Abstract
The purpose of this study was to perform a meta-analysis examining the association of isocitrate dehydrogenase (IDH)1/2 mutations with overall survival (OS) and progression-free survival (PFS) in patients with glioblastomas.
Medline, Cochrane, EMBASE, and Google Scholar were searched from inception to January 28, 2015, using combinations of the following keywords: IDH mutation, brain tumor, glioma, glioblastoma, oligodendroglioma, prognosis. Randomized controlled trials, and prospective and retrospective studies of patients with glioblastomas that provided IDH mutation and survival data were included. OS and PFS were used to evaluate the association of IDH1 and IDH1/2 mutations and prognosis. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for OS and PFS were calculated and compared between patients with and without mutations.
Of 165 studies that were identified, 136 nonrelevant studies were excluded. Twenty-nine full-text articles were assessed, and of these, 5 were excluded as they did not provide a quantitative outcome. Therefore, 24 studies were included in the qualitative synthesis. The pooled HR of 0.358 (95% CI 0.264–0.487, P < 0.001) indicated that IDH mutations were associated with better OS. Similarly, the pooled HR of 0.322 (95% CI 0.24200.455, P < 0.001) indicated that IDH mutations were associated with better PFS. When patients were stratified by surgery versus no surgery or IDH1 versus IDH1/2 mutations, the results also indicated that the presence of IDH mutations was associated with better OS and PFS.
The IDH mutations are associated with improved survival in patients with glioblastomas.
INTRODUCTION
Glioblastomas (glioblastoma multiforme [GBM]) are the most common and aggressive malignant brain tumor, with a median survival from diagnosis of approximately 12 to 14 months.1 The majority of glioblastomas (∼90%) occur without evidence of a less malignant precursor lesion (primary glioblastomas) in older patients, whereas secondary glioblastomas progress from low-grade diffuse astrocytoma or anaplastic astrocytoma, and occur in younger patients.2 Secondary glioblastomas have a significantly better prognosis than primary glioblastoma.2
Approximately 70% to 80% of secondary glioblastomas have somatic mutation in the isocitrate dehydrogenase 1 (IDH1) gene, which are absent in primary glioblastoma.3–5 Wild-type IDH1 protein is found in the cytoplasm, peroxisomes, and endoplasmic reticulum, and catalyzes the oxidative decarboxylation of isocitrate to α-ketogluterate.6–8 Mutations in IDH1 associated with glioblastomas map to the highly conserved residue R132 in the enzyme active site, and usually result in an Arg to His substitution, although other substitutions can also occur.8–12 The IDH1 R132 mutation occurs in 55% to 80% of grade II and III oligodendrogliomas and astrocytomas, but is rare in primary glioblastomas.12 To a lesser extent, glial tumors have somatic mutations in the corresponding codon (codon R172) of the IDH2 gene.9 The IDH2 protein has a similar function to IDH1, but is found in the mitochondria. Both the IDH1-R132 and IDH2-R172 mutations are thought to result in an accumulation of the oncometabolite 2-hydroxyglutarate instead of α-ketogluterate.13,14
It is unclear how a tumor's biology is affected by IDH1/2 mutations. IDH1/2 mutations may result in genome-wide epigenetic changes in human gliomas.4 Another hypothesis is that the mutations reduce the capacity of cells to produce NADPH, and consequently lowers the ability of the cell to scavenge oxygen species, making the tumor cells more susceptible to irradiation and chemotherapy. This increased sensitivity to treatments may result in increased patient survival.15
A number of studies have found that IDH1-R132 and IDH2-R172 mutations are linked to the genomic profile of the tumor, and are important prognostic markers in grade II to IV gliomas.16–20 However, other studies have not found an association of IDH1/2 mutations with prognosis in low-grade tumors.18,21 Therefore, the prognostic value of these genetic markers for survival is not clear.
The purpose of the current study was to perform a meta-analysis to examine the association of IDH1/2 mutations with overall survival (OS) and progression-free survival (PFS) in patients with glioblastomas.
METHODS
Literature Search Strategy
This systematic review and meta-analysis was conducted in accordance with the PRISMA guidelines.22 Medline, Cochrane, EMBASE, and Google Scholar were searched from inception to January 28, 2015, using combinations of the following keywords: IDH mutation, brain tumor, glioma, glioblastoma, oligodendroglioma, prognosis. Reference lists of relevant studies were hand-searched. Meta-analyses do not involve humans and do not require Institutional Review Board approval.23
Study Selection and Data Extraction
Inclusion criteria were as follows: randomized controlled trials (RCTs) and prospective and retrospective studies; patients with a malignant brain tumor (glioma, glioblastoma, anaplastic oligodendroglioma, etc); provided IDH mutation data; and contained survival analysis data. Letters, comments, editorials, case reports, proceedings, and personal communications were excluded, as were studies in which no survival analysis was performed. Studies were identified by the search strategy by 2 independent reviewers. When there was uncertainty regarding eligibility, a third reviewer was consulted.
The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of participants in each group, participants’ age and sex, diagnostic criteria, tumor type and World Health Organization (WHO) grade, treatments, and survival data.
