Abstract
The purpose of this study was to review the clinical features of maxillofacial space infection (MSI), and to identify the potential risk factors predisposing to life-threatening complications. A retrospective review of the medical charts of patients with MSI treated at Peking University School and Hospital of Stomatology from August 2008 to September 2013 was conducted. A total of 127 patients [75 men (59.1%) and 52 women (40.9%); mean age, 45.39 ± 21.18 years, with a range of 1–85 years] formed the study cohort. The most common cause of MSI was odontogenic infection (57.5%). The most common space involved was the submandibular space. All patients were treated by antibiotics as well as surgical incision and drainage. Sixteen patients developed life-threatening complications, and the dominant complication was respiratory obstruction. Multivariate logistic regression analysis revealed the percentage of neutrophils (NEUT%) upon hospital admission ≥85.0% to be associated with life-threatening complications (P < 0.05). Even though adequate antibiotic therapy and incision and drainage of abscess were given, MSI patients with NEUT% upon hospital admission ≥85.0% carry a higher risk of life-threatening complications. In these patients, an aggressive treatment strategy is mandatory.
Keywords: Complication, maxillofacial space infection, odontogenic infection, risk factor
Maxillofacial space infection (MSI) is one of the most common types of infection in the head and neck. Maxillofacial space infection has various causes, mainly odontogenic infection,1,2 and can involve different maxillofacial spaces. With the widespread use of antibiotics and improvement in oral hygiene, the incidence of MSI has decreased. If not treated promptly, life-threatening complications, however, can occur: respiratory obstruction, pneumonia, descending mediastinitis, septic shock, sepsis, necrotizing fasciitis, pericarditis, cavernous sinus thrombosis, and brain abscesses.1,3–7
Several studies have focused on the risk factors of life-threatening complications of MSI, but the results have differed. The current study was undertaken to review the clinical features of MSI and to identify the potential risk factors predisposing to life-threatening complications.
PATIENTS AND METHODS
Study Design
We reviewed the medical records of 127 patients with a diagnosis of MSI treated in the Department of Oral and Maxillofacial Surgery at the Peking University School and Hospital of Stomatology (Beijing, China) from August 2008 to September 2013. Owing to the retrospective nature of this study, it was granted an exemption in writing by the protocol review board of Peking University School and Hospital of Stomatology. Twenty-five patients with incomplete medical records or who were treated only by antibiotics were excluded. All patients were diagnosed and cared for using an identical protocol.
Data Collection
Clinical features studied were demography (age, sex, occupation), etiology, spaces involved, previous antibiotic treatment, systemic diseases, presentation upon hospital admission (symptoms and signs, temperature), laboratory data upon hospital admission (white blood cell [WBC] count, percentage of neutrophils [NEUT%], blood sugar), pus culture, treatment (medical and surgical), duration of hospital stay, and complications.
Statistical Analysis
Data were the mean ± standard error (SEM) and were analyzed using SPSS 19.0 (SPSS, Inc, Chicago, IL). Univariate logistic regression analysis was carried out to identify potential factors associated with life-threatening complications. A P value less than 0.05 (2-tailed) was considered statistically significant. Significant risk factors were analyzed further using multivariate logistic regression analysis.
RESULTS
Clinical Features
A total of 127 patients (75 men [59.1%] and 52 women [40.9%]; male-to-female ratio, 1.44/1) formed the study cohort. The mean age was 45.39 ± 21.18 years (range: 1–85 years) (Fig. 1). The most common occupation was staff (44 patients, 34.6%), followed by farmers (26 patients, 20%), workers (8 patients, 6.3%), students (8 patients, 6.3%), and “other” (41 patients, 32.3%).
FIGURE 1.
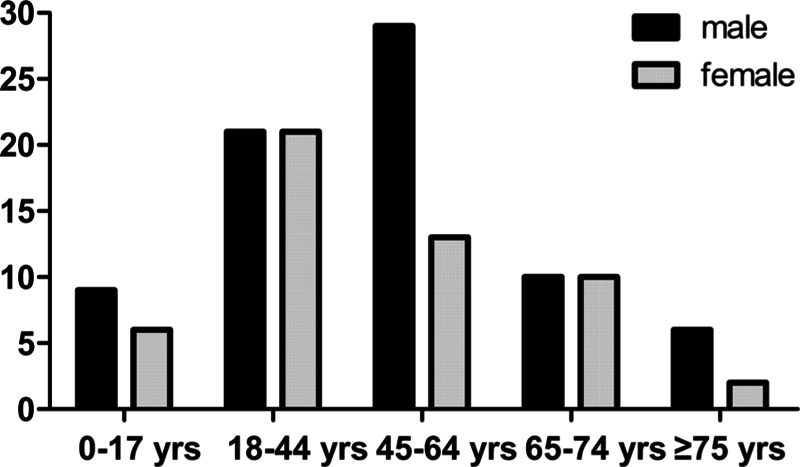
Distribution of age (n = 127).
