Abstract
This study aims to evaluate the role of volume-based positron emission tomography parameters as potential surrogate markers for tumor recurrence in resected pancreatic cancer.
Between January 2008 and October 2012, medical records of patients who underwent surgical resection for pancreatic ductal adenocarcinoma and completed 18F-fluorodeoxyglucose positron emission tomography/CT as a part of preoperative staging work-up were retrospectively reviewed. Not only clinicopathologic variables but also positron emission tomography parameters such as SUVmax, MTV2.5 (metabolic tumor volume), and TLG (total lesion glycolysis) were obtained.
Twenty-six patients were women and 31 were men with a mean age of 62.9 ± 9.1 years. All patients were preoperatively determined to resectable pancreatic cancer except 1 case with borderline resectability. R0 resection was achieved in all patients and 45 patients (78.9%) received postoperative adjuvant chemotherapy with or without radiation therapy. Median overall disease-free survival was 12.8 months with a median overall disease-specific survival of 25.1 months. SUVmax did not correlate with radiologic tumor size (P = 0.501); however, MTV2.5 (P = 0.001) and TLG (P = 0.009) were significantly associated with radiologic tumor size. In addition, MTV2.5 (P < 0.001) and TLG (P < 0.001) were significantly correlated with a tumor differentiation. There were no significant differences in TLG and SUVmax according to lymph node ratio; only MTV2.5 was related to lymph node ratio with marginal significance (P = 0.055). In multivariate analysis, lymph node ratio (Exp [β] = 2.425, P = 0.025) and MTV2.5 (Exp[β] = 2.273, P = 0.034) were identified as independent predictors of tumor recurrence following margin-negative resection. Even after tumor size-matched analysis, MTV2.5 was still identified as significant prognostic factor in resected pancreatic cancer (P < 0.05). However, preoperative neoadjuvant treatment attenuated adverse oncologic impact of high preoperative MTV2.5 (P = 0.210).
Preoperatively determined volume-based PET parameter, MTV2.5, can potentially be used as a surrogate marker to estimate tumor biology and tumor recurrence. Individual treatment strategies for pancreatic cancer can be suggested based on patients’ preoperative MTV2.5.
INTRODUCTION
Pancreatic cancer is one of the most lethal malignant tumors arising from the gastrointestinal tract. Margin-negative pancreatectomy is an essential step for cure of disease; however, most patients are diagnosed at advanced stages of pancreatic cancer that preclude curative resection. Even after R0 resection, most patients experience systemic tumor recurrence and finally die due to cancer progression. Therefore, further investigations are needed to determine optimal strategies for early diagnosis, safe margin-negative pancreatectomy, the stratification of patients in terms of recurrence risk, and effective adjuvant treatment.
For the purpose of accurate and reliable clinical and prognostic assessment, positron emission tomography (PET), which has the benefit for revealing the information about biological properties of tumors, has been frequently used in clinical practice. In fact, PET has been shown to provide several important clinical information about pancreatic cancer in terms of differential diagnosis between benign and malignant neoplasms,1,2 preoperative staging,3,4 the evaluation of therapeutic response,5,6 and the prediction of prognosis.7,8
Fluorine-18 fluorodeoxyglucose (18F-FDG) is metabolized similarly to glucose in tumor cells, which is transported into a cytoplasm through specific glucose transporters in cell membrane and phosphorylated by hexokinase. However, phosphorylated FDG cannot be metabolized further; therefore, it accumulates in tumor cells, forming the basis of tumor detection via increased FDG uptake. 18F-FDG PET/CT has become an important imaging modality in staging, restaging, and monitoring of treatment responses in many malignant tumors, as it can provide a quantification of tumor metabolic activity to clinicians. The standard uptake value (SUV) is a commonly used semiquantitative parameter for the interpretation of PET images. The maximum SUV (SUVmax), which can be calculated from a 1-pixel region of interest corresponding to the maximum pixel value in the tumor, is a very commonly used parameter for estimating the prognosis and assessing treatment responses. However, SUVmax is known to be an observer-dependent measurement that can be influenced by the region of interest, which is usually determined by an observer.9,10 Furthermore, a single pixel is unlikely to reflect the activity of metabolically heterogeneous tumors accurately. In order to resolve these clinical problems related to the use of SUVmax, several volume-based PET parameters have been introduced to estimate accurate and objective measurement of tumor biology. Recently, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were developed to measure the metabolic activity of an entire tumor.11
In this study, we correlated these 2 volume-based PET parameters with clinicopathologic characteristics of resected pancreatic cancer and sought to establish whether these recently developed PET parameters can estimate the risk of tumor recurrence in pancreatic cancer after curative resection.
