Abstract
The impact of psychosocial status at onset of antiretroviral therapy on changes in quality of life (QOL) and subjectively rated health (SRH) among adults on highly active antiretroviral therapy (HAART) in resource-limited settings is poorly understood. Therefore, we evaluate the association between stigma, anxiety, depression, and social support and change in QOL and SRH in HIV-infected Ugandan adults during an 18-month period.
Psychosocial indicators were assessed at enrollment using structured questionnaires. QOL and SRH measures were assessed at months 0, 6, 12, and 18 using the Medical Outcomes Survey-HIV. Linear mixed models determined risk estimated differences in QOL and SRH in relation to quartiles of each psychosocial status indicator. Repeated measures generalized estimating equations modeling was implemented to assess differences in likelihood of improved versus nonimproved SRH during follow-up.
QOL scores and SRH improved significantly for all participants over 18 months (P < 0.0001). The gain in QOL increased dose-dependently as baseline depressive symptoms (time∗depression P < 0.001) and anxiety levels (time∗anxiety P < 0.001) declined. Lower social support was associated with worse QOL at baseline (P = 0.0005) but QOL improvement during follow-up was not dependent on baseline level of social support (time∗social support P = 0.8943) or number of stigmatizing experiences (time∗stigma P = 0.8662). Psychosocial determinants did not predict changes in SRH in this study.
High levels of depression and anxiety symptoms at HAART initiation predicts lower gains in QOL for HIV-positive patients for as long as 18 months. Long-term QOL improvements in HIV-infected adults may be enhanced by implementation of psychosocial interventions to reduce depression and anxiety in HIV-infected adults.
INTRODUCTION
An estimated 25 million people were living with human immunodeficiency virus (HIV) in Sub-Saharan Africa (SSA) in 2013 contributing to over 70% of the HIV burden worldwide.1 The expansion of access to highly active antiretroviral therapy (HAART) among HIV-infected persons in resource-limited settings has been a key factor in prolonging life. HAART has been delivered to 65% of people who are eligible2 and has added over 9 million life-years in SSA.3 Uganda, a country in the region with a generalized HIV epidemic, has an estimated prevalence of 7.3% with the burden shifting from 30 to 34 year-olds to persons 35 years of age and older.4 Thus, as patients age with chronic HIV, survival at high levels of functioning has become of central importance in the clinical management of HIV.
Patients with chronic HIV navigate a host of physical (pain, energy, and vulnerability to infection), social (connections with friends or family and stigma), and emotional factors (problem-solving, concentration, and stress) that impact daily life. In the long-term, maintenance of optimal physical, social, and emotional health outcomes in this population may require reliance on psycho-spiritual strength or resiliency for coping, robust social support from friends, family and providers, compassionate care at the interface of health care delivery, and positive-reframing for self-management.5 High rates of psychosocial attributes such as anxiety, depression, and stigma are reported by HIV-infected patients regardless of race, sex, or sexual orientation and may affect long-term functional capacity in these populations.6
Among Brazilian people living with HIV (PLH), ease of performing activities of daily living, higher treatment adherence, physical activity, ability to self-care, and adequate social interaction have been associated with improved self-rated health (SRH) whereas intense feelings of worry and anxiety were associated with worse SRH.7 Similarly, higher levels of social support were associated with increased overall quality of life (QOL) in American men with HIV8 while depressive symptoms predicted worse role and emotional functioning scores, and general health outcomes overtime.8,9 The paucity of data on psychosocial determinants of QOL improvement in adults with chronic HIV in SSA has been recognized.6 Moreover, an analysis of long-term changes in QOL and SRH in patients on HAART in resource-limited settings is warranted. As access to HAART continues to expand in SSA, this information will be useful in identifying modifiable social barriers that may drive the persistence of health disparity among HIV-infected adults linked to clinical care. This study is designed to assess the impact of psychosocial determinants measured at enrollment (social support, anxiety, depression, and stigma) on change in QOL and SRH over 18 months among PLH receiving HAART in urban Uganda. We hypothesize that patients with high levels of social support and low levels of anxiety, depression, and stigma will realize greater improvements in SRH and QOL over 18 months on HAART.
METHODS
Study Population and Design
Data were analyzed from the Trial of Vitamins study, a randomized, double-blind, placebo-controlled trial designed to assess whether one recommended dietary allowance dose of multivitamin supplements (vitamins B-complex, C, and E) would improve immune function, weight gain, and overall QOL in PLH at least 18 years of age in Kampala, Uganda. Eligible subjects had to meet the following criteria: HIV-positive status, aged 18 years or more, initiating antiretroviral therapy during randomization or having been on HAART for 6 months or less, no intention of migrating or relocating more than 20 km outside of the Infectious Disease Institute HIV care clinic during the next 18 months, agree to allow home and follow-up visits, and provide written informed consent. Trial of Vitamins study design is presented in detail elsewhere.10
Measures
Primary Determinants
Four psychosocial factors were measured at baseline:
Cumulative stigma was defined as the patient's total score in response to questions on the following experiences since HIV diagnosis, with responses coded as reported “yes” (score = 1) versus “no” (score = 0): teased, insulted, or sworn at; loss of respect/standing with family/community; property loss; spousal abandonment; loss of customers or job; family abandonment/forced banishment to village/rural area; exclusion from social gatherings; and children taken away. For analytic purposes, the sum of stigmatizing experiences was categorized as 0, 1, and 2 or more.