Seasonal distribution of the presentation of MSI was summer (29.1%), winter (28.4%), autumn (22.8%), and spring (19.7%) (Fig. 2).
FIGURE 2.

The seasonal distribution of patients (n = 127). According to the admission season, the patients were divided into four groups.
Causes of MSI were identified based on clinical and radiologic evidence in 101 patients (79.5%). The most common cause was odontogenic infection (73 patients, 57.5%), followed by parotitis (6 patients, 4.7%), cyst in jaw (6 patients, 4.7%), and cyst in branchial cleft (5 patients, 3.9%). With respect to odontogenic infection, the most common origin was a periapical (60.3%), followed by pericoronitis (27.4%); 70 patients had an origin in the mandibles (95.9%), whereas 42 had an origin in the left mandible (57.5%), 71 had an origin in the molars (97.3%).
The spaces involved were identified according to clinical and imaging findings. The most common space involved was submandibular, and accounted for 54.6% and 28.7% for single-space infection and multiple-space infection, respectively (Fig. 3). Thirty-three patients had single-space infection (26.0%) and 94 patients had multiple-space infection (74.0%) (Fig. 3), and the mean number of involved spaces was 2.83 ± 1.79. An increasing number of involved sites showed an approximately downward trend in the number of patients presenting with MSI (Fig. 4).
FIGURE 3.
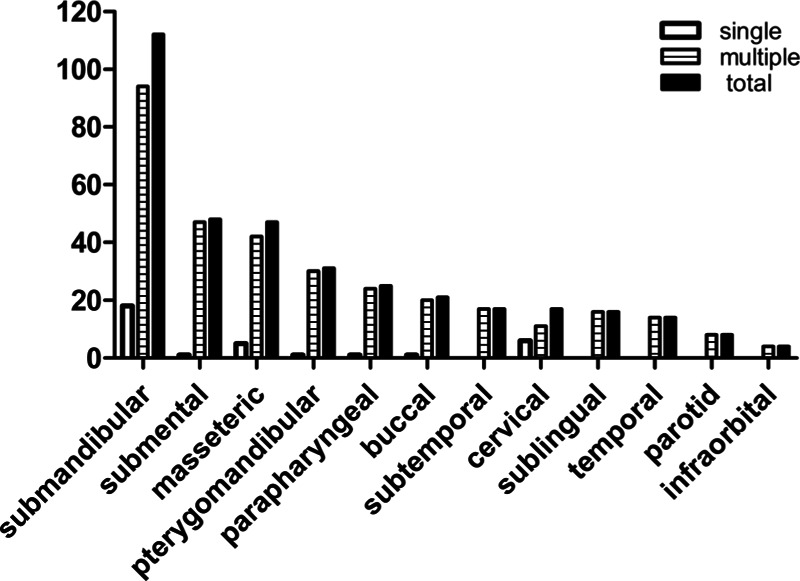
Distribution of maxillofacial spaces involved (n = 127). The figure described the proportion of each maxillofacial space in single-space infection, multiple-space infection, and the total maxillofacial space infection.
FIGURE 4.
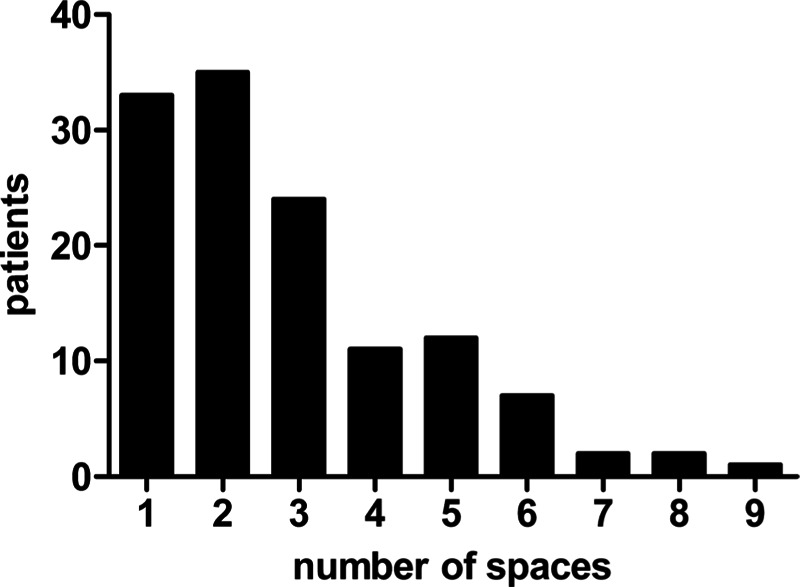
The number of maxillofacial spaces involved. The figure described the distribution of patients who had different number of spaces involved. An increasing number of involved sites showed an approximately downward trend in the number of patients presenting with maxillofacial space infection.