MATERIALS AND METHODS
Clinicopathologic Parameters
We retrospectively reviewed medical records of patients who underwent potentially curative resection of pancreatic ductal adenocarcinoma and completed preoperative 18F-FDG PET/CT as part of a staging work-up between January 2008 and October 2012. Those who received preoperative neoadjuvant treatment due to unresectable pancreatic cancer on preoperative imaging modalities or who had undergone palliative surgery were excluded from the study. However, the data set of patients with neoadjuvant treatment followed by pancreatectomy was used for evaluating the potential impact of neoadjuvant treatment on biologic impact of PET-based parameters. All patients underwent 18F-FDG PET/CT and conventional radiologic examinations including contrast-enhanced CT and/or magnetic resonance imaging (MRI). Additionally, serum CA19–9 levels were measured before treatment. This study was approved by the Institutional Review Board at our institution, and written informed consent was obtained from all patients. During the follow-up period, patients were clinically assessed every 3 to 6 months by blood tests including serum CA19–9 and contrast-enhanced abdomino-pelvic CT. If the clinical assessment or follow-up studies revealed abnormal findings, additional diagnostic studies and biopsy with histopathologic confirmation were performed to evaluate cancer recurrence. Clinicopathologic variables that were retrospectively collected regarding gender, age, tumor location, operation type, tumor size, grade (differentiation), pathologic tumor (pT) stage, presence of lymph node metastasis, lymph node ratio (total number of metastatic lymph nodes divided by total number of retrieved lymph node), microscopic perineural invasion, lymphovascular invasion, recurrence pattern, and time to recurrence, which was defined as the time from surgical resection to recurrence or last clinical follow-up visit at our medical center.
Volume-based PET parameter (MTV2.5 and TLG): 18F-FDG PET/CT scans were performed with a dedicated PET/CT scanner (Discovery STe, GE Healthcare; or Biograph TruePoint 40, Siemens Healthcare). All patients fasted for at least 6 hours before the PET/CT scan. A dose of ∼5.5 MBq/kg of 18F-FDG was intravenously injected 60 minutes before imaging. First, a CT scan was performed at 30 mA and 130 kVp for the Discovery STe instrument, and 36 mA and 120 kVp for Biograph TruePoint instrument without contrast-enhancement. After the CT scan was complete, a PET scan was performed for extending from the neck to the proximal thighs with an acquisition time of 3 min per bed position in 3D mode. PET images were reconstructed using ordered subset expectation maximization (OSEM) with an attenuation correction. 18F-FDG PET/CT images were reviewed by 2 nuclear medicine physicians using an Advantage Workstation 4.4 (GE Medical Systems). Maximum standardized uptake value (SUVmax) and MTV2.5 on PET images were measured using the volume viewer software. Each tumor was examined with a spherical-shaped volume of interest (VOI) that included the entire lesion in the axial, sagittal, and coronal planes. By using CT images, 18F-FDG uptake of normal organs such as the bowel, stomach, and liver was not included in the VOI. The SUVmax of the VOI was calculated as (decay-corrected activity/tissue volume)/(injected dose/body weight). MTV2.5 was defined as the total tumor volume with an SUV ≥ 2.5, and the MTV and mean SUV of the VOI were automatically calculated. TLG was calculated as (mean SUV) × (MTV). In patients with SUVmax < 2.5, MTV2.5 and TLG were not measured.
Statistics
Continuous variables were described as mean ± standard deviation, and categorical variables were described as a frequency (%). Student's t test, chi-squared tests with Fisher's exact tests, and linear regression analyses were performed. Survival curves were estimated using the Kaplan–Meier method to calculate cumulative recurrence-free survival rates. Statistical analyses were performed using SPSS 20.0 for Windows (SPSS Inc, Chicago, IL). P values < 0.05 were considered to be statistically significant. Propensity score matching was performed for reducing confounding bias between MTV2.5 and tumor size. Total populations were divided into 2 subgroups regarding small and large tumor groups according to mean value of pathologic tumor size which 2.4 cm (median and mean value of tumor size) was selected as cut-off value. And then, logistic regression was conducted to estimate propensity score of each patient. In each of the small and large tumor groups, the matching between high and low MTV2.5 patients were undergone by greedy algorithm based on calculated propensity score (1:2 ratio matching in small tumor group and 1:1 matching ratio in large tumor group). The comparisons of clinicopathologic factors between selected high and low MTV2.5 patients in each tumor size group were performed in terms of sex, age, tumor size, T stage, N stage, perineural invasion, lymphovascular invasion, tumor differentiation, lymph node ratio, and CA 19–9. Difference of disease-free survival between high and low MTV2.5 was calculated by the Kaplan–Meier method and log-rank test to estimate the prognostic effect of MTV2.5 value in small and large tumor groups.