Depressive symptoms were defined as the patient's total score to the following: lack of energy; loss of sexual interest/pleasure; loss of appetite; future hopelessness; feelings of loneliness; excessive worry; feeling that everything is an effort; and feelings of worthlessness. For each depression and anxiety symptom, severity was graded as: not at all (score = 0), a little (score = 1), quite a bit (score = 2), and extremely (score = 3). Anxiety level was defined by patient report of the following feelings: scared suddenly without reason; faintness, dizziness or weakness; nervousness or shakiness inside; trembling; tension; or restlessness. For each depression and anxiety symptom, severity was graded as: not at all (score = 0), a little (score = 1), quite a bit (score = 2), and extremely (score = 3).10 Social support was defined as receipt of the following: visits from friends and family; advice about important things in life; ability to talk to trusted person about personal/work problems; help with money during emergencies; and assistance when sick and love/affection. For social support components, patients rated support adequacy as: never (score = 0), much less than I would like (score = 1), less than I would like (score = 2), and as much as I would like (score = 3).10
Confounders: Clinical, Socio-Demographic, and Behavioral Characteristics
To determine CD4-cell count, an FACS Calibur flow cytometer (Becton-Dickinson, San Jose, CA) was used to measure absolute T-cell lymphocyte CD4 counts in cells/microliter. Body mass index (BMI) was calculated as the ratio of weight and the height squared for each participant. BMI values were used to define underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI >= 30).11
Age was measured in years and categorized as: ≤25, 26–35, 36–45, and 46+ years. Socioeconomic status was measured by years of education analyzed in 2 categories: above elementary versus ≤elementary educational level. Independent income source was included as an additional binary measure of SES based on response to the question “do you have your own income?” Employment status was defined by self-identification as unemployed, unskilled laborer, engagement in informal income generating activity (e.g., roadside vendor who sells inexpensive handicrafts or food items), driver/skilled laborer, and professional occupations (includes office worker, police, professional, bus owner).
Marital status was coded as: single/never married (reference for all analyses), married monogamously, married polygamously, cohabiting, divorced/separated, or widowed. Biological sex was defined as male or female with males the reference category in all analyses. History and current use of alcohol and cigarette smoking were self-reported by patients. For analytic purposes current versus no use of alcohol and ever versus never smoking status were the categories used.
Outcome Variables
Two health indicators (QOL and SRH) were measured at baseline as well as 3, 12, and 18 months of follow-up.
1. SRH: We defined SRH in line with extant literature12,13 by patients rating their overall health on a 5-point ordinal scale as excellent (score = 5), very good (score = 4), good (score = 3), fair (score = 2), or poor (score = 1) relative to an adult of same age and sex in their community. SRH was analyzed as a dichotomous outcome with low consisting of poor/fair and high including good/very good/ excellent, to accommodate the distribution of SRH rankings in our sample.
2. QOL: QOL was assessed with the Medical Outcomes Study HIV Health Survey (MOS-HIV) translated and culturally adapted for the study area.14,15 It includes 10 subscales: perceived health (5 items), pain (2 items), overall QOL (1 item), health transition (1 item), role functioning (2 items), social functioning (1 item), physical functioning (6 items), cognitive functioning (4 items), health distress (3 items), and energy (4 items). Variables were coded such that higher scores corresponded to better QOL. Within each subscale, a score was calculated as the sum of patient responses to individual questions. An overall QOL score included the sum of all subscale scores. We linearly transformed the overall score so that the highest score would be 100.
Statistical Analysis
The summed composite of depressive symptoms, anxiety, and social support were categorized based quartiles of respective score distribution in the sample. The 3 lower quartiles were each compared to the 4th quartile. QOL was analyzed as a linear outcome variable while SRH was evaluated as a dichotomous endpoint.
First, descriptive analyses were implemented to describe the socio-demographic, clinical, and behavioral characteristics of the study sample at enrollment by high versus low QOL and SRH. We estimated differences in the relative proportion or means of respective covariates using Chi-square or t-tests, respectively. All variables associated with respective outcomes at P of less than 0.2 were considered candidate confounders and further evaluated in multivariable analyses.
Secondly, we determined the bi-annual temporal trend in QOL and SRH scores from enrollment through month 18 of follow-up. Then we implemented 2 primary sets of analyses to determine whether the rate of change in QOL and high versus low SRH ratings varied by baseline psychosocial factors. To determine psychosocial status-related differences in QOL, we implemented linear repeated measures analyses using the SAS Proc Mixed procedure. We calculated baseline psychosocial status related differences in rate of QOL increase and corresponding 95% confidence intervals. We assumed an unstructured covariance matrix to address repeated QOL assessment within study participants. To assess differences in likelihood of improved versus nonimproved SRH during follow-up, we implemented repeated measures generalized estimating equations modeling. We assumed a binomial distribution for the dichotomous response and specified a logit link. From this model, the odds of high versus low SRH were determined in relation to baseline psychosocial predictors. Multivariable models adjusted for an extensive array of socio-demographic, behavioral, and clinical characteristics if warranted based on bivariate analyses (i.e., if P < 0.2). All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Ethical Approval
The parent study was approved by the Scientific Review Committee of the Infectious Diseases Institute at Makerere University College of Health Sciences; and the Institutional Review Boards of Harvard School of Public Health and that of Makerere University School of Public Health. The study was registered by the Uganda National Council for Science and Technology (UNCST).
