Abstract
For patients exposed to a massive blood loss during surgery, maintained coagulation competence is important. It is less obvious whether coagulation competence influences bleeding during elective surgery where patients are exposed to infusion of a crystalloid or a colloid.
This randomized controlled trial evaluates whether administration of 5% human albumin (HA) or lactated Ringer solution (LR) affects coagulation competence and in turn blood loss during cystectomy due to bladder cancer.
Forty patients undergoing radical cystectomy were included to receive either 5% HA (n = 20) or LR (n = 20). Nineteen patients were analyzed in the HA group and 20 patients in the lactated Ringer group.
Blinded determination of the blood loss was similar in the 2 groups of patients: 1658 (800–3300) mL with the use of HA and 1472 (700–4330) mL in the lactated Ringer group (P = 0.45). Yet, by thrombelastography (TEG) evaluated coagulation competence, albumin affected clot growth (TEG-angle 69 ± 5 vs 74° ± 3°, P < 0.01) and strength (TEG-MA: 59 ± 6 vs 67 ± 6 mm, P < 0.001) more than LR. Furthermore, by multivariate linear regression analyses reduced TEG-MA was independently associated with the blood loss (P = 0.042) while administration of albumin was related to the changes in TEG-MA (P = 0.029), aPPT (P < 0.022), and INR (P < 0.033).
This randomized controlled trial demonstrates that administration of HA does not affect the blood loss as compared to infusion of LR. Also the use of HA did not affect the need for blood transfusion, the incidence of postoperative complications, or the hospital in-stay. Yet, albumin decreases coagulation competence during major surgery and the blood loss is related to TEG-MA rather than to plasma coagulation variables.
INTRODUCTION
During surgery the circulation is supported by a crystalloid, often saline or lactated Ringer solution (LR), eventually supplemented by a colloid to delay when there is a need for blood transfusion. In contrast to crystalloids, colloids stay within the circulation and may even recruit fluid to the vasculature.1 It therefore remains an option to administer a colloid to bleeding patients perioperatively.2–4 On the other hand, synthetic colloids impair coagulation competence as exemplified by the use of hydroxyethyl starch (HES 130/0.4) during major surgery.5 A thrombelastography (TEG) analysis showed that HES reduced clot formation and strength (maximal amplitude [MA]) and doubled the perioperative blood loss when compared to the use of LR.5 Similarly, administration of Dextran reduced coagulation competence during cystectomy and clinically important hemorrhage (>1.5 L) appeared more often among the patients who received Dextran than LR.6
Human albumin (HA) might be expected to affect coagulation competence minimally but a meta-analysis addressing cardiac surgery7 found increased perioperative bleeding and need for transfusion with the use of albumin compared to administration of hydroxyethyl starch (HES 130/0.4). Albumin may reduce platelets activation and release of inflammatory mediators,8,9 but another meta-analysis found blood loss and need for blood transfusion increased by about 30% among patients who received HES 130/0.4 rather than albumin.10 One randomized controlled trial compared the effect of administration HA and LR during major surgery and found similar blood loss in the 2 groups of patients and yet increased need for transfusion of blood in the albumin treated group.11
The present randomized controlled study aimed to further explore the relationship between the perioperative blood loss and coagulation competence during cystectomy with the use of either HA or LR to support the circulation. The hypothesis was that HA affects coagulation competence to such an extent that it increases the blood loss as compared to use of LR. In addition, we tested the hypothesis that TEG analysis is superior to predict the blood loss compared to plasma coagulation variables.
MATERIALS AND METHODS
Trial Design
This randomized controlled trial was approved by ClinicalTrials.gov (NCT02270723), the Danish Health and Medicine Authority (EudraCT 2013-005350-29), and the local Ethics Committee in the Capital Regional of Denmark (H-1-2013-142) and was registered by the Danish Data Protection Agency. The trial was monitored by the Agency for Good Clinical Practice at the University of Copenhagen and the Declaration of Helsinki criteria were followed.12
Conduct of the trial and the safety of the participants were overseen by the authors who gathered the data that remained confidential throughout the process. The authors were involved in all stages of manuscript generation and vouched for completeness and accuracy. No third party influenced the study design, data analysis, or reporting.
