside the ventral stream, further studies might corroborate an involvement of frontal and parietal regions in the fusiform network in face processing.
Our meta-analysis on VBM findings revealed structural changes in ASD in the left anterior parietal lobe (Nickl-Jockschat et al. 2012), but this regions did not show an overlap with the fusiform network. However, a recent study reported cortical thinning that was more widespread in parietal regions (Wallace et al. 2010). While methodical differences could explain discrepant findings (please find a more detailed methodical discussion above), it is tempting to speculate that cortical thinning might be a discrete neuropathology underlying functional changes in parietal regions of the fusiform network. However, it needs to be emphasized that these results should be corroborated by additional studies, before reliable conclusions can be drawn.
Action-observation/imitation and the fusiform network
In our analysis, we found that several regions identified as part of the fusiform network were also found in a meta-analysis of the action-observation/imitation network, i.e., the MNS. This investigation was prompted by a close connection between face processing, imitative learning, and social cognition at the developmental and behavioral level: from the point of developmental psychology it has been argued that imitative behavior and the ensuing capacity of understanding other person's actions represent a key component in the development of social cognition (Rizzolatti and Craighero 2004). In this context, it is noteworthy that typically developing human infants imitate not only hand gestures, but also facial expressions (Meltzoff and Moore 1977). In fact, facial mimicry is one of the earliest social interactions seen in babies. Remarkably, while more complex aspects of action understanding (in particular inference on roles and goals of others) are found in human infants but not in, even adult, great apes (Tomasello and Carpenter 2005), the imitation of facial expressions seems to be an evolutionary conserved mechanism, since it is also found in infant chimpanzees (Myowa-Yamakoshi et al. 2004) and infant macaques (Ferrari et al. 2006). Only in humans, however, does imitation of facial expression and the subsequent development of the capacities to correctly interpret mimic behavior lay the foundation for “higher”, abstract social-cognitive processes related to theory of mind (Iacoboni and Dapretto 2006). The relevance of imitation or mimicry for social behavior is also underlined by the fact that adult humans tend to imitate each other automatically during social interaction (Williams et al. 2001), and seem to be more empathic the more they imitate (Chartrand and Bargh 1999).
In the current study, we found a substantial overlap between the fusiform network and the putative human MNS. We would hence argue that the neuronal correlates of face processing and action imitation are closely intertwined and, based on the behavioral evidence presented above, jointly contribute to social cognitive capacities and their development. The hypoactivation of the putative core node for processing visual-facial information in this network, i.e., the fusiform, in ASD patients may thus indicate a reduced input of face-related information into this system. It stands to be reasoned that such reduced or distorted input, if present during development as it may be assumed in a developmental disorder such as ASD, would have substantial effects on the shaping of capacities emerging from it. Given the behavioral evidence reviewed above, it may thus be argued that aberrant facial processing may lead to dysfunctional mechanisms for mimicry and intention understanding and ultimately deficits in complex social processes such as theory of mind, all of which have been shown to be abnormal in patients with ASD (Domes et al. 2008). Consequently and in synopsis with the earlier discussion of visual processing aberrations, we would argue that aberrant sensory (visual) processing may be a central pathophysiological aspect in ASD, whereas the clinically more prominent deficits in social processing are secondary effects of these.
It needs to be emphasized that our interpretation of these meta-analytic results certainly needs further corroboration by future targeted studies, since there are important limitations. Meta-analyses with data on a rather coarse scale and large functional voxels are more likely to show overlap than studies acquiring data with smaller functional voxels analyzed in individual subjects. Likewise, averaging across subjects and even studies will inevitably result in a spatial blurring. This limits the explanatory power of our conjunction analysis. Studies utilizing high-resolution fMRI, ideally at the single subject level, are thus needed to shed further light on a potential overlap between the fusiform network and the action observation/imitation network.
The fusiform network and brain structure anomalies in ASD
Reflecting on the above speculation, it is intriguing to note, that the only region in which the network related to the hypo-activated fusiform as delineated in the current study, the MNS (Caspers et al. 2010) and findings of a meta-analysis on structural atrophy in ASD (Nickl-Jockschat et al. 2012) converge is indeed in the visual system: area V5. Remarkably, a study reported cortical thinning in the ascending limb of the inferior temporal sulcus in ASD patients (Wallace et al. 2010), which is the typical location of V5 (Dumoulin et al. 2000). The fact that evidence for structural alterations in that region is also found by a study utilizing a different methodical approach appears to further corroborate our own findings of structural alterations in this region and highlights a potential role of this region in ASD pathophysiology.