RESULTS
Baseline Characteristics
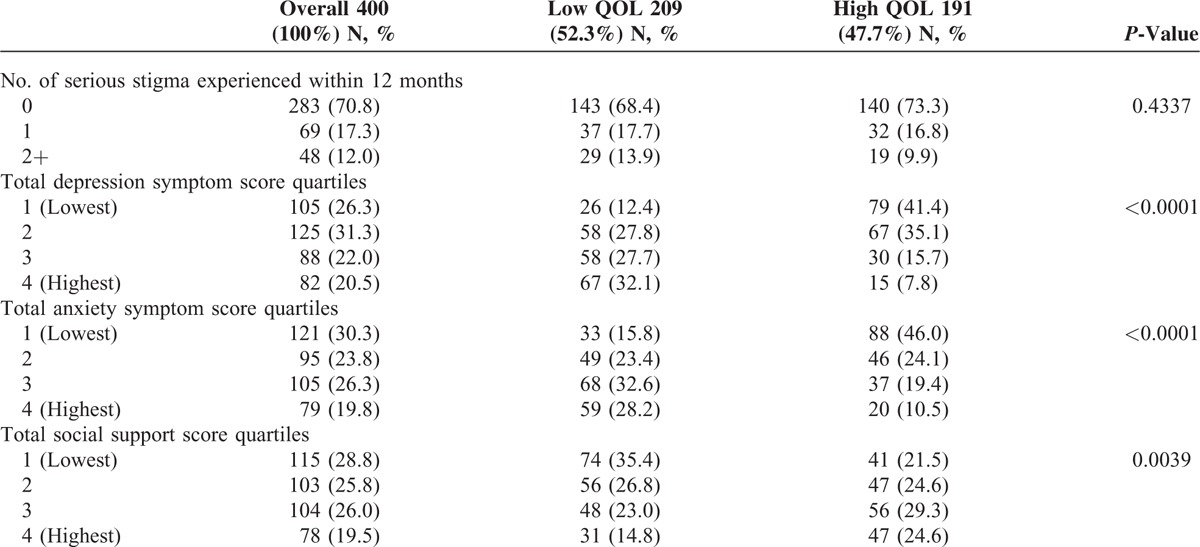
All 400 participants enrolled in the parent trial were included in this analysis. Participants were predominantly female (69.2%), aged 30 to 39 (45.2%), divorced or separated (30.8%), had their own income (74%), had an informally generating income (32%), and less than an elementary education (41%). History of cigarette smoke, current alcohol consumption, and multivitamin use were all low (17.5%, 20.2%, and 22%) at trial enrollment. Fifty percent (n = 200) were HAART naive. The average BMI and CD4 count at enrollment were 23.8 (SD = 4.4) kg/m2, and 148.9 (SD = 95.9) cells/μL, respectively. Over half of the sample experienced low QOL (52.3%) and poor/fair SRH (54.5%) at baseline (Tables 1 and 2).
TABLE 1.
Socio-Demographic Characteristics of Participants, by QOL at Baseline
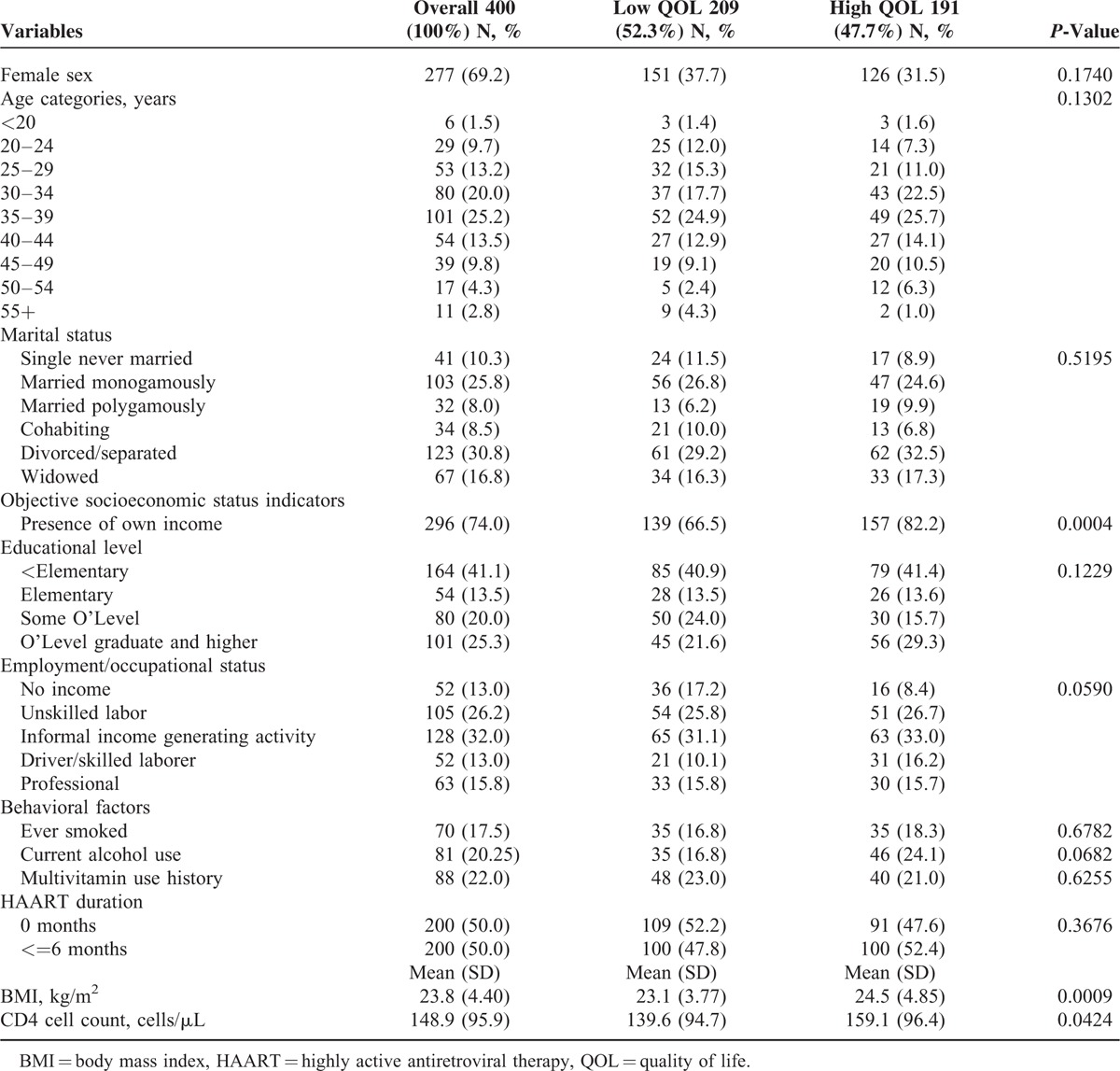
TABLE 2.