Quality Assessment
The quality of the included studies was assessed using the modified 18-items Delphi checklist, which is designed for assessing the quality of single-arm clinical studies.24 The quality assessment was also performed by 2 independent reviewers, and a third reviewer was consulted for any uncertainties.
Outcome Measures and Data Analysis
Overall survival and PFS were used to evaluate the association of IDH1 and IDH1/2 mutations, and prognosis for patients with malignant brain tumors. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for OS and PFS were calculated and compared between patients with and without mutations. Pooled HRs and 95% CIs were calculated for all studies combined, and for given subgroups (e.g., IDH mutation type or surgery vs no surgery). A HR value <1 indicates that mutations may prolong OS or PFS, whereas a HR value >1 indicates the absence of mutations may decrease OS or PFS. A HR value equal to 1 indicates there was no significant association of IDH1 or IDH1/2 mutations with OS or PFS.
Heterogeneity among the studies was evaluated by the Cochran Q and the I2 statistic. A Q statistic, with a P < 0.10, was considered to indicate statistically significant heterogeneity. The I2 statistic indicates the percentage of the observed between-study variability due to heterogeneity rather than chance, and a value >50% was considered to indicate significant heterogeneity. Random-effects models (DerSimonian–Laird method) were used if heterogeneity was detected (I2 > 50% or Q statistics P < 0.1). Otherwise, fixed-effects models (Mantel–Haenszel method) were utilized. Sensitivity analysis was performed using the leave-one-out approach. Publication bias was assessed by constructing funnel plots and by Egger test. The absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution, and a 1-tailed significance level of P > 0.05 (Egger test). All statistical assessments were 2-sided, and a value of P < 0.05 was considered as statistically significant. Statistical analyses were performed using the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ).
RESULTS
Search Results and Study Characteristics
A flow diagram of study selection is shown in Figure 1. A total of 165 studies were identified in the database search. After a review of the abstracts, 136 studies were excluded because they did not match the topic of the current analysis. Thus, 29 full-text articles were assessed for eligibility, and of these, 5 were excluded as they did not provide a quantitative outcome. Therefore, 24 studies were included in the qualitative synthesis.3,13,16–18,21,25–42 The characteristics and populations of the included studies are summarized in Table 1 , and OS and PFS data are summarized in Table 2. Studies that reported median OS or PFS time were not considered for the analysis because most of the included studies were presented as HR. The study by Mukasa et al21 was not included in the analysis because the HRs were reported by tumor stage.
FIGURE 1.
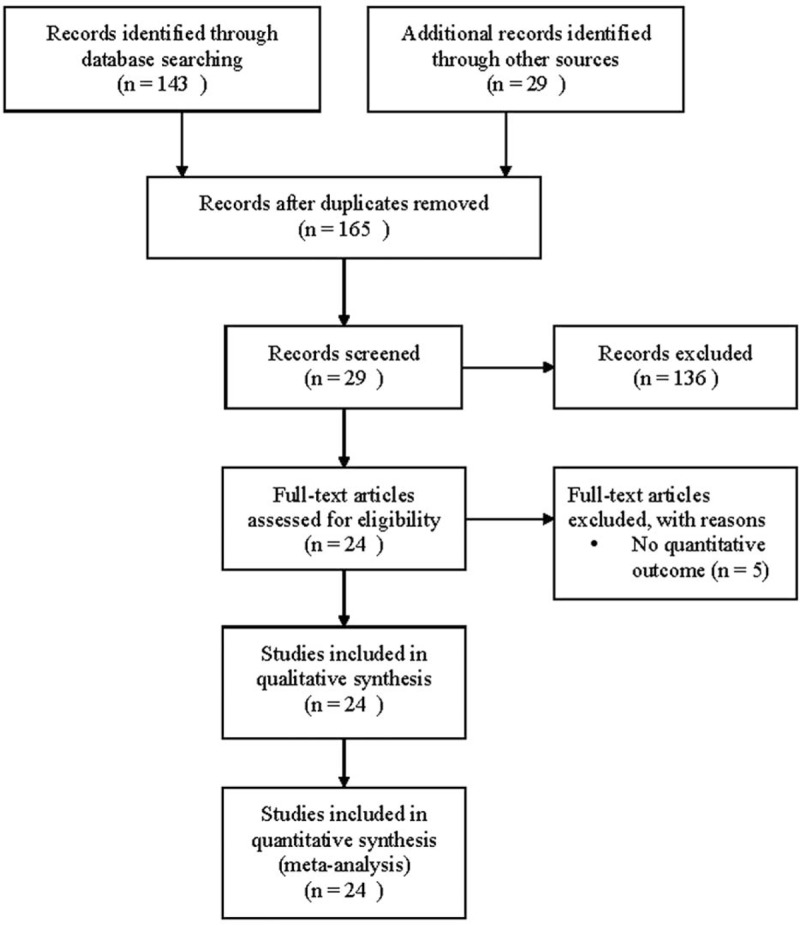
Flow diagram of study selection.
TABLE 1.