RESULTS
Patient Demographics
Twenty-six patients were women and 31 were men with an average age of 62.9 ± 9.1 years. Only 1 patient was determined to have a borderline resectable pancreatic cancer, and the others were all resectable lesions. All patients underwent margin-negative pancreatectomy. Most patients required pancreaticoduodenectomy (41 patients, 71.9%). The clinicopathologic characteristics are described in Table 1. Forty-five patients (78.9%) received postoperative adjuvant chemotherapy with or without radiation therapy. Median overall disease-free survival was 12.8 months (95% confidence interval: 9.2–16.3) and median overall disease-specific survival was 25.1 months (95% confidence interval: 15.3–34.9).
TABLE 1.
Clinicopathologic Characteristics of Patients
Correlation Between Radiologic Tumor Size and PET Parameters
When correlating PET parameters and radiologic tumor size, SUVmax had no association with radiologic tumor size (SUVmax = 0.419 × radiologic tumor size (cm) + 4.557, R2 = 0.010, P = 0.501). However, volume-based PET parameters MTV2.5 (MTV2.5 = 3.177 × radiologic tumor size (cm) – 2.343, R2 = 0.213, P = 0.001) and TLG (TLG = 12.737 × radiologic tumor size (cm) – 9.596, R2 = 0.149, P = 0.009), were both significantly associated with radiologic tumor size (Figure 1).
FIGURE 1.
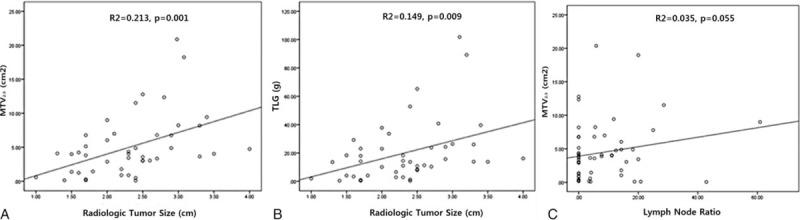
Correlation between volume-based PET parameters and radiologic tumor size (A), (B) and potential associations between MTV2.5 and lymph node ratio (C). PET = positron emission tomography, MTV = metabolic tumor volume.
Correlation Between Lymph Node Ratio and PET Parameters
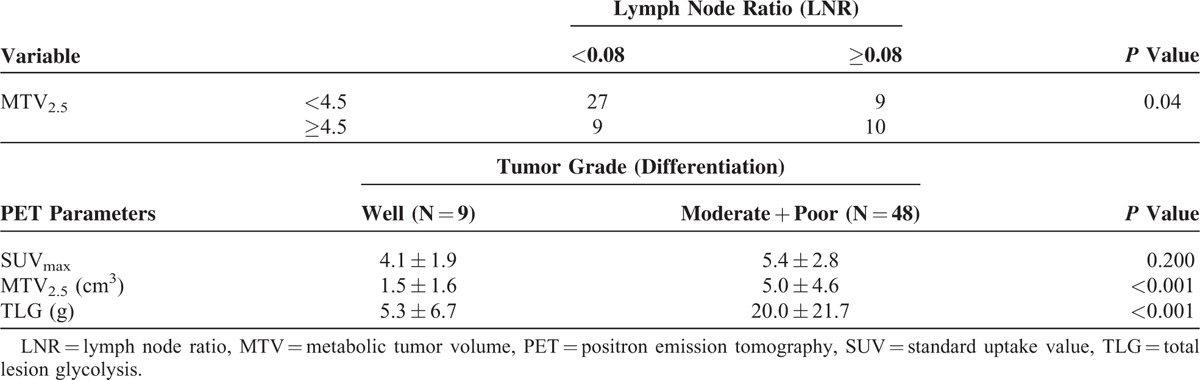
There were no significance differences in SUVmax (5.3 ± 2.8 vs 5.1 ± 2.6, P = 0.749), TMV2.5 (3.6 ± 3.4 vs 5.2 ± 5.1, P = 0.93), or TLG (13.1 ± 14.5 vs 21.7 ± 24.6, P = 0.124) according to lymph node metastasis. However, the lymph node ratio (LNR) was correlated with MTV2.5 (TMV2.5 = 0.11 × LNR + 1.260, R2 = 0.035, P = 0.055, Figure 1 and Table 2), but not with TLG (R2 = 0.037, P = 0.158) and SUMmax (R2 = 0.006, P = 0.555).