Baseline Sample Description According to Psychosocial Status Indicators – Overall and Within Strata of High Versus Low Quality of Life (QOL)
QOL and SRH Trends Overtime
The number of participants present at months 0, 6, 12, and 18 were 400, 380, 369, and 366, respectively, reflecting a 91.5% rate by study end. The greatest improvement occurred between baseline and 6 months for mean QOL scores (6.2 points) and high-SRH (29% difference). After 6 months, mean QOL and percent classified as high SRH was sustained over 18 months.
Effect of Baseline Psychosocial Status on QOL
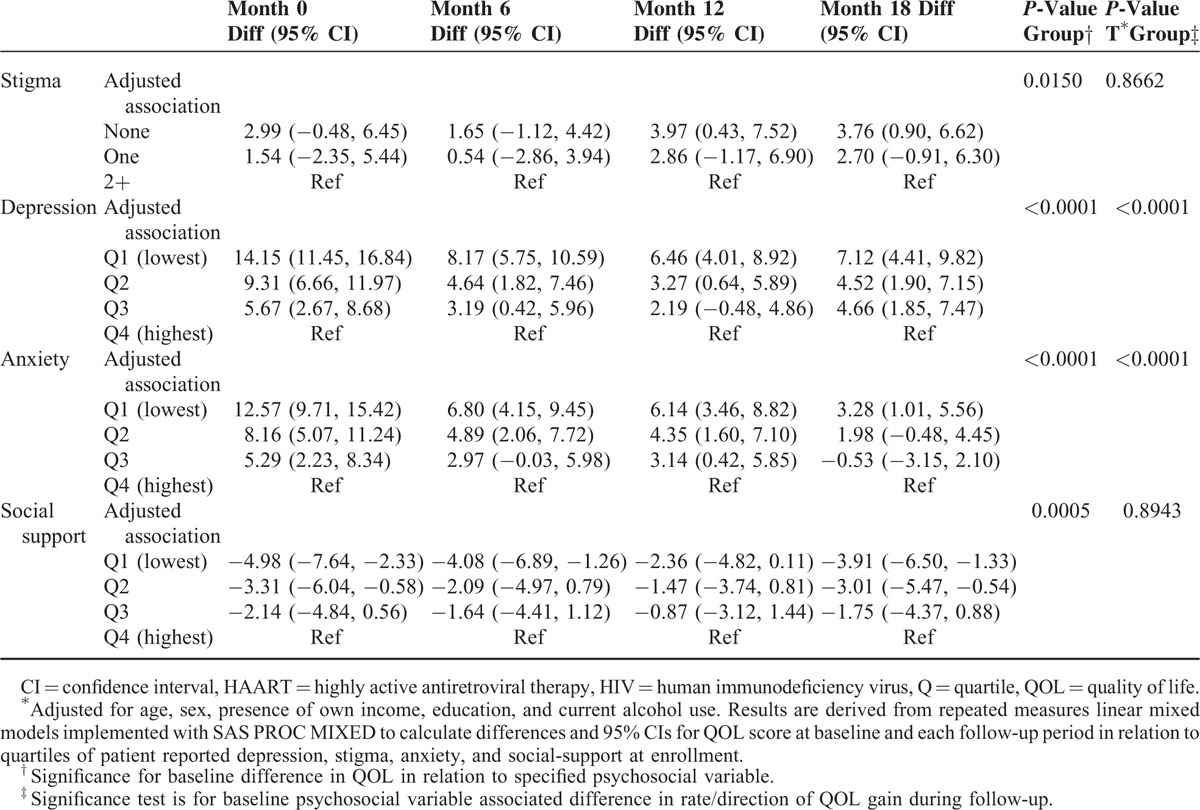
Table 3 shows the difference in the rate of patients’ QOL overtime within each psychosocial determinant level for those with lesser amounts of stigma compared to 2 or more events and the 3 lower quartiles for depression, anxiety, and social support compared to the highest amount. Adjusted models controlled for age, sex, presence of own income, education, and current alcohol use. The change in the rate of QOL declined dose-dependently and significantly as stigmatizing events increased (P-value = 0.0150). At all follow-up periods, low stigma was associated with QOL advantage with significant differences evident for patients reporting none versus 2 or more stigmatizing events at 12 (QOL difference = 4.3, 95% CI: 0.7–7.9) and 18 (QOL difference = 4.1, 95% CI: 1.1–7.5) months. Similarly, improvement in QOL declined dose-dependently and significantly at each follow-up period as depression and anxiety worsened (time∗group P < 0.0001). Overall, the disadvantage of lower QOL for patients with high depression and high anxiety at HAART onset was observed at 18 months of follow-up. Finally, patients reporting lower social support had dose-dependently and significantly worse QOL at baseline (P = 0.0005). However, individuals reporting lower levels of social support had lower QOL scores on average at all follow-up intervals. By month 18, the QOL scores were significantly lower for patients in the least (QOL difference = −3.9, 95% CI: −6.5, −1.3) and 2nd lowest quartile (QOL difference = −3.0, 95% CI: −5.5, −0.5) of social support compared to the highest quartile. Finally, the rate of QOL improvement during follow-up did not differ significantly by baseline level of social support (time∗group P = 0.8943) and stigma (P = 0.8662).