Study Characteristics
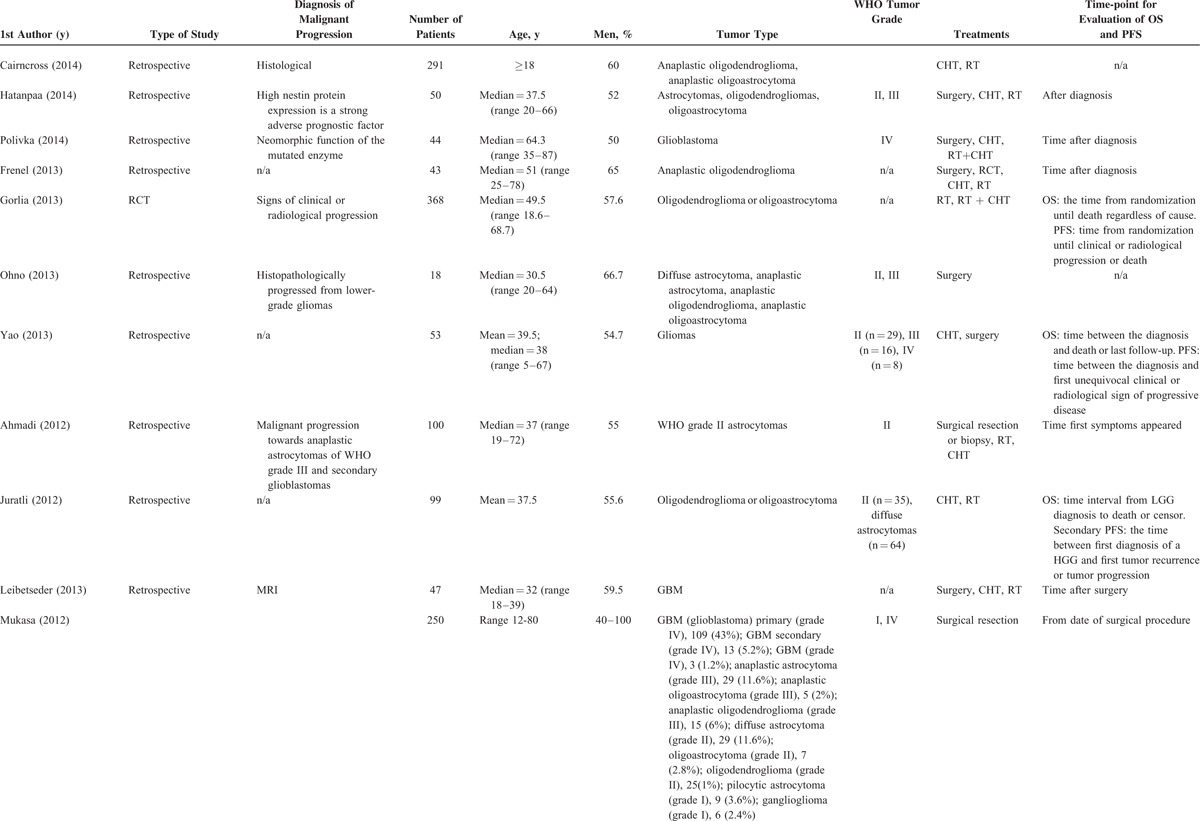
TABLE 1 (Continued).
Study Characteristics
Association of IDH1 or IDH2 Mutations With OS
A total of 15 studies with completed data of OS were included in the analysis.3,13,16,18,25–29,32–34,36,37,42 Significant heterogeneity was noted (I2 = 59.23%, Q statistic = 34.336, P = 0.002); therefore a random-effects model was used. The pooled HR of 0.358 (95% CI 0.264–0.487, P < 0.001) indicated that IDH1 or IDH1/2 mutations were associated with better OS (Figure 2). When patients were stratified by surgery versus no surgery or IDH1 versus IDH1/2 mutations, the results also indicated that the presence of IDH mutations was associated with better OS.
FIGURE 2.
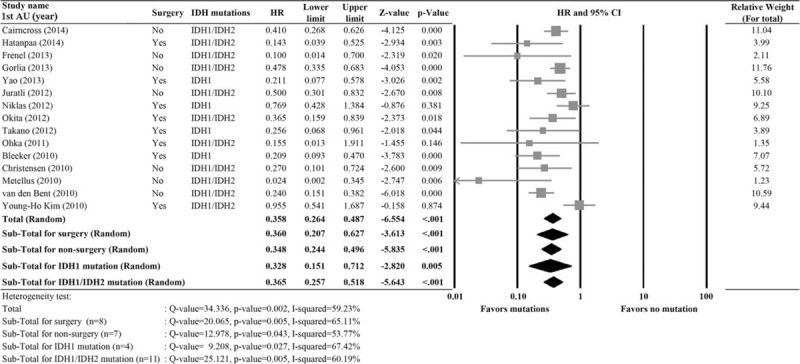
Meta-analysis for the association of IDH1/IDH2 mutations versus overall survival (OS). 1st AU = first author, 95% CI = 95% confidence interval, HR = hazard ratio, IDH = isocitrate dehydrogenase, lower limit, upper limit of HR.