Forty-three patients had diabetes mellitus (DM). Of these DM patients: 3 patients also had hypertension and coronary heart disease; 15 patients also had hypertension; 2 patients also had coronary heart disease; 1 patient also had systemic lupus erythematosus. With regard to non-DM patients: 1 patient had hypertension and coronary heart disease; 2 patients had hypertension; 1 patient had cerebral thrombosis; 1 patient had rheumatoid arthritis. No patient had a known primary or acquired immunodeficiency.
A total of 188 subjects (92.9%) received antibiotic treatment before hospital admission.
Patterns of symptoms and signs are shown in Figure 5. Swelling was present in all patients (100.0%) (Fig. 6). Other common findings were pain (96.0%), limited opening of mouth (89.0%), fever (42.5%), local pitting edema (22.0%), dyspnea (21.3%), and dysphagia (18.9%). Mean duration of hospital stay was 11.89 ± 15.33 days (range: 2–90 days). Mean temperature upon hospital admission was 37.29 ± 0.99 °C (range: 35.50–41.00 °C).
FIGURE 5.
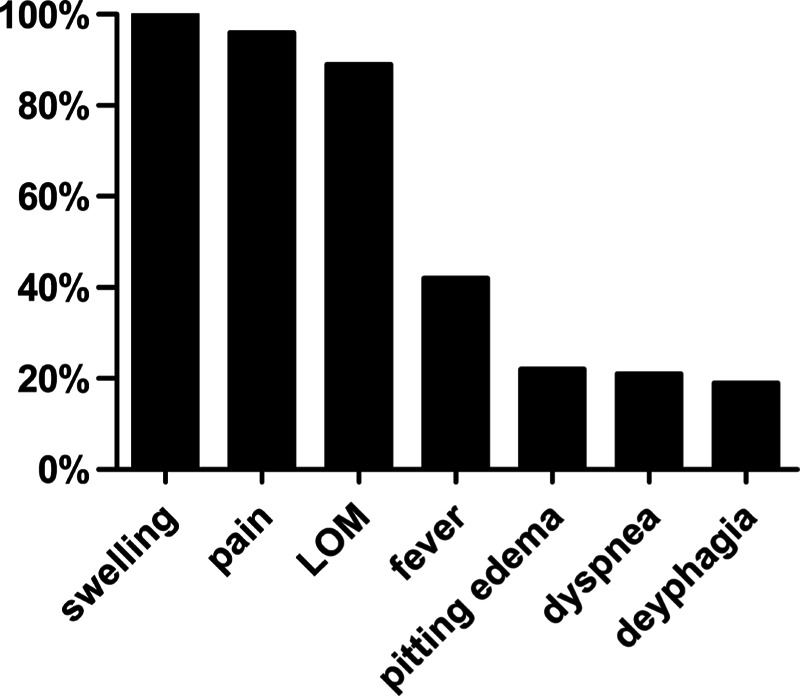
Symptoms and signs. LOM, limited opening of mouth.
FIGURE 6.
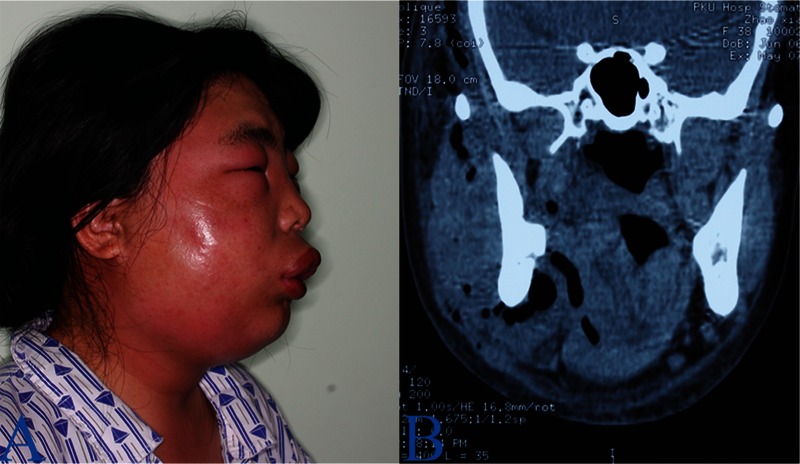
A 38-year-old woman with infections involving infratemporal, masticatory, submandibular, parapharyngeal, and pterygomandibular regions on right side. A, Edematous swelling with erythema is evident in the submandibular and masticatory regions. B, computed tomography scan demonstrates extensive soft tissue swelling and gas formation in the affected regions.
Mean WBC count upon hospital admission was 14.25 ± 7.09 ×109/L. Mean NEUT% upon hospital admission was 78.26 ± 15.00%.
Prevalence of positive pus culture was 66.4% (73/110) and the delivery rate was 86.6% (110/127). The most common causative organism was viridans group streptococci (53/110, 48.2%).
Treatment
All patients underwent surgical incision and drainage in tandem with intravenous administration of antibiotics (Fig. 7). All patients received intravenous administration of primary empiric antibiotics upon hospital admission, and most adult patients received second-generation or third-generation cephalosporins, along with metronidazole or ornidazole for anaerobic cover. Mean duration of intravenous administration of antibiotics was 7.24 ± 4.34 days (range: 1–28 days).