TABLE 3.
Baseline Psychosocial Status in Relation to Change in Quality of Life Over 18 Months Follow-Up, Among HIV Infected Adults Starting HAART in Kampala, Uganda∗
Effect of Baseline Psychosocial Status on SRH
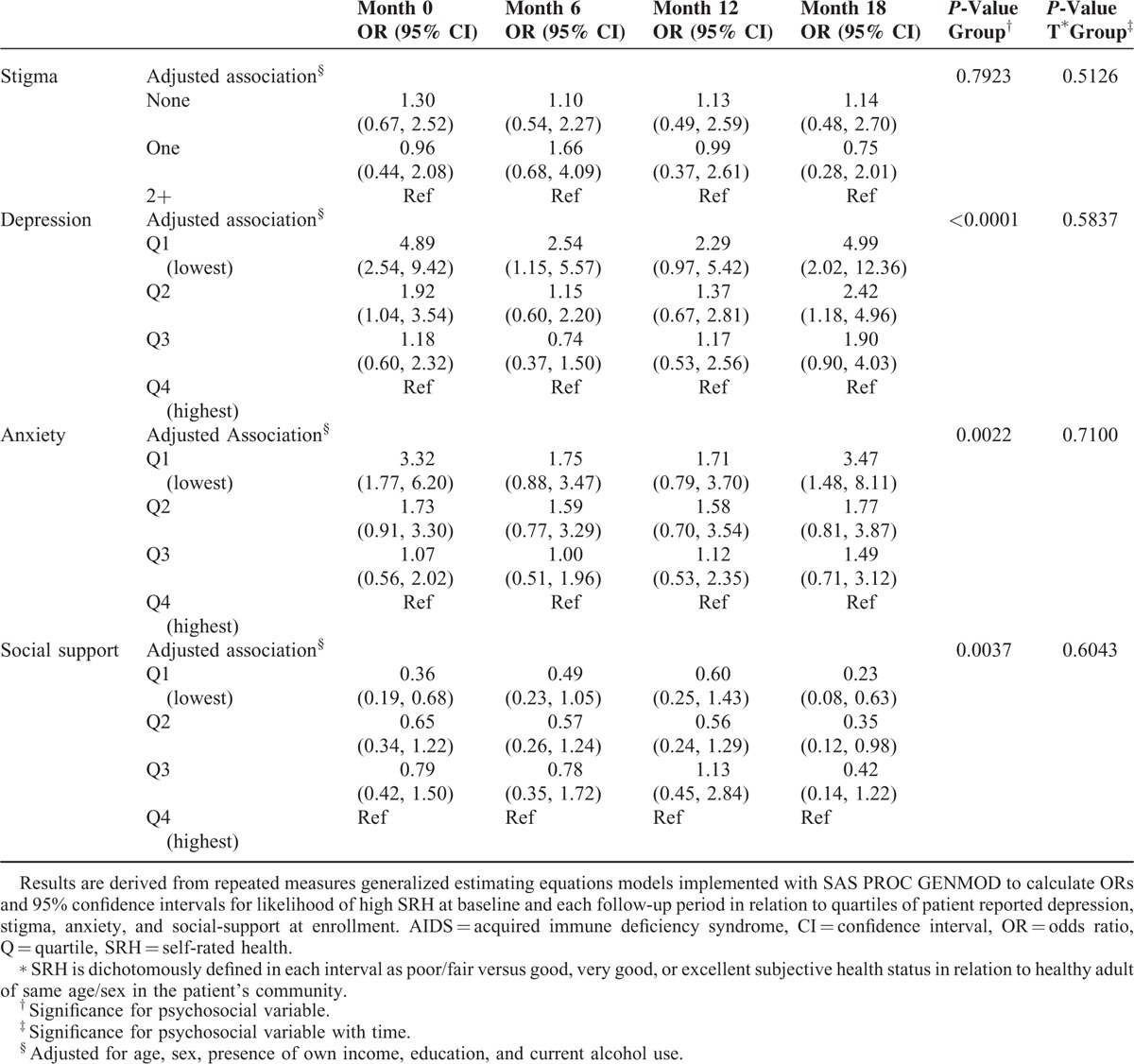
The likelihood of high SRH at baseline and overtime in relationship to baseline psychosocial determinants is shown in Figure 1. The odds of a high versus low SRH at baseline increased dose-dependently for participants in the lower quartiles of depressive symptoms (P < 0.0001) and lower quartiles of anxiety (P = 0.002). By study end, the difference in odds of high SRH was significantly higher for patients in the lowest versus highest quartile of anxiety (OR = 3.5, 95% CI: 1.5, 8.1), and the 2 lowest versus highest quartiles of depressive symptoms (Q1 vs Q4: OR = 5.0, 95% CI: 2.0, 12.4). Similarly, as baseline quartiles of social support decreased, the odds of a high versus low reported health status declined dose-dependently and significantly (P = 0.0037). By study end, however, participants in the 2 lowest versus highest quartile of SRH (Q1 vs Q4, OR = 0.23, 95% CI: 0.08, 0.63) were at significantly lower odds rating their health as high compared to participants reporting the highest level of social support at enrollment. Overall, associations between psychosocial variables and high versus low SRH were not significant overtime (Table 4).