Association of IDH1 or IDH2 Mutations With PFS
A total of 10 studies with completed data of PFS were included in the analysis.13,16,25–29,32,36,37 Significant heterogeneity was noted (I2 = 53.53%, Q statistic = 19.369, P = 0.022); therefore a random-effects model was used. The pooled HR of 0.322 (95% CI 0.242–0.455, P < 0.001) indicated that IDH1 or IDH1/2 mutations were associated with better PFS (Figure 3). When patients were stratified by surgery versus no surgery or IDH1 versus IDH1/2 mutations, the results also indicated that the presence of IDH mutations was associated with better PFS.
FIGURE 3.
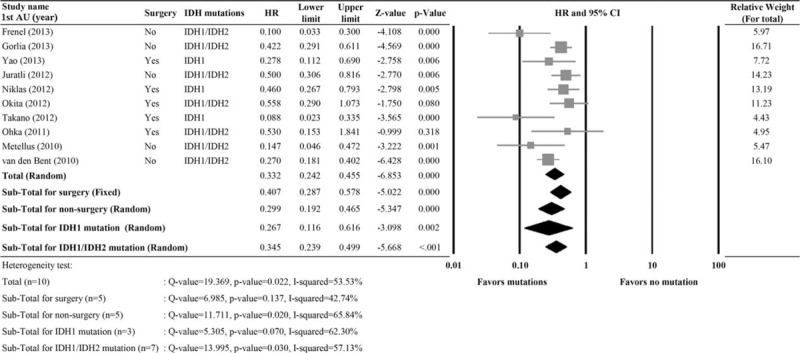
Meta-analysis for the association of IDH1/IDH2 mutations versus progression-free survival (PFS). 1st AU = first author, 95% CI = 95% confidence interval, HR = hazard ratio, lower limit, upper limit of HR.
Sensitivity Analysis
Results of the sensitivity analysis using the leave-one-out approach for OS and PFS are shown in Figure 4. For both OS and PFS, the pooled estimates with each of the studies removed in turn remained statistically significant, indicating that the meta-analysis had good reliability for both measures (HRs for OS: range 0.33–0.38, all P values <0.001; HRs for PFS, range 0.31–0.37, all P values <0.001).
FIGURE 4.
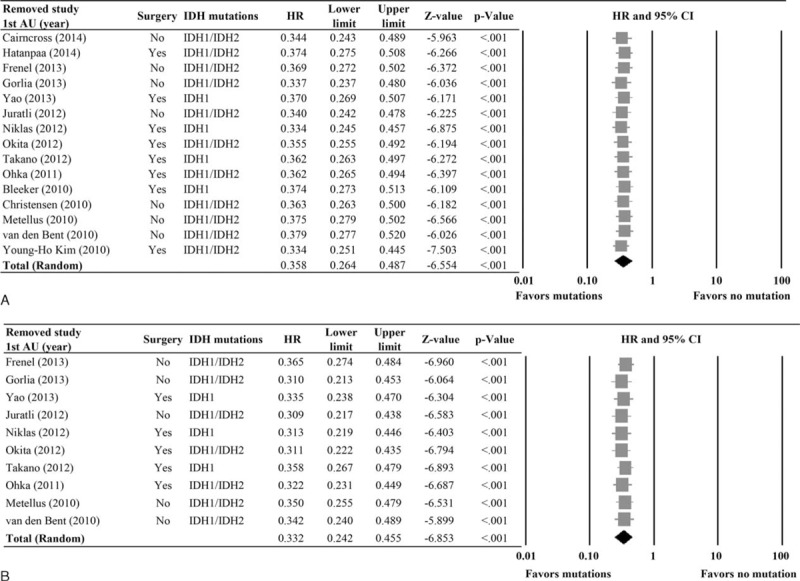
Sensitivity analysis using the leave-one-out approach for (A) overall survival (OS) and (B) progression-free survival (PFS). 1st AU = first author, 95% CI = 95% confidence interval, HR = hazard ratio, IDH = isocitrate dehydrogenase, lower limit, upper limit of HR.
Publication Bias Analysis
Results of the evaluation of publication bias for OS and PFS are shown in Figure 5. For both measures, the funnel plots were symmetric (both P < 0.001; classic fail-safe test). However, Egger test indicated that the intercepts of the funnel plots did not obtain statistical significance (OS: 1-tailed, P = 0.037; PFS: 1-tailed, P = 0.075, respectively). Hence, publication bias may exist with respect to OS.
FIGURE 5.