The area hOc5 is commonly regarded as cytoarchitectonic correlate of V5 (Malikovic et al. 2007; Wilms et al. 2005). Thus, our allocation of the mentioned cluster to V5 is based on probabilistic histological maps in standard space, not on the use of functional data. V5 has long been known as the part of the visual cortex that is probably most sensitive to motion (Watson et al. 1993; Tootell et al. 1995; Wilms et al. 2005), including the processing of biological motion in the context of action observation (Grosbras et al. 2012). In other words, in contrast to other regions more specialized for processing geometric features or biological objects, area V5 seems to be sensitive to motion per se, independently of the conveyed image content (but see Kolster et al. 2010 for a discussion on the potential internal heterogeneity of the V5 region). Intriguingly, this presumed role of V5 and the convergent findings from the current study align very well with neuropsychological and clinical characteristics of ASD. Many previous studies have provided convergent evidence for a wide range of deficits in ASD patients with respect to motion processing, ranging from reduced sensitivity for motion coherence to deficient processing of complex motion stimuli, such as random dot kinematograms and biological motion (Gepner et al. 1995; Spencer et al. 2000; Milne et al. 2002; Dakin and Frith 2005; Bertone et al. 2003; Blake et al. 2003). In turn, the correct interpretation of mimics, the subjective reward gained from facial cues such as smiles, the automatic and later goal-directed imitation of these, and ultimately the development of higher-order concepts about intentionality strongly rely on the correct processing of the movements by different parts of the face and the face as a whole (Schneider et al. 1990). From this perspective, structural and/or functional alternations of the V5 region may thus explain or at least contribute to many neuropsychological and psychopathological features of ASD.
As in the previous study on brain structure anomalies in ASD (Nickl-Jockschat et al. 2012), probabilistic cytoarchitectonic maps in standard space allocated the cluster in the right TOC with highest probability to area hOC5, the putative correlate of functionally defined V5 (Malikovic et al. 2007; Wilms et al. 2005). Anatomical relations in the TOC, however, are intricate, with limb-, but also face-selective areas bordering immediately on V5 (Weiner and Grill-Spector 2013). Thus, given the probabilistic approach utilized and the rather coarse level of resolution at a meta-analytic level, we cannot fully rule out the possibilities that proximate regions are also affected. Given these limitations, to elucidate the precise relations between the cluster obtained in our conjunction analysis and the face-selective regions surrounding V5/MT will be an important objective for future studies.
Conclusion and outlook
Combining several meta-analyses (function in ASD, structure in ASD, action observation/imitation in healthy subjects) with the normative delineation of functional brain networks by two independent approaches, the present study not only revealed dysfunctional face processing in the fusiform in ASD, but also highlighted a potential role of the visual cortex and in particular area V5 in the pathophysiology of ASD. It now remains to be investigated how structure, function, connectivity, and clinical impairment within the delineated network model relate to each other at the level of individual patients.
Contributor Information
Thomas Nickl-Jockschat, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Juelich-Aachen Research Alliance Brain, Juelich/Aachen, Germany.
Claudia Rottschy, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Juelich-Aachen Research Alliance Brain, Juelich/Aachen, Germany; Department of Neuroscience and Medicine, INM-1, Research Center Jülich, Jülich, Germany.
Johanna Thommes, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany.
Frank Schneider, Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Juelich-Aachen Research Alliance Brain, Juelich/Aachen, Germany.
Angela R. Laird, Department of Physics, Florida International University, Miami, USA
Peter T. Fox, Research Imaging Institute, University of Texas Health Sciences Center, San Antonio, USA
Simon B. Eickhoff, Department of Neuroscience and Medicine, INM-1, Research Center Jülich, Jülich, Germany Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany.