FIGURE 1.
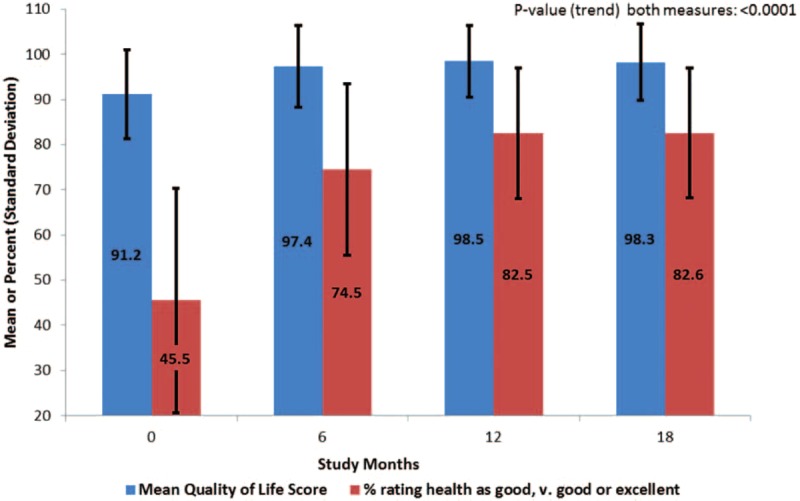
Mean quality of life score and percent high self-rated health scores at months 0, 6, 12, and 18 among adult human immunodeficiency virus (HIV)-infected patients from Kampala, Uganda.
TABLE 4.
Baseline Psychosocial Status and Likelihood of High Self-Rated Health∗ Over 18 Months Among Adult AIDS Patients Starting HAART in Kampala, Uganda
DISCUSSION
We demonstrate that the average QOL score at enrollment increased with social support score but declined with increasing number of reported stigmatizing events, depressive symptoms, and anxiety level. Patients reporting lower social support levels at enrollment were less likely to subjectively rate their health as high. Conversely participants with the lower levels of depressive symptoms and anxiety at enrollment were more likely to highly rate their health and had higher QOL scores. These observations are in support of similar cross-sectional reports among HIV-infected adults from India and Ethiopia.16,17 The depression-related differences in QOL we observe at enrolment is consistent with a previous report among Ugandan PLH.18
As expected, we found that QOL scores and participant subjective rating of their health increased during follow-up with highest increases observed by the 6th month and maintained over the remaining 12 months of follow-up. This observation is in line with specific investigations among HIV-infected patients from South Africa and Uganda.18–20 Over 18 months of follow-up, patients that reported lower levels of depression and anxiety at HAART initiation experienced dose-dependently greater QOL improvement compared to the most depressed and the most anxious participants. Low levels of social support and the number of stigmatizing experiences were associated with lower QOL scores and lower likelihood of high SRH in some follow-up intervals – particularly when the extremes of respective psychosocial measures are compared. However, the overall trend in the change of QOL and SRH during follow-up did not differ significantly by reported stigma and social support level at participant enrollment.
We demonstrate sustained dose-dependent QOL disadvantage over 18 months of follow-up for patients who began HAART with high levels of depression and anxiety or have high levels of depression and anxiety within 6 months of HAART initiation. Our finding of the inverse association between patient anxiety, depression symptoms, and QOL gain is in line with observations in HIV-infected US populations.21 Our findings also correspond with 1 longitudinal study with 12 months follow-up implemented in rural Uganda among HIV-infected adults with WHO disease stages between 1 and 4 all HAART naive at enrolment.18 We replicate the depression-dependent difference in QOL and confirm that the magnitude of disadvantage may decline with time; however, we also show that the QOL disadvantage associated with high depressive symptoms at HAART initiation does not disappear altogether but persists for as long as 18 months.18 We highlight important differences in samples between these studies. Ours included an urban sample of patients with World Health Organization stage ≥3 disease while Stangl et al18 enrolled rural HIV patients of all WHO disease stages. Advanced HIV disease stage is known to be associated with depression and lower QOL.20 Hence, our sample could be especially vulnerable to depression related reduced QOL during HAART. In addition, some differences in the prevalence of stigma, social support, and their associated relationships with QOL may exist between our urban sample and the rural sample of adults investigated by Stangl et al.22 Long-term prospective studies in both urban and rural settings with repeated assessment of psychosocial status indicators will clarify how psychosocial status evolves during HAART and its impact on patient wellbeing. This information will be of utility for devising targeted interventions to enhance QOL in PLWHA. In this same sample, we previously demonstrated that CD4 cell count increased overtime as patients benefitted from HAART. However, differences in immune recovery persisted for those vitamin D deficient compared to those sufficient at enrollment.23 The present study suggests that in addition to HAART, a multidisciplinary clinical management approach including interventions to reduce micronutrient deficiencies, anxiety, and depression may be important complimentary therapies for holistic management of patients living with chronic HIV infection.