FIGURE 7.
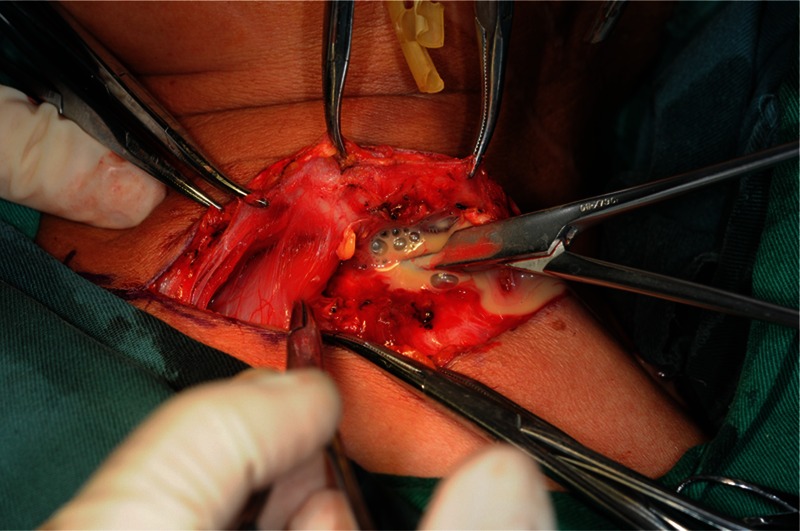
A 62-year old woman with infections involving superior mediastinum region. Two days after the first incision and drainage for temporal, masticatory, submandibular, parapharyngeal, pterygomandibular regions, the second incision and drainage for cervical and superior mediastinum regions was performed.
All patients underwent incision and drainage of involved spaces. A total of 119 patients received only 1 surgical intervention (93.7%), whereas 8 patients underwent a second surgical intervention because of disease progression (6.3%). Surgical intervention was done only under general anesthesia in 20 patients (15.7%), only under local anesthesia for 105 patients (82.7%), and under local anesthesia and general anesthesia for 2 patients (1.6%). Most patients had only extraoral drainage, and 4 patients (3.1%) had intraoral drainage combined with extraoral drainage. Eighty-six patients had submandibular incisions, whereas 21 of 86 patients had submandibular incisions combined with other types of incisions.
Outcome
Sixteen patients developed life-threatening complications. Twelve patients had respiratory obstruction, of which 4 patients underwent emergency tracheotomy, 8 patients had pneumonia, 4 patients had sepsis, 2 patients had septic shock, and 1 patient had descending mediastinitis (Table 1). Only one patient had no underlying systemic disease but suffered from respiratory obstruction, pneumonia, and septic shock: he died (mortality, 0.8%).
TABLE 1.
Characteristics of Patients With Life-threatening Complications of Maxillofacial Space Infection
| Patient | Sex | Age/yrs | Space | Comorbidities | Complications | Procedure | Outcome |
| 1 | Male | 60 | SMS, SM, M, PM, SL, IT | DM | Respiratory obstruction | I&D | Good |
| 2 | Male | 49 | SMS, SM, PP | HTN | Sepsis | I&D | Good |
| 3 | Male | 59 | SMS, SM | Nil | Respiratory obstruction | I&D | Good |
| 4 | Male | 75 | SMS, SM, SL | DM | Respiratory obstruction, sepsis | I&D, tracheotomy | Good |
| 5 | Female | 76 | SMS, SM, SL | DM,HTN | Respiratory obstruction, pneumonia | I&D, tracheotomy | Good |
| 6 | Female | 62 | SMS, M, PM, PP, C, T | Nil | Pneumonia, descending mediastinitis | I&D | Good |
| 7 | Male | 69 | SMS, M, PM, P, B, T | Nil | Respiratory obstruction, pneumonia | I&D, tracheotomy | Good |
| 8 | Male | 56 | SMS, M, PM | Nil | Respiratory obstruction | I&D | Good |
| 9 | Male | 70 | SMS, PP, B | DM | Respiratory obstruction | I&D | Good |
| 10 | Female | 57 | SMS, M, PM, IT, T, B, IO | DM | Pneumonia | I&D | Good |
| 11 | Male | 42 | SMS, SM, SL | DM | Respiratory obstruction | I&D | Good |
| 12 | Male | 51 | SMS, SM, M, PM, PP, SL, P | Nil | Respiratory obstruction | I&D | Good |
| 13 | Male | 83 | SMS, M, PP, IT, T, B, IO | DM, HTN | Pneumonia | I&D | Good |
| 14 | Male | 70 | SMS, SM, PP, C | Nil | Respiratory obstruction, sepsis, pneumonia, septic shock | I&D, tracheotomy | Death |
| 15 | Female | 54 | M, PM, PP, IT, T | Nil | Respiratory obstruction, sepsis, pneumonia, septic shock | I&D | Good |
| 16 | Male | 37 | SMS, SM, PP, C | Nil | Respiratory obstruction, pneumonia | I&D | Good |
B, buccal space; C, cervical space; DM, diabetes mellitus; HTN, hypertension; I&D, incision and drainage; IO, infraorbital space; IT, infratemporal space; M, masticatory space; MSI, maxillofacial space infection; P, parotid space; PM, pterygomandibular space; PP, parapharyngeal space; SL, sublingual space; SM, submental space; SMS, submandibular space; T, temporal space.