References
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB. Definition and characterization of an extended social-affective default network. Brain Struct Funct. 2014 doi: 10.1007/s00429-013-0698-0. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Avidan G, Behrmann M. Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Curr Biol. 2009;19(13):1146–1150. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a “complex” issue. J Cogn Neurosci. 2003;15:218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31(4):1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14(6):922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34(12):3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, Lawrence K, Mandy W, Mitra C, Jeyakuma L, Skuse D. Meanings in motion and faces: developmental associations between the processing of intention from geometrical animations and gaze detection accuracy. Dev Psychopathol. 2006;18(1):99–118. doi: 10.1017/S0954579406060068. [DOI] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Rainville C, Chouinard S. Do high functioning persons with autism present superior spatial abilities? Neuropsychologia. 2004;42:467–481. doi: 10.1016/j.neuropsychologia.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Amunts K, Laird AR, Fox PT, Eickhoff SB. Functional characterization and connectivity of two cytoarchitectonic visual areas on the human posterior fusiform gyrus. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22364. doi:10.1002/hbm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Luke L, Waldman S, Andrade A, Fletcher PC, Ring H. An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. Eur J Neurosci. 2011;33:558–567. doi: 10.1111/j.1460-9568.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- Chan AW, Downing PE. Faces and eyes in human lateral prefrontal cortex. Front Hum Neurosci. 2011;5:51. doi: 10.3389/fnhum.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: the perception-behavior link and social interaction. J Personal Soc Psychol. 1999;76(6):893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23(11):2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Çukur T, Huth AG, Nishimoto S, Gallant JL. Functional subdomains within human FFA. J Neurosci. 2013;33(42):16748–16766. doi: 10.1523/JNEUROSCI.1259-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly EM, Surguladze S, Page L, Toal F, Robertson D, Curran S, Giampietro V, Seal M, Brammer MJ, Andrew C, Murphy K, Phillips ML, Murphy DG. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol Psychiatry. 2007;62(3):207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35(3):1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Kumbier E, Herpertz-Dahlmann B, Herpertz SC. Social cognition in autism. A survey of functional imaging studies. Nervenarzt. 2008;79(3):261–274. doi: 10.1007/s00115-008-2409-2. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10(5):454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30(18):6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti IM, Schuh J, Mencl E, Schultz RT, Paul R. The neural underpinnings of prosody in autism. Child Neuropsychol. 2011;18(6):600–617. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135:949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gepner B, Mestre D, Masson G, de Schonen S. Postural effects of motion vision in young autistic children. NeuroReport. 1995;6:1211–1214. doi: 10.1097/00001756-199505300-00034. [DOI] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthélémy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W. The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci. 1998;10(5):1563–1573. doi: 10.1046/j.1460-9568.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, Martin A. The perils of global signal regression for group comparisons: a case study of autism spectrum disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Tesink C, Petersson KM, van Berkum J, van der Gaag RJ, Hagoort P, Buitelaar JK. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb Cortex. 2010;20:1937–1945. doi: 10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Beaton S, Eickhoff SB. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Hum Brain Mapp. 2012;33(2):431–454. doi: 10.1002/hbm.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Taylor JM, Grupe DW, Curby KM, Schultz RT. Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage. 2011;56(4):2348–2355. doi: 10.1016/j.neuroimage.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22363. doi:10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bölte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Failure to deactivate in autism: the co-constitution of self and other. Trends Cogn Sci. 2005;10(10):431–433. doi: 10.1016/j.tics.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Ishai A. Let's face it: it's a cortical network. Neuroimage. 2008;40(2):415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage. 2012;60(4):2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. Connectivity of the primate sensory-motor cortex. In: Jones EG, Peters A, editors. Cerebral cortex, vol 5. Sensory-motor areas and aspects of cortical connectivity. Plenum; New York: London: 1986. pp. 113–83. [Google Scholar]
- Joseph RM, Tager-Flusberg H. An investigation of attention and affect in children with autism and Down syndrome. J Autism Dev Disord. 1997;27(4):385–396. doi: 10.1023/a:1025853321118. [DOI] [PubMed] [Google Scholar]
- Kana RK, Wadsworth HM. “The archeologist's career ended in ruins”: hemispheric differences in pun comprehension in autism. Neuroimage. 2012;62:77–86. doi: 10.1016/j.neuroimage.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2893. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku K, Hashimoto K, Muraki S, Miura K, Takahashi T, Kawano K. Retinotopic hemodynamic activation of the human V5/MT area during optokinetic responses. NeuroReport. 2001;12(18):3891–3895. doi: 10.1097/00001756-200112210-00007. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. NeuroReport. 1999;10(1):183–187. doi: 10.1097/00001756-199901180-00035. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, Aylward E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2008;54:697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc. 2008;14:967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci. 2010;30(29):9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, Saumier D. Face perception in high-functioning autistic adults: evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology. 