Of note, we find no evidence that the overall trend in likelihood of patients reporting high versus low SRH was dependent on baseline psychosocial measures. The incongruence of associations between psychosocial determinants and QOL and SRH as outcomes was not expected. This divergence could in part be explained by the greater robustness of QOL (a composite measure including several questions) versus SRH which is assessed by a single question as measures of well-being.10,24 Similarly, we were surprized that baseline stigma did not predict the overall trend in rate of change in QOL or SRH during follow-up given the described negative impact of stigma in populations with chronic HIV-infection6,25,26 and its reported high prevalence in Ugandan adults with HIV.27 Our data show that patients reporting none versus 2 or more stigmatizing experiences start treatment with relative advantage that persists and is widened to significance by months 12 and 18. Thus, given HAART, the rate of increase in QOL does not appear to vary by level of reported stigma at beginning of therapy. However, the stigma-related disadvantage in wellbeing at baseline is sustained for as much as 18 months and may continue to contribute to the disparity of patients’ subjective wellbeing.
Our finding that the overall trend in QOL change over 18 months was not significantly dependent on baseline level of social support is inconsistent with prospective findings among HIV infected men from the United States reported8 and with findings from cross-sectional epidemiologic studies implemented in United States, Canada, and Venezuela.28–32 Epidemiologic studies among US veterans have shown that a greater magnitude of the positive impacts of social support on QOL is indirectly exerted by buffering the negative effects of adverse psychosocial determinants such as depression and anxiety on HRQOL.28,32 A greater perception of social support directly and indirectly contribute to improved wellbeing by creating environments that make recipients more likely to engage in behavioral and psychological processes or physical activities33,34 that decrease psychosocial and physiologic stress35 with downstream salutatory impacts on overall QOL. Despite a significant baseline social support dependent change in QOL over 18 months, our data show that the low social-support associated disadvantage present at baseline persisted throughout follow-up for least versus most supported quartiles and suggests that enhancement of social support is likely to contribute to enhancement of overall wellbeing among HIV-infected adults on HAART.
Our study has limitations that must be considered in the interpretation of our data. First psychosocial determinants were only assessed at baseline; thus, significant associations between stigma and QOL may be a result of clinical status improvements (i.e., HAART and social integration) overtime. Thus, the complex relationship between stigma and QOL among PLH was difficult to understand. Specifically, psychosocial determinants were measured by patient report in the context of face-to-face interview with study personnel. Therefore, differential recall of experiences and/or social desirability bias leading to an over reporting or underreporting of psychosocial and behavioral attributes may have occurred.
The prospective nature of this investigation which allows for temporal inference, our use of culturally adapted QOL questionnaire with good psychometric properties for assessment of wellbeing in HIV-positive adults, investigation within a clinically well characterized large sample of HIV-infected adults with up to 18 months of follow-up, and rigorous control for important socio-demographic confounders are key strengths this study. Of importance for the psychosocial measures and outcomes under investigation, we eliminate the confounding effect differential access to healthcare by restricting investigation to patients already connected to care while analytically controlling for baseline health indicators such as nutritional status.
In summary, we report, a dose-dependent and sustained QOL disadvantage over 18 months of follow-up in relation to high depressive symptoms and anxiety in HIV-infected Ugandan adults on HAART at baseline. Interventions such as cognitive behavioral stress management have shown improvement in QOL for African American women living with acquired immune deficiency syndrome36 and have reduced various mental health disorders (anxiety, depression, distress, and fatigue), while improving QOL in white male HIV patients.37 Thus, in an era of long-term survival with chronic HIV, interventions that combine stress management and psychosocial support programs within clinical practice are likely to enhance overall well-being of patients on HAART.
Acknowledgments
The authors thank study participants and their families who participated in the original trial. The authors also acknowledge the support provided by all the Infectious Diseases Institute staff who contributed in various ways to provide support to the conduct of the trial.
Footnotes
Abbreviations: BMI = body mass index, HAART = highly active antiretroviral therapy, PLH = people living with HIV, QOL = quality of life, SRH = self-rated health, SSA = Sub-Saharan Africa.
AEE and MNW contributed equally to this work.
AE, DG, YCM, WWF, and SMK contributed to study design, data interpretation, drafting, and revising of the manuscript for important intellectual content. AE and MNW conceived of the study question, analyzed the data, interpreted data, drafted the manuscript, and revised it critically for important intellectual content. DB contributed to analysis, study design, interpretation of data, and revised the paper critically for important intellectual content. All authors were involved in the final approval of the version to be published.