Screening and randomization took place between April 8th 2014 and July 8th 2015. At least 24 hours before surgery written informed consent was obtained from the patients. We excluded patients from this investigator-initiated, prospective, blinded trial if consent was withdrawn. Forty patients scheduled for elective cystectomy were randomized to receive the recommended maximum volume (25 mL/kg) of either HA (Albumin, human, CSL Behring, GmbH, Marburg, Germany) or LR by computer-generated allocation sequence without blocks produced by the biostatistics Department (Figure 1). We included patients older than 18 years scheduled for elective cystectomy with no history of heart or hepatic insufficiency, disability of coagulation, intracerebral hemorrhage, or hemodialysis. If a patient used medication that was considered to affect coagulation competence, that medication was paused 5 days prior to surgery according to national guidelines.13
FIGURE 1.
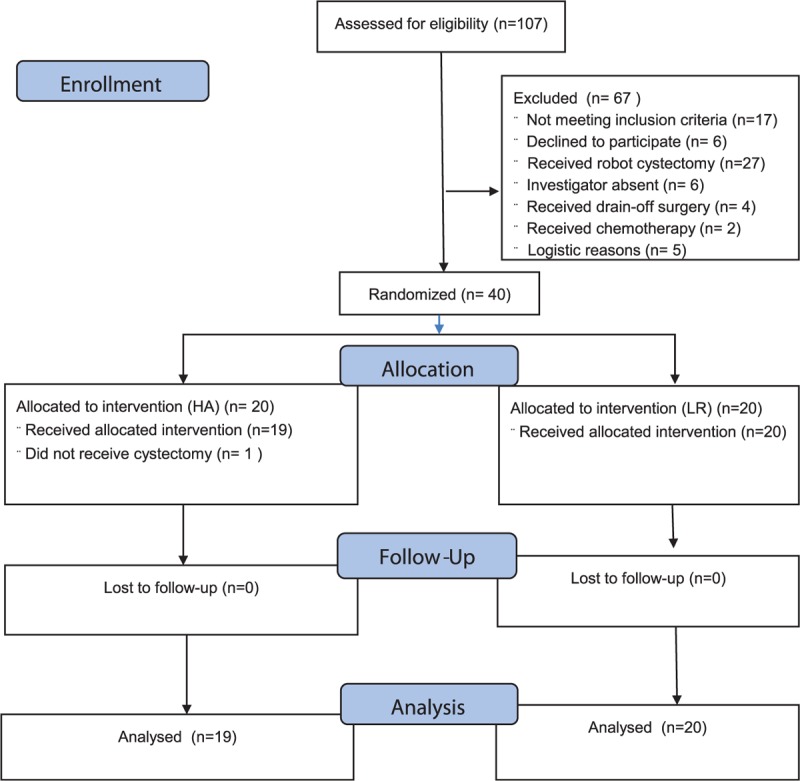
Consort study flow diagram.
Interventions
The primary design of intervention has been published previously.5,6 An intravenous line flushed with the randomized fluid was placed in an opaque bag together with the intravenous set to blind the surgical nurse who recorded the volume of lost blood. The patients were in general anesthesia by remifentanil and propofol administration and monitored with a periphery arterial catheter connected to Nexfin device besides a central venous and epidural catheter – the latter for postoperative pain treatment.14
Normovolemia was established according to “goal directed fluid therapy” (GDT) criteria.15 Hemodynamic data were recorded after induction of anesthesia and insertion of the arterial catheter (T0), before start of surgery (T1), after resection of the urinary bladder (T2), at the end of anesthesia (T3), and 2 hours thereafter in the recovery room (T4).