2006;20:30–41. doi: 10.1037/0894-4105.20.1.30. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, McKay DR, Bzdok D, Laird RW, Robinson JL, Turner JA, Turkeltaub PE, Lancaster JL, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macko KA, Jarvis CD, Kennedy C, Miyaoka M, Shinohara M, Sololoff L, Mishkin M. Mapping the primate visual system with [2-14C]deoxyglucose. Science. 1982;218(4570):394–397. doi: 10.1126/science.7123241. [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT?: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17:562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Byrne CE, Maunsell JH. Does primate motion perception depend on the magnocellular pathway? J Neurosci. 1991;11(11):3422–3429. doi: 10.1523/JNEUROSCI.11-11-03422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. J Child Psychol Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6(1):57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134:2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mottron L, Belleville S, Ménard E. Local bias in autistic subjects as evidenced by graphic tasks: perceptual hierarchization or working memory deficit? J Child Psychol Psychiatry. 1999;40:743–755. [PubMed] [Google Scholar]
- Müller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Müller VI, Habel U, Derntl B, Schneider F, Zilles K, Turetsky BI, Eickhoff SB. Incongruence effects in crossmodal emotional integration. Neuroimage. 2011;54(3):2257–2266. doi: 10.1016/j.neuroimage.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front Hum Neurosci. 2013;12(7):268. doi: 10.3389/fnhum.2013.00268. doi:10.3389/fnhum.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes). Dev Sci. 2004;7(4):437–442. doi: 10.1111/j.1467-7687.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. Brain structure anomalies in autism spectrum disorder–a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33(6):1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14(2):239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156:450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2(2):140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(Pt 12):2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Child Psychol Psychiatry. 1999;40:733–742. [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74(3):1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci USA. 2009;106(6):1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB. Investigating function and connectivity of morphometric findings—exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17). Neuroimage. 2012;62(3):1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Kleiman A, Dogan I, Langner R, Mirzazade S, Kronenbuerger M, Werner C, Shah NJ, Schulz JB, Eickhoff SB, Reetz K. Diminished activation of motor working-memory networks in Parkinson's disease. PLoS One. 2013a;8(4):e61786. doi: 10.1371/journal.pone.0061786. doi:10.1371/journal.pone.0061786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Caspers S, Roski C, Reetz K, Dogan I, Schulz JB, Zilles K, Laird AR, Fox PT, Eickhoff SB. Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Struct Funct. 2013b;218(6):1551–1567. doi: 10.1007/s00429-012-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Heimann H, Himer W, Huss D, Mattes R, Adam B. Computer-based analysis of facial action in schizophrenic and depressed patients. Eur Arch Psychiatry Clin Neurosci. 1990;240(2):67–76. doi: 10.1007/BF02189974. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Koch K, Backes V, Amunts K, Shah NJ, Bilker W, Gur RE, Habel U. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163(3):442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, Piefke M. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Soc Neurosci. 2011;6(1):1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? J Child Psychol Psychiatry. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Spencer J, O'Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. NeuroReport. 2000;11:2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Tesink CM, Buitelaar JK, Petersson KM, van der Gaag RJ, Kan CC, Tendolkar I, Hagoort P. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132:1941–1952. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M. The emergence of social cognition in three young chimpanzees. Monogr Soc Res Child Dev. 2005;70(1):vii–132. doi: 10.1111/j.1540-5834.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Dale AM, Look RB, Sereno MI, Malach R, Brady TJ, Rosen BR. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Rosenblum AC, Gazzaniga MS, Macrae CN. Seeing John Malkovich: the neural substrates of person categorization. Neuroimage. 2005;24(4):1147–1153. doi: 10.1016/j.neuroimage.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131(Pt 4):987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschläger AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund HJ, Rizzolatti G, Fink GR. Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. Neuroimage. 2007;37(4):1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133(Pt 12):3745–3754. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou T, Qiu M, Du A, Cai K, Wang Z, Zhou C, Meng M, Zhuo Y, Fan S, Chen L. Relationship between ventral stream for object vision and dorsal stream for spatial vision: an fMRI ? ERP study. Hum Brain Mapp. 1999;8(4):170–181. doi: 10.1002/(SICI)1097-0193(1999)8:4<170::AID-HBM2>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Am Acad Child Adolesc Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. Neuroimage. 2010;52(4):1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Neural representations of faces and limbs neighbor in human high-level visual cortex: evidence for a new organization principle. Psychol Res. 2013;77(1):74–97. doi: 10.1007/s00426-011-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, Amunts K, Grill-Spector K. The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage. 2014;84:453–465. doi: 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Wilms M, Eickhoff SB, Specht K, Amunts K, Shah NJ, Malikovic A, Fink GR. Human V5/MT?: comparison of functional and cytoarchitectonic data. Anat Embryol (Berl) 2005;210:485–495. doi: 10.1007/s00429-005-0064-y. [DOI] [PubMed] [Google Scholar]
- Zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60(1):162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]