Research findings reported in this publication were supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD060333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.UN Joint Programme on HIV/AIDS. Regional Fact Sheet 2014: Sub-Saharan Africa. December 2014. [Google Scholar]
- 2.UN Joint Programme on HIV/AIDS. Global report: UNAIDS Report on the Global AIDS Epidemic 2013. December 2013. [Google Scholar]
- 3.UN Joint Programme on HIV/AIDS. Regional Fact Sheet 2012: Sub-Saharan Africa. December 2012. [Google Scholar]
- 4.Uganda AIDS Commission. HIV and AIDS Uganda Country Progress Report 2013. March 31, 2013. [Google Scholar]
- 5.Slomka J, Lim JW, Gripshover B, et al. How have long-term survivors coped with living with HIV? J Assoc Nurses AIDS Care 2013; 24:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whetten K, Reif S, Whetten R, et al. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom Med 2008; 70:531–538. [DOI] [PubMed] [Google Scholar]
- 7.Souza Junior PR, Szwarcwald CL, Castilho EA. Self-rated health by HIV-infected individuals undergoing antiretroviral therapy in Brazil. Cadernos de Saude Publica 2011; 27 Suppl 1:S56–S66. [DOI] [PubMed] [Google Scholar]
- 8.Jia H, Uphold CR, Wu S, et al. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care STDS 2005; 19:395–405. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Johnson L, Ostrow D, et al. Predictors for lower quality of life in the HAART era among HIV-infected men. J Acquir Immune Defic Syndr (1999) 2006; 42:470–477. [DOI] [PubMed] [Google Scholar]
- 10.Guwatudde D, Ezeamama AE, Bagenda D, et al. Multivitamin supplementation in HIV infected adults initiating antiretroviral therapy in Uganda: the protocol for a randomized double blinded placebo controlled efficacy trial. BMC Infect Dis 2012; 12:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavazzotto TG, Brasil MR, Oliveira VM, et al. Nutritional status of children and adolescents based on body mass index: agreement between World Health Organization and International Obesity Task Force. Rev Paul Pediatr 2014; 32:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nwabueze SA, Adogu PO, Adinma ED, et al. Determinants of subjective health status of HIV positive mothers in NAUTH Nnewi. Nigerian J Med 2012; 21:381–386. [PubMed] [Google Scholar]
- 13.Mosack KE, Weinhardt LS, Kelly JA, et al. Influence of coping, social support, and depression on subjective health status among HIV-positive adults with different sexual identities. Behav Med 2009; 34:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stangl AL, Bunnell R, Wamai N, et al. Measuring quality of life in rural Uganda: reliability and validity of summary scores from the medical outcomes study HIV health survey (MOS-HIV). Qual Life Res 2012; 21:1655–1663. [DOI] [PubMed] [Google Scholar]
- 15.Wu AW, Revicki DA, Jacobson D, et al. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res 1997; 6:481–493. [DOI] [PubMed] [Google Scholar]
- 16.Peter E, Kamath R, Andrews T, et al. Psychosocial determinants of health-related quality of life of people living with HIV/AIDS on antiretroviral therapy at Udupi District, Southern India. Int J Prev Med 2014; 5:203–209. [PMC free article] [PubMed] [Google Scholar]
- 17.Mekuria LA, Sprangers MA, Prins JM, et al. Health-related quality of life of HIV-infected adults receiving combination antiretroviral therapy in Addis Ababa. AIDS Care 2015; 27:934–945. [DOI] [PubMed] [Google Scholar]
- 18.Stangl AL, Wamai N, Mermin J, et al. Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care 2007; 19:626–636. [DOI] [PubMed] [Google Scholar]
- 19.Jelsma J, Maclean E, Hughes J, et al. An investigation into the health-related quality of life of individuals living with HIV who are receiving HAART. AIDS Care 2005; 17:579–588. [DOI] [PubMed] [Google Scholar]
- 20.Mutabazi-Mwesigire D, Katamba A, Martin F, et al. Factors that affect quality of life among people living with HIV attending an urban clinic in Uganda: a cohort study. PloS One 2015; 10:e0126810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008; 70:539–545. [DOI] [PubMed] [Google Scholar]
- 22.Heckman TG, Somlai AM, Kalichman SC, et al. Psychosocial differences between urban and rural people living with HIV/AIDS. J Rural Health 1998; 14:138–145. [DOI] [PubMed] [Google Scholar]
- 23.Ezeamama AE, Guwatudde D, Wang M, et al. Vitamin-D deficiency impairs CD4+T-cell count recovery rate in HIV-positive adults on highly active antiretroviral therapy: A longitudinal study. Clin Nutr (Edinburgh, Scotland) 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mast TC, Kigozi G, Wabwire-Mangen F, et al. Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS Care 2004; 16:81–94. [DOI] [PubMed] [Google Scholar]
- 25.Vanable PA, Carey MP, Blair DC, et al. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav 2006; 10:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ammirati RJ, Lamis DA, Campos PE, et al. Optimism, well-being, and perceived stigma in individuals living with HIV. AIDS Care 2015; 1–8. [DOI] [PubMed] [Google Scholar]
- 27.Kuteesa MO, Wright S, Seeley J, et al. Experiences of HIV-related stigma among HIV-positive older persons in Uganda – a mixed methods analysis. SAHARA J 2014; 11:126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia H, Uphold CR, Wu S, et al. Health-related quality of life among men with HIV infection: effects of social support, coping, and depression. AIDS Patient Care STDS 2004; 18:594–603. [DOI] [PubMed] [Google Scholar]
- 29.Bastardo YM, Kimberlin CL. Relationship between quality of life, social support and disease-related factors in HIV-infected persons in Venezuela. AIDS Care 2000; 12:673–684. [DOI] [PubMed] [Google Scholar]
- 30.Cowdery JE, Pesa JA. Assessing quality of life in women living with HIV infection. AIDS Care 2002; 14:235–245. [DOI] [PubMed] [Google Scholar]
- 31.Stewart KE, Cianfrini LR, Walker JF. Stress, social support and housing are related to health status among HIV-positive persons in the deep south of the United States. AIDS Care 2005; 17:350–358. [DOI] [PubMed] [Google Scholar]
- 32.Bekele T, Rourke SB, Tucker R, et al. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care 2013; 25:337–346. [DOI] [PubMed] [Google Scholar]
- 33.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull 1996; 119:488–531. [DOI] [PubMed] [Google Scholar]
- 34.Berkman LF, Glass T, Brissette I, et al. From social integration to health: Durkheim in the new millennium. Social Sci Med (1982) 2000; 51:843–857. [DOI] [PubMed] [Google Scholar]
- 35.Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci 2014; 32:149–162. [DOI] [PubMed] [Google Scholar]
- 36.Lechner SC, Antoni MH, Lydston D, et al. Cognitive-behavioral interventions improve quality of life in women with AIDS. J Psychosom Res 2003; 54:253–261. [DOI] [PubMed] [Google Scholar]
- 37.Scott-Sheldon LA, Kalichman SC, Carey MP, et al. Stress management interventions for HIV+ adults: a meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol 2008; 27:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]