Nine of the 16 patients had 1 life-threatening complication, 5 patients had 2 life-threatening complications, and 2 patients had 4 life-threatening complications. Eight of the 16 patients had underlying systemic diseases, 5 patients had only DM, 1 patient had hypertension, and 2 patients had DM and hypertension. Mean age of the 16 patients was 60.63 ± 12.66 years, whereas the mean age of the remaining patients was 43.20 ± 21.29 years.
Results of univariate logistic regression analysis suggested that factors associated with a significant increased risk of development of life-threatening complications were (upon hospital admission): temperature ≥39.0 °C (P < 0.05); WBC count ≥15.0 × 109/L (P < 0.05); and NEUT% ≥ 85.0% (P < 0.05) (Table 2).
TABLE 2.
Univariate Logistic Regression Analyses of the Clinical Features and Life-threatening Complications of Maxillofacial Space Infection
| Variable | Categories | With/Without Complication | OR | 95% CI | P |
| Sex | Male | 12/63 | 0.44 | 0.13–1.44 | 0.174 |
| Female | 4/48 | ||||
| Age, years | ≥65 | 6/22 | 2.43 | 0.80–7.40 | 0.119 |
| <65 | 10/89 | ||||
| Occupation | Staff | 4/40 | 0.30 | 0.05–2.01 | 0.215 |
| Farmers | 4/22 | 0.00 | 0.00 | 0.999 | |
| Workers | 2/6 | 0.51 | 0.08–3.17 | 0.474 | |
| Students | 0/8 | 0.55 | 0.08–3.73 | 0.537 | |
| Other | 6/35 | ||||
| Number of spaces involved | Multiple | 16/78 | 0.00 | 0.00 | 0.998 |
| Single | 0/33 | ||||
| Duration of stay, days | ≥7 | 6/66 | 0.41 | 0.14–1.21 | 0.105 |
| <7 | 10/45 | ||||
| Treatment before HA | Present | 14/104 | 0.47 | 0.09–2.50 | 0.376 |
| Absent | 2/7 | ||||
| Temperature upon HA, °C | ≥39.0 | 3/6 | 6.38 | 1.14–35.58 | 0.035 |
| 38.0–38.9 | 5/20 | 3.19 | 0.78–13.09 | 0.108 | |
| 37.0–37.9 | 4/34 | 1.50 | 0.35–6.41 | 0.584 | |
| <37.0 | 4/51 | ||||
| WBC upon HA, × 109/L | ≥15.0 | 11/42 | 3.61 | 1.17–11.13 | 0.025 |
| <15.0 | 5/69 | ||||
| NEUT% upon HA, % | ≥85.0 | 12/33 | 12.00 | 1.48–97.63 | 0.020 |
| 75.0–84.9 | 3/45 | 2.20 | 0.22–22.10 | 0.503 | |
| <75.0 | 1/33 | ||||
| Diabetes mellitus | Present | 7/36 | 1.62 | 0.56–4.70 | 0.374 |
| Absent | 9/75 |
CI, confidence interval; HA, hospital admission; MSI, maxillofacial space infection; OR, odds ratio.
Results of multivariate logistic regression analysis confirmed that NEUT% ≥ 85.0% upon hospital admission was an independent predictor of life-threatening complications (odds ratio, 7.09; 95% confidence interval, 2.13–23.60; P < 0.05) (Table 3). It, however, was not confirmed that temperature ≥39.0 °C or WBC count ≥15.0 × 109/L upon hospital admission were independent predictors of life-threatening complications.
TABLE 3.
Multivariate Logistic Regression Analyses of the Clinical Features and Life-threatening Complications of Maxillofacial Space Infection
| Variable (upon hospital admission) | OR | 95% CI | P |
| WBC count | 2.21 | 0.64–7.66 | 0.210 |
| Temperature, °C | 2.55 | 0.51–12.64 | 0.252 |
| NEUT% | 7.09 | 2.13–23.60 | 0.001 |
CI, confidence interval; MSI, maxillofacial space infection; NEUT%, the percentage of neutrophils; OR, odds ratio; WBC, white blood cell count.
Mean duration of hospital stay was 7.91 ± 4.69 (range: 1–31) days. Univariate logistic regression analysis suggested that no factors were associated significantly with duration of hospital stay.