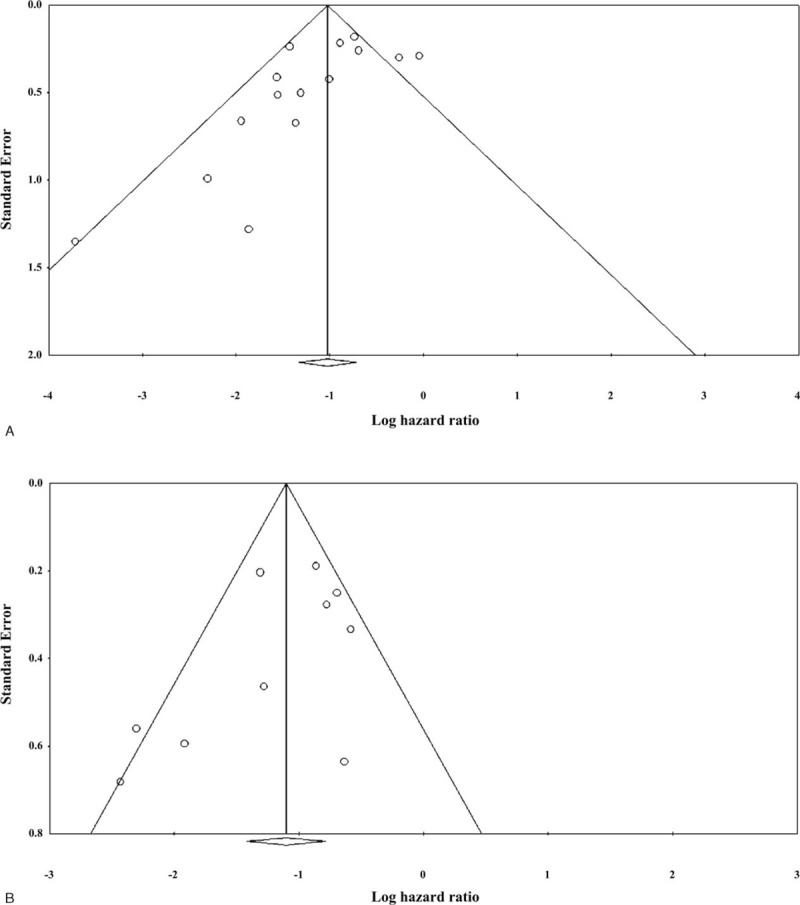
Evaluation of publication bias by funnel plot and the Egger test for (A) overall survival (OS) and (B) progression-free survival (PFS).
Quality Assessment
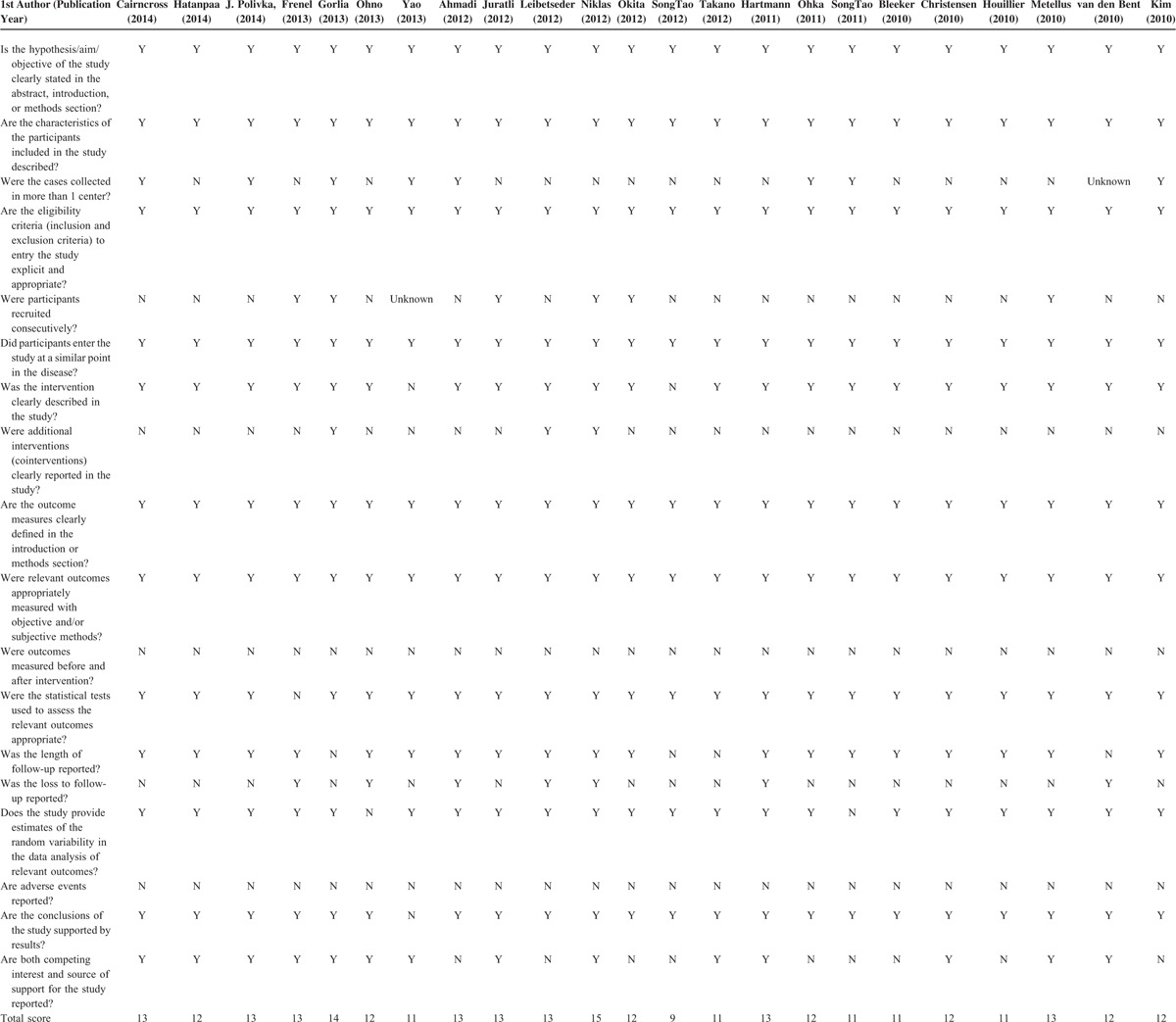
Results of the quality assessment using the modified 18-item Delphi checklist are shown in Table 3. All of the included studies clearly stated the aim of the study in the abstract or introduction, and described the characteristics of the included participants. The eligibility criteria of all the studies were explicit and appropriate, and outcome measures were all well-defined. The final total Delphi checklist scores of the studies ranged from 9 to 15 (maximum possible score of 18). Overall, the results indicate the studies are of good quality.