TABLE 2.
Association Between MTV2.5 and Lymph Node Ratio (LNR) and Difference in PET Parameters According to Tumor Grade
Correlation Between Tumor Grade and PET Parameters
Tumor grade was also significantly correlated with volume-based PET parameters. The values of MTV2.5 and TLG in well-differentiated pancreatic cancer were much lower than those in moderately or poorly differentiated pancreatic cancer (P < 0.05); however, there was no difference in SUVmax between the 2 groups (P = 0.200, Table 2).
Predicting Tumor Recurrence in Resected Pancreatic Cancer Without Neoadjuvant Treatment
The results of univariate analysis indicate that gender (P = 0.636), age (P = 0.301), lymph node status (pN-stage, P = 0.558), lymphovascular invasion (P = 0.705), perineural invasion (P = 0.838), tumor grade (P = 0.643), and postoperative adjuvant treatment (P = 0.998) were not predictive of tumor recurrence. In contrast, SUVmax (<5 vs ≥ 5, median 13.6 months vs 8.9 months, P = 0.077), TLG (<18 vs ≥18, median 14.7 months vs 8.8 months, P = 0.017), MTV2.5 (<4.5 vs ≥4.5, median 12.9 months vs 8.8 months, P = 0.011), radiologic tumor size (<2.5 vs ≥2.5, median 17.5 vs 7.3 months, P = 0.035), operation mode (pancreaticoduodenectomy vs pylorus preserving pancreaticoduodenectomy vs distal pancreatectomy with splenectomy vs total pancreatectomy, median 3.3 months vs 12.2 months, vs 14.7 months, vs 5.3 months, P = 0.01), lymph node ratio (<0.08 vs ≥0.08, median 15.0 months vs 8.8 months, P = 0.07), and both volume-based PET parameters below a certain threshold (TLG < 18 AND MTV2.5 < 4.5 vs TLG ≥18 OR MTV2.5 ≥ 4.5, median 14.7 months vs 8.8 months, P = 0.017) were found to predict tumor recurrence after curative pancreatectomy.
In multivariate analysis, only lymph node ratio (≥0.08) and MTV2.5 (≥4.5) were found to be independent prognostic factors predicting tumor recurrence in resected pancreatic cancer without neoadjuvant chemo ± radiation therapy (Table 3). In addition, disease-free survival varied significantly according to these 2 risk factors (Combination 0; LNR < 0.08 AND MTV2.5 < 4.5, median 14.7 months, Combination 1; LNR≥0.08 AND MTV2.5 < 4.5, OR LNR < 0.08 AND MTV2.5≥4.5, median 12.2 months, Combination 2, LNR≥0.08 AND MTV2.5≥4.5, median 6.1 months, P = 0.001, Figure 2).
TABLE 3.
Multivariate Analysis for Tumor Recurrence in Resected Pancreatic Cancer
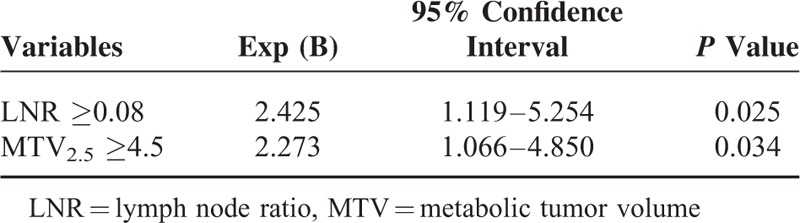
FIGURE 2.
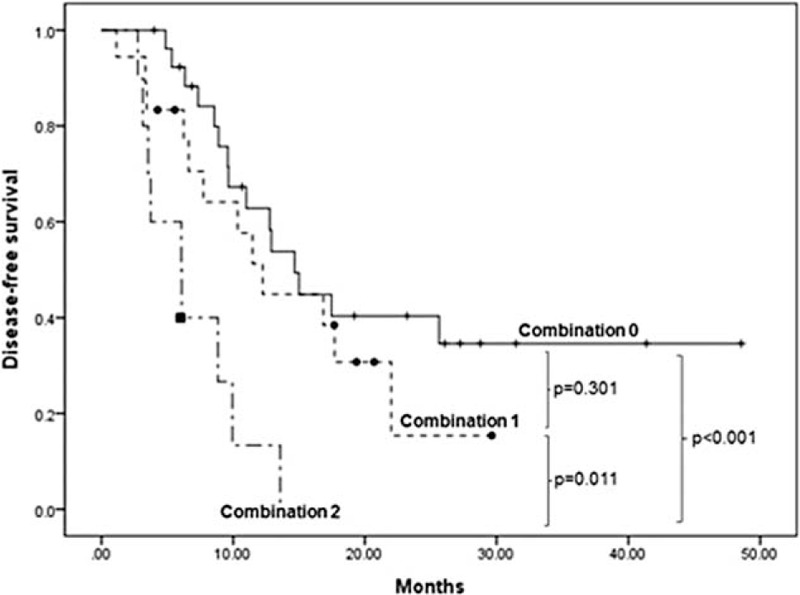
Disease-free survivals according to a combination of independent prognostic factors (MTV2.5 and LNR): Combination 0, MTV2.5 < 4.5 AND LNR < 0.08; Combination1, MTV2.5 < 4.5 AND LNR≥0.08,n MTV2.5 ≥ 4.5 AND LRN < 0.08; Combination 2, MTV2.5 ≥ 4.5 AND LNR ≥0.08. LNR = lymph node ratio, MTV = metabolic tumor volume.