DISCUSSION
The most common cause of MSI in our study was odontogenic infections (57.5%). This finding was consistent with studies demonstrating that odontogenic infections are the most common type of infections of the head and neck among adults.1,2,6,8 Nevertheless, the prevalence of odontogenic infections has been reported to differ in different studies. This phenomenon could be because: odontogenic infections cannot be managed by antibiotics alone (antibiotics are an adjunct to definitive treatment); some patients may self-medicate; and some general practitioners may administer suboptimal management.3
Huang et al6 revealed a prevalence of deep-neck infections of 50% in 185 patients in Taiwan in 2004. Wang et al2 demonstrated a prevalence of 62.8% of MSI in 250 subjects in the USA in 2005. Eftekharian et al8 showed a prevalence of deep-neck infections of 49% in 112 patients in Iran in 2009. Zhang et al1 revealed a prevalence of MSI of 56.1% in 212 individuals in China in 2010. Cause of infection was not found in 26.0% of patients in our study despite extensive clinical and imaging studies, probably because infectious foci had resolved by the time of presentation or subclinical infectious foci were present.9,10
Male and female adults accounted for 52.0% and 36.2% of the study cohort, respectively, and ≈12% were children aged <18 years. This result corresponds with studies demonstrating that MSIs occur mainly in adults. This finding was probably because of the higher prevalence of underling systemic diseases that compromise immunity (43.2% of adults had underlying systemic disease in our study, whereas all children were essentially healthy with no underlying disease), neglect of oral health, and an increase in the incidence of pericoronitis of wisdom teeth and other odontogenic infections.3 The current study showed male dominancy (male-to-female ratio, 1.44/1), a finding that is consistent with the literature. There is some evidence that women tend to have better oral health and seek oral healthcare more frequently.11 Slight preponderance of patients presenting in the summer (29.1%) and winter (28.4%) was observed. Bakir et al7 reported an apparent preponderance of patients presenting in the autumn (43.4%).
The most common involved space was submandibular, a finding that is in accordance with the observations of Marioni et al12 and Zhang et al.1 Buccal,13 masticatory,14 parapharyngeal,6 and pterygomandibular spaces,15 however, have also been reported to be the most common involved spaces. Odontogenic infections usually spread contiguously from the mandible or maxilla into the sublingual, submandibular, or masticatory spaces, and then spread into the parapharyngeal space directly.9,16 In the current study, 74.0% of patients had multiple-space infections. Reasons for this finding could be: self-medication (which has become a big issue in China)17,18; delay in hospital admission (mean duration of hospital stay was 11.89 ± 15.33 days); high prevalence of underling systemic disease (which compromises immunity); rapid spread of infection between spaces.
All patients in our study presented with swelling, and 96.0% of patients presented with pain. Swelling of the tissues surrounding the spaces in the floor of the mouth or larynx is extremely dangerous, and can lead to respiratory obstruction. Bridgeman et al19 observed that 98% of patients presented for care only if there was a sudden onset of swelling. Most patients had limited opening of the mouth upon hospital admission, suggesting that the infection may have involved the masticatory spaces and could disturb the upper airway abruptly. Also, close monitoring of patients with trismus is necessary in case of the symptoms and signs of upper-airway compromise (eg, tongue elevation, stridor, difficulty in swallowing saliva, breathlessness).3
Prevalence of positive pus culture was 66.4% (73/110) in the current study. The reason for an absence of growth in pus culture could be: collection of samples after antibiotic administration; inappropriate collection and delivery of specimens; absence of routine anaerobic cultures; difficulty in culturing of anaerobes.3
Management of MSI comprises: antibiotic administration; surgical incision and drainage; underlying treatment of systemic disease; management of complications; supportive treatment. Empiric antibiotic therapy should be administered without waiting for the results of pus culture when patients are admitted to hospital. Empiric antibiotics must cover Gram-positive and Gram-negative aerobic and anaerobic pathogens. Second- or third-generation cephalosporins as primary empiric antibiotic therapy, along with metronidazole or ornidazole, are usually employed. Thirty-three patients changed antibiotics during their stay in hospital, or had another antibiotic added, as a result of lack of clinical resolution of infection rather than as a result of culture and sensitivity.20 Wang et al21 and Bakir et al,7 however, preferred penicillin and metronidazole for initial antibiotic therapy, and clindamycin for severe deep-neck infection (which provides adequate cover against penicillin-resistant anaerobes). In their studies, second- or third-generation cephalosporins were used instead if poor clinical response was noted or if complications had developed.7 Mathew et al3 preferred amoxicillin with clavulanate potassium and metronidazole for initial antibiotic therapy. With regard to the time of antibiotic treatment, Kirse et al22 suggested antibiotic therapy should be employed until drug-resistant bacteria develop, whereas Santos et al23 suggested the intravenous antibiotic therapy should be used for 2 to 3 weeks. We employed antibiotic therapy until apparent resolution of the underlying medical condition and location of infection (eg, temperature was normal, there were no symptoms or signs of dyspnea and dysphagia, and WBC count and NEUT% were normal).20