TABLE 2.
Overall Survival and Progression-free Survival in Patients With and Without IDH1/IDH2 Mutations
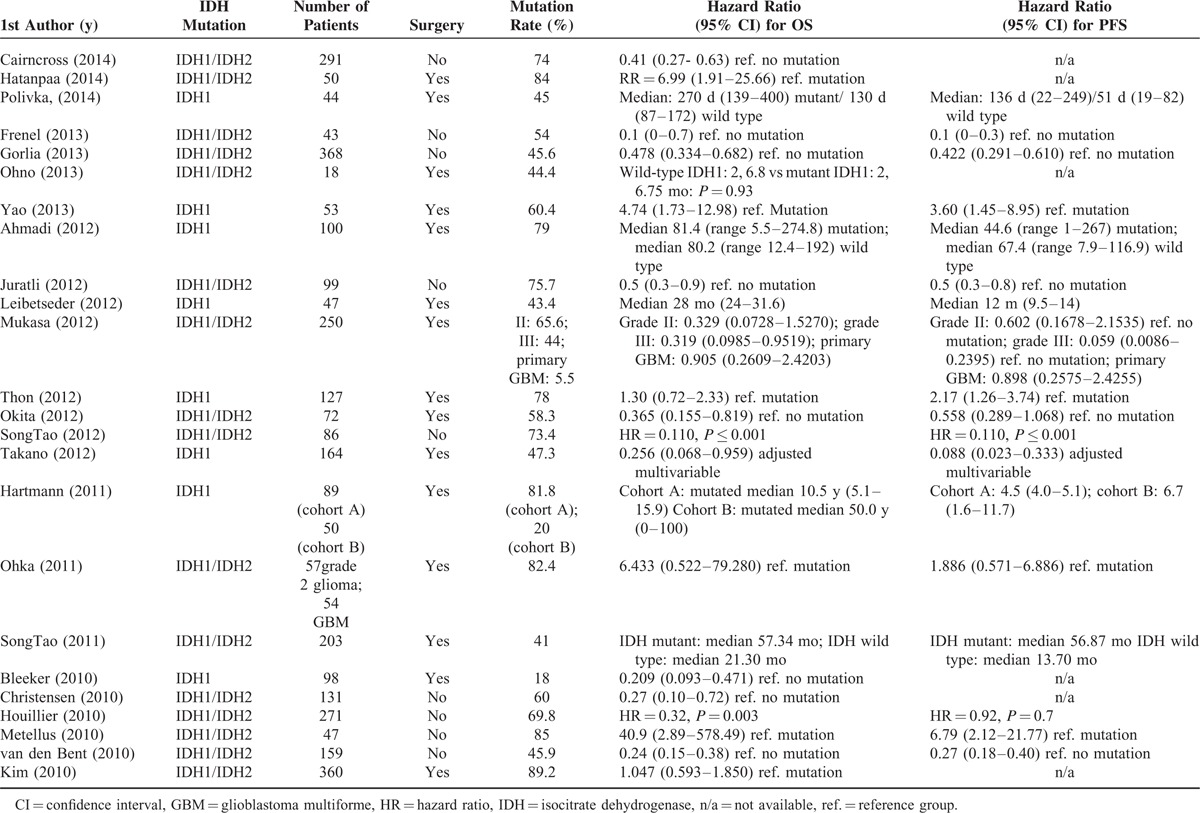
TABLE 3.
Quality Assessment Results
DISCUSSION
The purpose of this meta-analysis was to evaluate the prognostic value of IDH1/2 mutations with respect to OS and PFS in patients with glioblastoma. The results showed that the presence of IDH1/2 mutations was associated with longer OS and PFS, and this result was seen in both patients treated with surgery and those treated nonsurgically (e.g., radiotherapy), as well as in patients with IDH1 and IDH1/2 mutations.