Tumor-Size Matched Analysis to Validate Oncologic Impact of MTV2.5
Oncologic impact of MTV2.5 was evaluated again by tumor-size matched analysis using propensity scores. There was no significant clinicopathologic difference according to MTV2.5 (Table 4). However, regardless of tumor size, it was found that MTV2.5 still played a significant role in determining tumor recurrence in resected pancreatic cancer (P = 0.048 in small sized pancreatic cancer (<2.5 cm), Figure 3(A), and P = 0.001 in large sized pancreatic cancer (≥2.5 cm), Figure 3(B)).
TABLE 4.
Propensity Score Matched Comparisons of Clinicopathologic Characteristics Between High and Low MTV Value in Small and Large Tumor Group
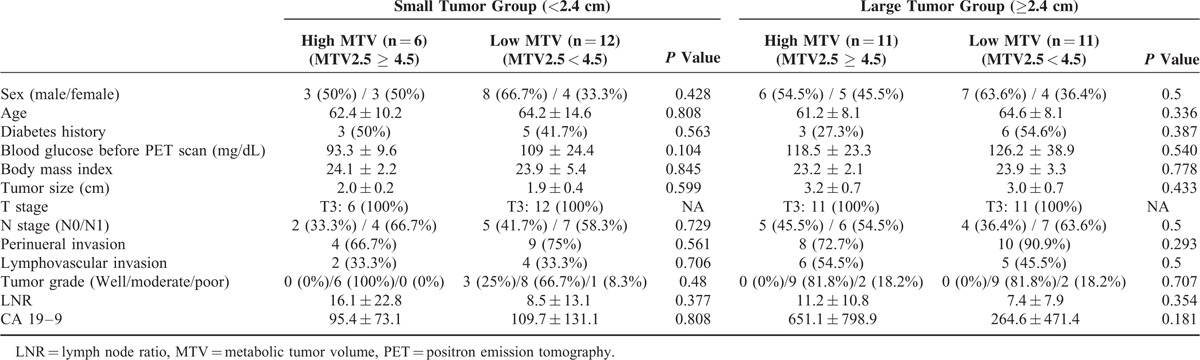
FIGURE 3.
Size-dependent propensity score matched analysis for disease-free survival between high MTV and low MTV in small (<2.4 cm) (A) and large (≥2.4 cm) tumor group (B). MTV = metabolic tumor volume.
Attenuating Adverse Oncologic Impact of MTV2.5≥4.5 by Neoadjuvant Chemoradiation Therapy: a Pilot Study
During the same study period, 30 patients with resectable pancreatic cancer who underwent preoperative CT image were found to undergo pancreatectomy following neoadjuvant chemoradiation therapy. It was noted that adverse oncologic impact of MTV2.5≥4.5 were attenuated to show comparable oncologic outcome with those with MTV2.5 < 4.5 (disease-free survival, 24.9 months (95% CI: 15.9–34.1) vs 16.4 months (95% CI: 8.1–24.7), P = 0.210, Figure 4).
FIGURE 4.