All patients underwent surgical incision and drainage. We employed antibiotic treatment and surgical drainage in inpatients with cellulitis and abscesses because the difference between them is not clinically relevant and because drainage can help to avoid the spread of inflammation.3,24,25 The indications for surgical incision and drainage, however, remain controversial (especially for cellulitis). Different management of cellulitis is based on the preantibiotic era, when it was believed that surgical intervention could worsen the condition.25 Bakir et al7 used only antibiotic treatment primarily for cellulitis and surgical drainage for abscesses. Surgical exploration may be required if there is airway compromise, clinical signs of sepsis, or if there is poor response to antibiotic treatment within the first 48 hours.8,12
Compared with extraoral drainage, the advantages of intraoral drainage are better cosmetic outcome, easier postoperative care, and no risk of injury to surrounding nerves.26 Safety and efficacy of an intraoral approach have been confirmed by Amar and Manoukian27 as well as Choi et al28 in their studies of patients whose parapharyngeal abscesses were drained successfully in this way. Only 4 patients (3.1%) in the current study, however, had intraoral drainage combined with extraoral drainage, and 3 of them had an infection in the parapharyngeal space. Patients with an infection in the parapharyngeal space also had an infection in other spaces. With regard to MSI around the jaws (eg, infections in submandibular and submental spaces), extraoral drainage could make drainage and irrigation easier without the risk of fistula formation, blunting of the vestibular sulcus, difficulty in early feeding, or injury to the mental nerve.26
Peters et al20 showed that the underlying medical condition and location of the infection to be the best predictors of the duration of hospital stay. White blood cells count and temperature were important indicators for the severity of infection in the current study. Wang et al2 reported that the WBC count upon hospital admission appears to have a positive correlation with the duration of hospital stay. Rao et al29 reported that DM patients had a significantly longer duration of hospital stay. Univariate logistic regression analyses in the current study, however, failed to confirm those findings.
Diabetes mellitus,1,30 multiple-space infection,3,30 WBC count upon hospital admission ≥15.0 × 109/L,3 age ≥ 65 years,1 self-medication,1 respiratory difficulties,1 and temperature upon hospital admission ≥39.0°C1 have been noted to be significantly associated with life-threatening complications. Multivariate logistic regression analysis in the current study suggested that NEUT% upon hospital admission was an independent predictor of life-threatening complications, and that NEUT% ≥ 85.0% upon hospital admission was closely associated with MSI severity. Univariate or multivariate logistic regression analyses in the current study failed to confirm that DM, multiple-space infection, temperature upon hospital admission ≥39.0 °C, and WBC count upon hospital admission ≥15.0 × 109/L were independent predictors of life-threatening complications. It has been shown that well-managed DM can lead to a prognosis similar to that of non-DM individuals with a similar severity of MSI.29,31We must also pay more attention to patients with underlying systemic disease, and administration of antibiotics may be different for patients who do not have an underlying systemic disease.21 White blood cell count may be associated with the response to the treatment of infection, but may not be associated with the severity of infection.32
CONCLUSIONS
Although the prevalence and complication incidences of MSI has decreased with advancement of diagnostic techniques, availability of effective antibiotics and improvement in oral hygiene, MSI remain potentially life threatening. Even though adequate antibiotic therapy and incision and drainage of abscess were given, patients with NEUT% upon hospital admission ≥85.0% carry a higher risk of life-threatening complications, and the treatment strategy of these patients should be aggressive.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Zhang C, Tang Y, Zheng M, et al. Maxillofacial space infection experience in West China: a retrospective study of 212 cases. Int J Infect Dis 2010; 14:e414–e417. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Ahani A, Pogrel MA. A five-year retrospective study of odontogenic maxillofacial infections in a large urban public hospital. Int J Oral Maxillofac Surg 2005; 34:646–649. [DOI] [PubMed] [Google Scholar]