IDH1 mutations have been reported in secondary GBM, diffuse astrocytoma, oligodendrogliomas, anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic oligoastrocytomas, and rarely in primary GBM, and have not been reported in pilocytic astrocytomas, ependymonmas, and medulloblastomas.43 Mutations have also been reported in other cancers including acute myeloid leukemia and colorectal and prostate cancer.43
Prior studies have found that IDH1/2 mutations may influence the prognosis of patients with secondary or greater than grade II gliomas; however, these studies have differed in design and the results have not always been consistent.16–21,44
Evidence has generally shown that IDH1 mutations are associated with improved OS and PFS, particularly in patients with high-grade gliomas.9,13,27 The prognostic value in low-grade gliomas is, however, less clear. For example, Sanson et al19 showed that the IDH1 mutation had a significant prognostic value for OS in gliomas, whereas Kim et al18 reported the IDH1/IDH2 mutation was of no prognostic value in 360 low-grade gliomas. Interestingly, although IDH1 mutations have generally been shown to be a prognostic indicator, their presence is not necessarily predictive of response to therapy.9,13,19,40 Reasons for these findings may have to do with the association of IDH1 mutations with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status.42 For example, Molenaar et al45 reported that the combination of IDH1 mutations and MGMT methylation status predicted survival in patients with glioblastomas better than either IDH1 or MGMT status alone. Though the reasons for the associations between survival, and IDH1 and MGMT methylation status remain to be determined, it has been suggested there may be mechanistic link between IDH1 mutations and MGMT methylation.46
Prior studies have suggested that chemoradiotherapy may be effective for a subset of patients with gliomas, as the addition of procarbazine, lomustine, and vincristine (PCV) chemotherapy to radiotherapy conferred a significant increase in OS and PFS.47,48 Among the studies included in the current analysis, Okita et al28 suggested IDH1/2 mutations were predictive for response to chemoradiotherapy, but not radiotherapy alone in patients with grade II gliomas. However, van den Bent et al13 reported that IDH1 mutations were predictive of both OS and PFS for patients treated with radiotherapy and radiotherapy/PVC. It has also been reported that patients with low-grade gliomas were sensitive to temozolomide.17 In the current meta-analysis, we did not evaluate the predictive value of IDH1/2 mutations with respect to radiotherapy, chemotherapy, or chemoradiotherapy. This was due to the heterogeneity across the studies, and because few studies directly evaluated this question.
Other prior meta-analyses have evaluated the association of IDH mutations and survival in patients with glioblastomas. An analysis by Cheng et al49 included 9 studies with a total of 1669 patients with glioblastomas, and, similar to our results, found that IDH1 mutations were associated with improved OS. Zou et al50 performed a meta-analysis including 12 studies with a total of 2190 patients, and reported HRs for OS and PFS in patients with IDH mutations were 0.33 (95% CI 0.25–0.42) and 0.38 (95% CI 0.21–0.68), respectively, as compared with glioma patients with the wild-type IDH gene. Subgroup analyses based on tumor grade also showed that the presence of IDH mutations was associated with better outcomes.
There are several limitations to this analysis that should be considered when interpreting the results. We did not evaluate whether the histological subtype or tumor grade influenced the association of IDH1/2 mutations with the survival outcomes of patients with secondary GBM. As mentioned above, we also did not evaluate whether the type of treatment regimen influenced the prognostic value of IDH1/2 mutations of patients with secondary GBM. Furthermore, significant heterogeneity was present among the studies for both OS and PFS with respect to tumor type and grade, treatments, method for calculating endpoints, and method for determining the presence of mutations. Publication bias may be present as well, for those studies without significance might not be submitted or published.
CONCLUSIONS
In summary, the results of this meta-analysis indicate that IDH1/2 mutations are associated with improved survival in patients with glioblastomas.
Footnotes
Abbreviations: CI = confidence interval, GBM = glioblastoma multiforme, HR = hazard ratio, IDH = isocitrate dehydrogenase 1, OS = overall survival, PCV = procarbazine, lomustine, and vincristine, PFS = progression-free survival, RCT = randomized controlled trial, WHO = World Health Organization.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Urbańska K, Sokołowska J, Szmidt M, et al. Glioblastoma multiforme: an overview. Contemp Oncol (Pozn) 2014; 18:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013; 19:764–772. [DOI] [PubMed] [Google Scholar]
- 3.Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1 (R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol 2010; 119:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo C, Pirozzi CJ, Lopez GY, et al. Isocitrate dehydrogenase mutations in gliomas: mechanisms, biomarkers and therapeutic target. Curr Opin Neurol 2011; 24:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander BM, Mehta MP. Role of isocitrate dehydrogenase in glioma. Expert Rev Neurother 2011; 11:1399–1409. [DOI] [PubMed] [Google Scholar]
- 6.Koshland DE, Jr, Walsh K, LaPorte DC. Sensitivity of metabolic fluxes to covalent control. Curr Top Cell Regul 1985; 27:13–22. [DOI] [PubMed] [Google Scholar]
- 7.Geisbrecht BV, Gould SJ. The human PICD gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent isocitrate dehydrogenase. J Biol Chem 1999; 274:30527–30533. [DOI] [PubMed] [Google Scholar]
- 8.Margittai E, Banhegyi G. Isocitrate dehydrogenase: a NADPH-generating enzyme in the lumen of the endoplasmic reticulum. Arch Biochem Biophys 2008; 471:184–190. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009; 360:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 2008; 116:597–602. [DOI] [PubMed] [Google Scholar]