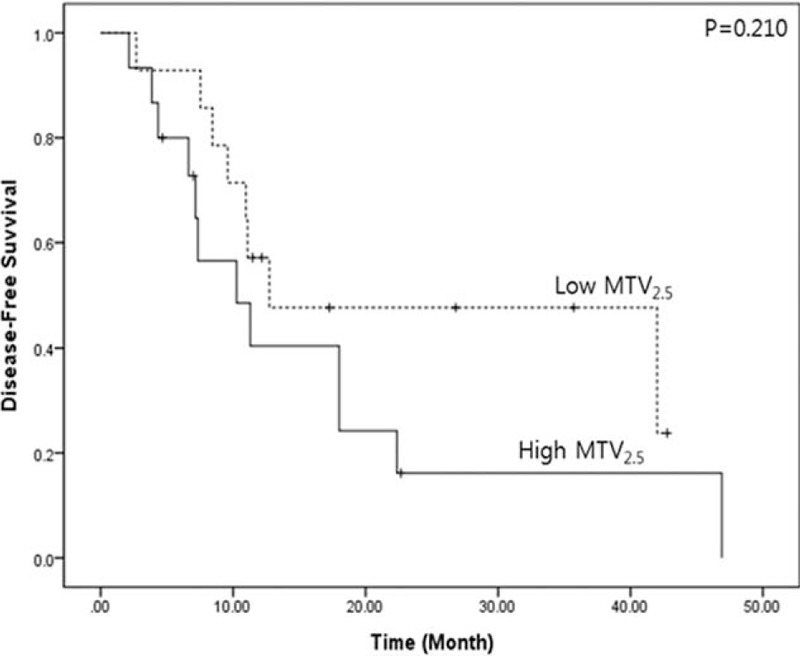
Potential oncologic effect of neoadjuvnat treatment in preoperative resectable pancreatic cancer with high MTV2.5 (MTV2.5≥4.5). Neoadjuvant chemoradiation therapy attenuated negative impact of preoperative high MTV2.5 on pancreatic cancer recurrence following radical pancreatectomy. MTV = metabolic tumor volume.
DISCUSSION
In clinical oncology, biologic behavior of the cancer is usually determined on the basis of pathologic examination of resected surgical specimens. Increased tumor size,12 positive lymph node metastasis,13 increased lymph node ratio,14 high tumor grade,15 positive lymphovascular16 or perineural invasion,17 and positive margin-status18 represent aggressive tumor behaviors in pancreatic cancer that lead to early treatment failure and resistance to conventional chemotherapy. Determining these prognostic factors is a key component of developing an effective and reasonable treatment strategy based on accurate cancer biologic behavior. Considering the high frequency of advanced and potentially systemic disease at the time of initial diagnosis of pancreatic cancer,19 preoperative prediction of early recurrence and aggressive biologic behavior of tumor can inform the prognosis and help patients avoid unnecessary surgery.
It was hypothesized that PET parameters could reflect not only tumor burden, but also tumor biology, because 18F-FDG uptake correlates with cellular proliferation20,21 and tumor behavior6,7,22,45 in pancreatic cancer. According to our results, preoperative volume-based PET parameters MTV2.5 and TLG were found to represent some tumor biologic characteristics in resected pancreatic cancer. In the present study, the conventional PET parameter, SUVmax, did not correlate with radiologic tumor size, lymph node ratio, or tumor differentiation. In contrast, MTV2.5 and TLG were closely related to radiologic tumor size, lymph node ratio, and tumor grade, suggesting the clinical usefulness of these volume-based PET parameters for predicting tumor recurrence and tailoring the treatment of pancreatic cancer.
In this study, preoperative MTV2.5 and TLG were found to reflect tumor biologic behavior and were shown to be prognostic factors determining tumor recurrence in univariate analysis. However, only MTV2.5 was identified as an independent prognostic factor for predicting tumor recurrence among preoperatively detectable clinical parameters. Tumors with MTV2.5 ≥ 4.5 were revealed to have a higher risk of recurrence compared with tumors <4.5 (Exp (B) = 2.273, P = 0.034), a similar risk to that found for lymph node ratio in multivariate analysis (Exp (B) = 2.425, P = 025). Recently, the distribution of metastatic lymph nodes among all retrieved lymph nodes (lymph node ratio) has become a powerful prognostic factor for pancreatic cancer.14,23–28 However, the LNR can only be obtained from pathologic examination of resected surgical specimens. Therefore, the preoperative volume-based PET parameter, MTV2.5, can be a useful surrogate marker that can predict tumor recurrence before surgical intervention, and may even help oncologists counsel against surgery that would be unlikely to benefit the patient. For example, even in “resectable” pancreatic cancer,29 neoadjuvant chemotherapy with or without radiation therapy can be proposed as an alternative treatment for pancreatic cancer with a preoperative MTV2.5 ≥ 4.5, or an oncologist may recommend postoperative adjuvant treatment regardless of their pathological parameters. In fact, patients (N = 2) with 2 independent prognostic factors (MTV2.5 ≥ 4.5 and LNR ≥0.08), who had no postoperative adjuvant treatment typically showed early recurrence within 3 months from curative resection, whereas recurrence was found to be delayed in patients (N = 8) who had both 2 risk factors and received postoperative adjuvant chemotherapy (data not shown, P < 0.001).