Arterial blood specimens were analyzed by TEG16 and we also analyzed blood specimens for hemoglobin, creatinine, plasma coagulation, and blood gas variables. Both groups of patients received either LR or SAG M if considered in need after infusion of the allocated fluid (nonstudy fluid). Fluid balance was determined at T2, T3, and T4. The very same 2 surgeons carried out the operative procedures.
The primary outcome variables were coagulation competence besides the perioperative blood loss. We also compared the use of HA and LR on patient outcome (need for postoperative treatment of cardiopulmonary, infectious, or surgical complications). A straight postoperative track was defined as length of hospital stay ≤7 days without complications requiring treatment.
Statistical Methods
Patients were analyzed in the group to which they were assigned (Table 1). The sample size was based on data from the study comparing the effect on hemorrhage during cystectomy with the use of HES 130/0.4 versus LR. In that study, the blood loss was 2181 versus 1370 mL with an SD of 1190 versus 603 mL.5 The study was powered to detect a difference in blood loss of 800 mL between administration of HA and LR with a 2-sided alpha level of 0.05 and a power of 80% for t-test with correction for multiple comparisons. Consequently, a sample size of 20 patients per group was included.
TABLE 1.
Patient Characteristics and Baseline Laboratory Values and Perioperative Events
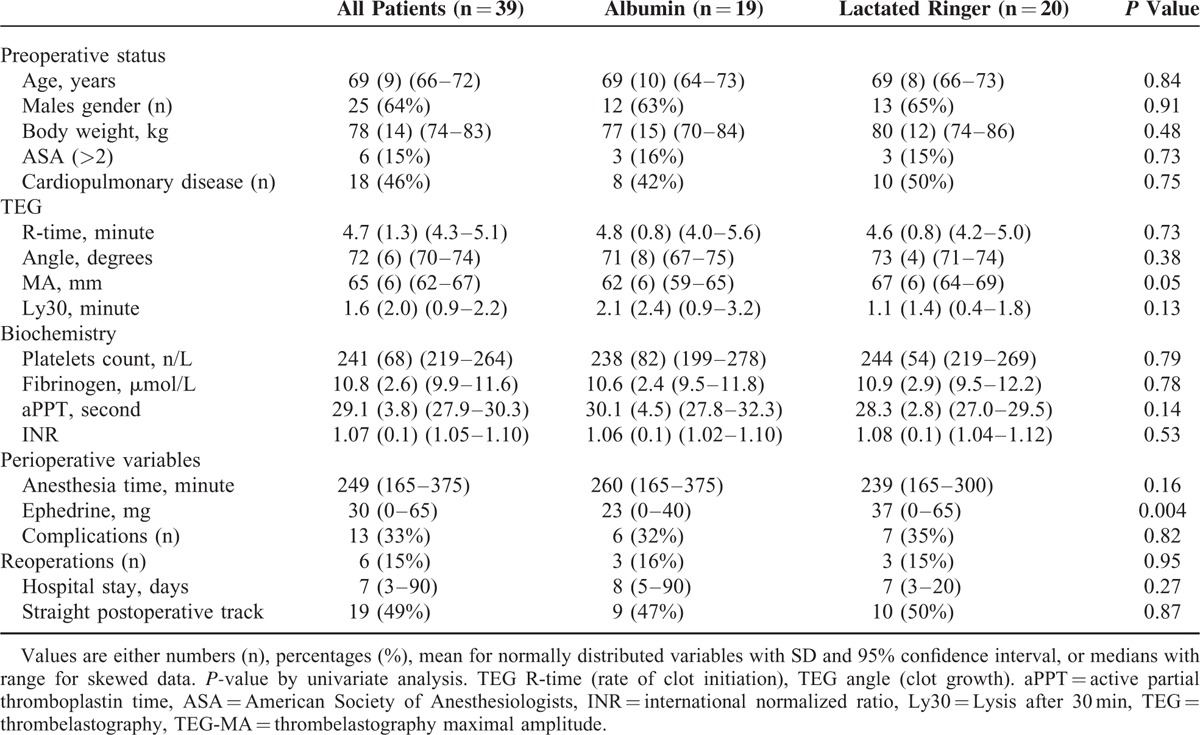
The statistical analyses were performed before breaking the randomization code, and data were analyzed from the modified intention-to-treat population, defined as all randomly assigned patients except for those who could be excluded without the risk of bias (Figure 1). We used 2-sided or unadjusted Chi-square tests with odds ratio (OR), t-test, and Fisher exact test for continuous and dichotomous variables, respectively (SPSS version 20.0). Evaluation of differences was performed using χ2 test for categorical data and analysis of variance or Mann–Whitney U-test and Wilcoxon signed ranks test for continuous data when appropriate. Results are presented as mean (SD) or median with range for skewed variables and a 2-sided P value < 0.05 was considered to indicate statistical significance. Correlations were evaluated by Spearman coefficient. A backwards multivariate linear regression analysis with Bonferroni correction