- 3.Mathew GC, Ranganathan LK, Gandhi S, et al. Odontogenic maxillofacial space infections at a tertiary care center in North India: a five-year retrospective study. Int J Infect Dis 2012; 16:e296–e302. [DOI] [PubMed] [Google Scholar]
- 4.Steiner M, Grau MJ, Wilson DL. Odontogenic infection leading to cervical emphysema and fatal mediastinitis. J Oral Maxillofac Surg 1982; 40:600–604. [DOI] [PubMed] [Google Scholar]
- 5.Colmenero Ruiz C, Labajo AD, Yañez Vilas I, et al. Thoracic complications of deeply situated serous neck infections. J Craniomaxillofac Surg 1993; 21:76–81. [DOI] [PubMed] [Google Scholar]
- 6.Huang TT, Liu TC, Chen PR, et al. Deep neck infection: analysis of 185 cases. Head Neck 2004; 26:854–860. [DOI] [PubMed] [Google Scholar]
- 7.Bakir S, Tanriverdi MH, Gün R, et al. Deep neck space infections: a retrospective review of 173 cases. Am J Otolaryngol 2012; 33:56–63. [DOI] [PubMed] [Google Scholar]
- 8.Eftekharian A, Roozbahany NA, Vaezeafshar R, et al. Deep neck infections: a retrospective review of 112 cases. Eur Arch Otorhinolaryngol 2009; 266:273–277. [DOI] [PubMed] [Google Scholar]
- 9.Huang TT, Tseng FY, Liu TC, et al. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg 2005; 132:943–947. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed Y, Al Dousary S. Deep-neck space abscesses. J Otolaryngol 1996; 25:227–233. [PubMed] [Google Scholar]
- 11.Zakrzewska JM. Women as dental patients: are there any gender differences? Int Dent J 1996; 46:548–557. [PubMed] [Google Scholar]
- 12.Marioni G, Staffieri A, Rinaldi R, et al. Deep neck infection with dental origin: analysis of 85 consecutive cases (2000–2006). Acta Otolaryngol 2008; 128:201–206. [DOI] [PubMed] [Google Scholar]
- 13.Sato FR, Hajala FA, Filho FW, et al. Eight-year retrospective study of odontogenic origin infections in a postgraduation program on oral and maxillofacial surgery. J Oral Maxillofac Surg 2009; 67:1092–1097. [DOI] [PubMed] [Google Scholar]
- 14.Akinbami BO, Akadiri O, Gbujie DC. Spread of odontogenic infections in Port Harcourt, Nigeria. J Oral Maxillofac Surg 2010; 68:2472–2477. [DOI] [PubMed] [Google Scholar]
- 15.Flynn TR, Shanti RM, Levi MH, et al. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg 2006; 64:1093–1103. [DOI] [PubMed] [Google Scholar]
- 16.Yonetsu K, Izumi M, Nakamura T. Deep facial infections of odontogenic origin: CT assessment of pathways of space involvement. AJNR Am J Neuroradiol 1998; 19:123–128. [PMC free article] [PubMed] [Google Scholar]
- 17.Li XP. Analysis and suggestion to antibiotics abuse (In Chinese). Med Philos 2005; 26:20–44. [Google Scholar]
- 18.Li H, Zeng XJ. Epidemiological study on self-medication in China (In Chinese). Chinese J Epidemiol 1996; 16:55–58. [Google Scholar]
- 19.Bridgeman A, Wiesenfeld D, Hellyar A, et al. Major maxillofacial infections. An evaluation of 107 cases. Aust Dent J 1995; 40:281–288. [DOI] [PubMed] [Google Scholar]
- 20.Peters ES, Fong B, Wormuth DW, et al. Risk factors affecting hospital length of stay in patients with odontogenic maxillofacial infections. J Oral Maxillofac Surg 1996; 54:1386–1391. [DOI] [PubMed] [Google Scholar]
- 21.Wang LF, Kuo WR, Tsai SM, et al. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol 2003; 24:111–117. [DOI] [PubMed] [Google Scholar]
- 22.Kirse DJ, Roberson DW. Surgical management of retropharyngeal space infections in children. Laryngoscope 2001; 111:1413–1422. [DOI] [PubMed] [Google Scholar]
- 23.Santos Gorjón P, Blanco Pérez P, Morales Martín AC, et al. Deep neck infection. Review of 286 cases. Acta Otorrinolaringol Esp 2012; 63:31–41. [DOI] [PubMed] [Google Scholar]
- 24.Gupta M, Singh V. A retrospective study of 256 patients with space infection. J Maxillofac Oral Surg 2010; 9:35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uluibau IC, Jaunay T, Goss AN. Severe odontogenic infections. Aust Dent J 2005; 50:S74–81. [DOI] [PubMed] [Google Scholar]
- 26.Ardehali MM, Jafari M, Hagh AB. Submandibular space abscess: a clinical trial for testing a new technique. Otolaryngol Head Neck Surg 2012; 146:716–718. [DOI] [PubMed] [Google Scholar]
- 27.Amar YG, Manoukian JJ. Intraoral drainage: recommended as the initial approach for the treatment of parapharyngeal abscesses. Otolaryngol Head Neck Surg 2004; 130:676–680. [DOI] [PubMed] [Google Scholar]
- 28.Choi SS, Vezina LG, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otolaryngol Head Neck Surg 1997; 123:1271–1275. [DOI] [PubMed] [Google Scholar]
- 29.Rao DD, Desai A, Kulkarni RD, et al. Comparison of maxillofacial space infection in diabetic and nondiabetic patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:e7–e12. [DOI] [PubMed] [Google Scholar]
- 30.Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis 2009; 13:327–333. [DOI] [PubMed] [Google Scholar]
- 31.Peralta G, Sanchez MB, Roiz MP, et al. Diabetes does not affect outcome in patients with Enterobacteriaceae bacteremia. BMC Infect Dis 2009; 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storoe W, Haug RH, Lillich TT. The changing face of odontogenic infections. J Oral Maxillofac Surg 2001; 59:739–748. [DOI] [PubMed] [Google Scholar]