It is one of the outstanding points of current study that MTV2.5 was again evaluated by propensity score matching analysis according to tumor size. It is well known that tumor size can be one of the prognostic factors to impact on oncologic outcome of resected pancreatic cancer.12,30–32 In the present study, MTV2.5 also showed strong relationship with radiologic tumor size (R2 = 0.213, P = 0.001, Figure 1(A)). Radiologic tumor size was found to be one of the prognostic factors to predict tumor recurrence in univariate analysis (<2.5 vs ≥2.5, median 17.5 vs 7.3 months, P = 0.035). Although MTV2.5 was identified as an independent prognostic factor in multivariate analysis, tumor size-matched analysis was performed again to remove all possible confounding bias from radiologic tumor size. MTV2.5 was still analyzed as significant determinant in predicting disease-free survival in resected pancreatic cancer (Table 4, and Figure 3). There have been several studies evaluating the oncologic significance of MTV2.5 and TLG in lung cancer,33 head and neck cancer,34,35 esophageal cancer,36 ovarian cancer,37 osteosarcoma,38 and colorectal cancer.39,40 Several previous studies have showed the efficacy of MTV2.5 in predicting oncologic outcomes in various cancers. However, to the best of the authors’ knowledge, there have been only a few published clinical researches on the prognostic value of MTV2.5 in patients with resected pancreatic cancer. Even recent studies41–43 successfully showing the potential oncologic role of MTV2.5 in pancreatic cancer did not concern this possible confounding effect from tumor size. On top of that, our study showed that neoadjuvant chemoradiation therapy could attenuate potential adverse effect of high MTV on recurrence of resectable pancreatic cancer (Figure 4). Although this pilot study is based on a small number of selected patients, it is suggesting that preoperatively determined “resectable” pancreatic cancer can be treated in a different way according to preoperative PET-based parameter. In general, neoadjuvant chemoradiation therapy has performed usually based on anatomic relationship between tumor and major vessel.44 Beyond this anatomic relationship, clinical parameters representing tumor biology, such as PET-based parameter, and CA 19–9 could be possibly used even in preoperative “resectable” pancreatic cancer for selecting patients who would be benefit from neoadjuvant treatment followed by pancreatectomy. This effort will be a back bone of patient-oriented surgical approach to improve survival outcome and further study is mandatory to prove our observation.
There are several limitations to the present study, including retrospective study design, which can lead to significant selection bias even after case-matched comparisons. Therefore, these encouraging results should be validated based on large-scale prospective clinical data in the near future. Our study demonstrated that preoperative MTV2.5 functions as a new, clinically detectable surrogate marker can predict the recurrence of pancreatic cancer following curative resection. In addition, differences in molecular expression according to volume-based PET parameters such as MTV2.5 would be interesting topic to be further investigated to identify molecular mechanism in determining recurrence risk in resected pancreatic cancer.
Footnotes
Abbreviations: CT = computed tomography, FDG = fluorodeoxyglucose, MRI = magnetic resonance imaging, MTV = metabolic tumor volume, PET = positron emission tomography, SUV = standard uptake value, TLG = total lesion glycolysis.
MY equally contributed to this study.