for multiple comparisons was performed, including variables that were either found to be correlated with or expected to influence TEG and plasma hemostatic variables that were independently associated with albumin infusion during anesthesia. The variables included in the multivariate analyses were TEG-angle, TEG-maximum amplitude (TEG-MA), platelets, fibrinogen, aPPT, and INR. Results are presented as regression coefficients (β) with 95% confidence interval and t and P values.
RESULTS
There was no significant intergroup difference in baseline data including preoperative diseases between the 2 groups of patients (Figure 1 and Table 1). After induction of anesthesia, 3 patients (16%) in the albumin group were intravascular hypovolemic according to GDT criteria as compared to 1 patient (5%) in the crystalloid group (P = 0.34). In the colloid group, patients received 23.5 mL/kg (1125–2500) mL HA and 4 patients did not receive the full scheduled volume, because they were considered hemodynamic stable before the intended volume (25 mL/kg) was infused.
Fluid Administration and Blood Loss
The perioperative blood loss was 1658 (800–3300) mL in the albumin group and not significantly different in the LR group: 1472 (700–4330) mL, P = 0.45, Table 2. Furthermore, the number of patients exposed to what we considered a significant blood loss (>1500 mL) was similar in the albumin and in the LR group: 6 patients (33%) versus 5 patients (28%), P = 1.00. Table 2 also presents the IV administration of study and nonstudy fluids. Transfusion was initiated at a hemoglobin of 3.8 to 5.5 mmol/L and the transfusion of packed red blood cells was 235 (0–980) versus 80 (0–1100) mL in the albumin and LR group, respectively (P = 0.14). Thus, 6 patients (33%) in both groups were provided with blood transfusion.
TABLE 2.
Perioperative Fluid Administration, Blood Loss, and Fluid Balance (mL) in 2 Groups of Patients Undergoing Cystectomy
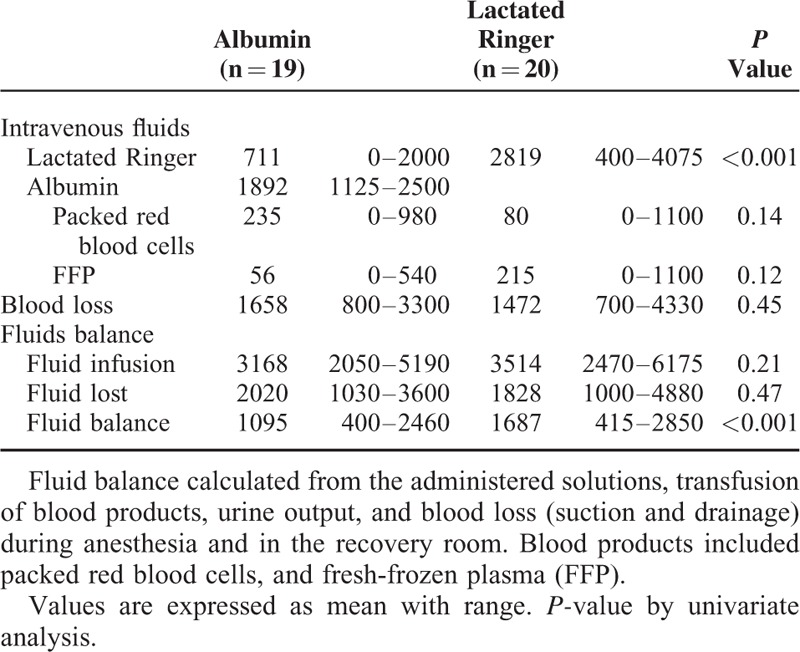
The fluid balance was positive by 1095 (400–2460) mL in the albumin group and by 1687 (415–2850) mL in the LR group (P < 0.001), also with more patients in the LR group having a positive fluid balance that we considered significant, that is, exceeding 2000 mL: 9 patients versus 1 patient, OR = 14.7 (1.6–132.6), P = 0.016. Furthermore, the albumin group received less ephedrine than the LR group, 23 (0–40) mg versus 37 (0-65) mg, P = 0.004).