- 11.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol 2009; 11:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009; 118:469–474. [DOI] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res 2010; 16:1597–1604. [DOI] [PubMed] [Google Scholar]
- 14.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009; 462:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldewpersad Tewarie NM, Burgers IA, Dawood Y, et al. NADP+-dependent IDH1 R132 mutation and its relevance for glioma patient survival. Med Hypotheses 2013; 80:728–731. [DOI] [PubMed] [Google Scholar]
- 16.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol 2010; 120:719–729. [DOI] [PubMed] [Google Scholar]
- 17.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 2010; 75:1560–1566. [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol 2010; 177:2708–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 2009; 27:4150–4154. [DOI] [PubMed] [Google Scholar]
- 20.Nobusawa S, Watanabe T, Kleihues P. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 2009; 15:6002–6007. [DOI] [PubMed] [Google Scholar]
- 21.Mukasa A, Takayanagi S, Saito K, et al. Significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients. Cancer Sci 2012; 103:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan GM. Irb 101. J Grad Med Educ 2011; 3:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moga C, Guo B, Schopflocher D, et al. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics; 2012. [Google Scholar]
- 25.Yao Y, Chan AK, Qui ZY, et al. Mutation analysis of IDH1 in paired gliomas revealed IDH1 mutation was not associated with malignant progression but predicted longer survival. PLoS One 2013; 8:e67421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorlia T, Delattre JY, Brandes AA, et al. New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of European Organisation for Research and Treatment of Cancer Brain Tumour Group Study 26951. Eur J Cancer 2013; 49:3477–3485. [DOI] [PubMed] [Google Scholar]
- 27.Juratli TA, Kirsch M, Geiger K, et al. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol 2012; 110:325–333. [DOI] [PubMed] [Google Scholar]
- 28.Okita Y, Narita Y, Miyakita Y, et al. IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol 2012; 41:1325–1336. [DOI] [PubMed] [Google Scholar]
- 29.Ohka F, Natsume A, Motomura K, et al. The global DNA methylation surrogate LINE-1 methylation is correlated with MGMT promoter methylation and is a better prognostic factor for glioma. PLoS One 2011; 6:e23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann C, Hentschel B, Tatagiba M, et al. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res 2011; 17:4588–4599. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi R, Stockhammer F, Becker N, et al. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol 2012; 109:15–22. [DOI] [PubMed] [Google Scholar]
- 32.Takano S, Kato Y, Yamamoto T, et al. Immunohistochemical detection of IDH1 mutation, p53, and internexin as prognostic factors of glial tumors. J Neurooncol 2012; 108:361–373. [DOI] [PubMed] [Google Scholar]
- 33.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014; 32:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatanpaa KJ, Hu T, Vemireddy V, et al. High expression of the stem cell marker nestin is an adverse prognostic factor in WHO grade II-III astrocytomas and oligoastrocytomas. J Neurooncol 2014; 117:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polivka J, Polivka J, Jr, Rohan V, et al. Isocitrate dehydrogenase-1 mutations as prognostic biomarker in glioblastoma multiforme patients in West Bohemia. Biomed Res Int 2014; 2014:735659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thon N, Eigenbrod S, Kreth S, et al. IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer 2012; 118:452–460. [DOI] [PubMed] [Google Scholar]
- 37.Frenel JS, Leux C, Loussouarn D, et al. Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J Neurooncol 2013; 114:85–91. [DOI] [PubMed] [Google Scholar]
- 38.Ohno M, Narita Y, Miyakita Y, et al. Secondary glioblastomas with IDH1/2 mutations have longer glioma history from preceding lower-grade gliomas. Brain Tumor Pathol 2013; 30:224–232. [DOI] [PubMed] [Google Scholar]
- 39.Leibetseder A, Ackerl M, Flechl B, et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: a study of the Society of Austrian Neurooncology (SANO). Neuro Oncol 2013; 15:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SongTao Q, Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 2012; 103:269–273. [DOI] [PubMed] [Google Scholar]
- 41.SongTao Q, Yu L, Lu YT, et al. IDH mutations occur frequently in Chinese glioma patients and predict longer survival but not response to concomitant chemoradiotherapy in anaplastic gliomas. Oncol Rep 2011; 26:1479–1485. [DOI] [PubMed] [Google Scholar]
- 42.Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst 2011; 103:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodges TR, Choi BD, Bigner DD, et al. Isocitrate dehydrogenase 1: what it means to the neurosurgeon: a review. J Neurosurg 2013; 118:1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartmann C, Hentschel B, Simon M, et al. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res 2013; 19:5146–5157. [DOI] [PubMed] [Google Scholar]
- 45.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 2014; 16:1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 2013; 81:1515–1522. [DOI] [PubMed] [Google Scholar]
- 47.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol 2013; 31:344–350. [DOI] [PubMed] [Google Scholar]
- 48.Cairncross G, Wang M, Shaw E, et al. A phase 3 trial of chemoradiotherapy for anaplastic oligodendroglioma: long term results of RTOG 9402. J Clin Oncol 2013; 31:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng HB, Yue W, Xie C, et al. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumour Biol 2013; 34:3555–3559. [DOI] [PubMed] [Google Scholar]
- 50.Zou P, Xu H, Chen P, et al. IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: a meta-analysis. PLoS One 2013; 8:e68782. [DOI] [PMC free article] [PubMed] [Google Scholar]