Funding: this study was supported by a faculty research grant of Yonsei University College of Medicine (6-2013-0163).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nagamachi S, Nishii R, Wakamatsu H, et al. The usefulness of (18)F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: comparison with (18)F-FDG PET/CT. Ann Nucl Med 2013; 27:554–563. [DOI] [PubMed] [Google Scholar]
- 2.Sperti C, Bissoli S, Pasquali C, et al. 18-Fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2007; 246:932–937.discussion 937–939. [DOI] [PubMed] [Google Scholar]
- 3.Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009; 250:957–963. [DOI] [PubMed] [Google Scholar]
- 4.Asagi A, Ohta K, Nasu J, et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013; 42:11–19. [DOI] [PubMed] [Google Scholar]
- 5.Kittaka H, Takahashi H, Ohigashi H, et al. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg 2013; 37:169–178. [DOI] [PubMed] [Google Scholar]
- 6.Bang S, Chung HW, Park SW, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol 2006; 40:923–929. [DOI] [PubMed] [Google Scholar]
- 7.Moon SY, Joo KR, So YR, et al. Predictive value of maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med 2013; 38:778–783. [DOI] [PubMed] [Google Scholar]
- 8.Grassetto G, Rubello D. Role of FDG-PET/CT in diagnosis, staging, response to treatment, and prognosis of pancreatic cancer. Am J Clin Oncol 2011; 34:111–114. [DOI] [PubMed] [Google Scholar]
- 9.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med 2007; 48:932–945. [DOI] [PubMed] [Google Scholar]
- 10.Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med 2012; 53:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999; 2:159–171. [DOI] [PubMed] [Google Scholar]
- 12.Fortner JG, Klimsta DS, Senie RT, et al. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg 1996; 223:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaki H, Hiraoka T, Mizumoto R, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Jpn J Surg 1999; 29:16–22. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007; 141:610–618. [DOI] [PubMed] [Google Scholar]
- 15.Hruban RH, Goggins M, Parsons J. Progression model for pancreatic cancer. Clin Cancer Res 2000; 6:2969–2972. [PubMed] [Google Scholar]
- 16.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma. Ann Surg 2003; 237:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Ishikura H, Motohara T, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol 1997; 65:164–170. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004; 8:935–949.discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Wolff RA, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363:1049–1057. [DOI] [PubMed] [Google Scholar]
- 20.Hu SL, Yang ZY, Zhou ZR, et al. Role of SUV(max) obtained by 18F-FDG PET/CT in patients with a solitary pancreatic lesion: predicting malignant potential and proliferation. Nucl Med Commun 2013; 34:533–539. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann K, Eckel F, Schmidt S, et al. In vivo characterization of proliferation for discriminating cancer from pancreatic pseudotumors. J Nucl Med 2008; 49:1437–1444. [DOI] [PubMed] [Google Scholar]
- 22.Choi HJ, Kang CM, Lee WJ, et al. Prognostic value of (18)f-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J 2013; 54:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008; 15:165–174. [DOI] [PubMed] [Google Scholar]
- 24.Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009; 13:1337–1344. [DOI] [PubMed] [Google Scholar]
- 25.Peschaud F, Benoist S, Julie C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg 2008; 248:1067–1073. [DOI] [PubMed] [Google Scholar]
- 26.John BJ, Naik P, Ironside A, et al. Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford) 2013; 15:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatti I, Peacock O, Awan AK, et al. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg 2010; 34:768–775. [DOI] [PubMed] [Google Scholar]
- 28.La Torre M, Cavallini M, Ramacciato G, et al. Role of the lymph node ratio in pancreatic ductal adenocarcinoma. Impact on patient stratification and prognosis. J Surg Oncol 2011; 104:629–633. [DOI] [PubMed] [Google Scholar]
- 29.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014; 12:1083–1093. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas 2008; 36:e15–20. [DOI] [PubMed] [Google Scholar]
- 31.Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012; 41:985–992. [DOI] [PubMed] [Google Scholar]
- 32.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012; 118:2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, Lin WY, Kao CH, et al. Prognostic value of preoperative metabolic tumor volumes on PET-CT in predicting disease-free survival of patients with stage I non-small cell lung cancer. Anticancer Res 2012; 32:5087–5091. [PubMed] [Google Scholar]
- 34.Chung MK, Jeong HS, Park SG, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009; 15:5861–5868. [DOI] [PubMed] [Google Scholar]
- 35.Park GC, Kim JS, Roh JL, et al. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol 2013; 24:208–214. [DOI] [PubMed] [Google Scholar]
- 36.Roedl JB, Colen RR, Holalkere NS, et al. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol 2008; 89:278–286. [DOI] [PubMed] [Google Scholar]
- 37.Chung HH, Kwon HW, Kang KW, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann Surg Oncol 2012; 19:1966–1972. [DOI] [PubMed] [Google Scholar]
- 38.Im HJ, Kim TS, Park SY, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging 2012; 39:39–49. [DOI] [PubMed] [Google Scholar]
- 39.Fendler WP, Philippe Tiega DB, Ilhan H, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med 2013; 54:1202–1208. [DOI] [PubMed] [Google Scholar]
- 40.Melton GB, Lavely WC, Jacene HA, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg 2007; 11:961–969.discussion 969. [DOI] [PubMed] [Google Scholar]
- 41.Xu HX, Chen T, Wang WQ, et al. Metabolic tumour burden assessed by (1)(8)F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging 2014; 41:1093–1102. [DOI] [PubMed] [Google Scholar]
- 42.Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014; 55:898–904. [DOI] [PubMed] [Google Scholar]
- 43.Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014; 89:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang CM, Hwang HK, Choi SH, et al. Controversial issues of neoadjuvant treatment in borderline resectable pancreatic cancer. Surg Oncol 2013; 22:123–131. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015; 22:677–684. [DOI] [PubMed] [Google Scholar]