Hemodynamics
During anesthesia changes in MAP were similar in the 2 groups of patients, whereas HR increased more in the LR group (68 ± 18 to 81 ± 10) beats/min compared to the albumin group (67 ± 15 to 68 ± 14) beats/min, P < 0.002. SV increased in the albumin group (61 ± 14 to 76 ± 13) mL/beats compared to LR group (67 ± 14 to 66 ± 14) mL/beats, P = 0.038; however, CO was not significantly different between the 2 groups of patients.
Coagulation
The baseline values of TEG and biochemistry variables are given in Table 1. After cystectomy (T2) in the LR group, TEG-angle increased to 74 ± 3 compared to 69 ± 5 degrees in the albumin group, P < 0.01. The lowest values of TEG-MA was observed in the albumin group (60 ± 7 vs 68 ± 6) mm, P < 0.002. APPT decreased in the LR group to 27.9 ± 2.7 and to 31.4 ± 3.9 seconds in the albumin group, P < 0.003. There was no significant differences between the groups regarding R-time, Lysis after 30 min (Ly30), platelets, INR, or coagulation factor II, VII, X.
At the end of anesthesia (T3) TEG-angle in the LR group increased to 74 ± 5 compared with 69 ± 5 degrees in the albumin group, P < 0.003. The lowest values of TEG-MA was observed in the albumin group (59 ± 6 vs 67 ± 6) mm, P < 0.001. Platelets decreased in both groups of patients, mainly in the albumin group (185 ± 51) 109/L compared to the LR group (232 ± 49) 109/L, P < 0.008. APPT decreased in the LR group to 26.0 ± 32.7 seconds compared to the albumin group 31.8 ± 6.2 seconds, P < 0.001, and INR increased more in the albumin group (to 1.34 ± 0.20) compared with the LR group (to 1.14 ± 0.10), P < 0.001. There was no significant difference in R-time and Ly30, between the groups of patients.
In the recovery room (T4) in the LR group, the TEG-angle was 74 ± 4 compared with 71 ± 4 degrees in the albumin group, P < 0.03. The lowest values of TEG-MA was observed in the albumin group compared to the LR group (60 ± 6 vs 65 ± 7) mm, P < 0.008. Platelets decreased in both groups of patients, mainly after albumin (185 ± 51 vs 223 ± 48) 109/L, P < 0.03. APPT decreased in the LR group to 25.0 ± 3.2 seconds compared to the albumin group (30.6 ± 4.9) seconds, P < 0.001, whereas INR increased more in the albumin group (to 1.26 ± 0.10) compared with the LR group (to 1.14 ± 0.08), P < 0.001. Likewise, in the recovery room, the R-time and Ly30 were without differences between the groups. The decrease in hemoglobin was not significantly different in the albumin group compared to the LR group (7.4 ± 1.3 to 5.9 ± 0.8) versus (7.8 ± 1.1 to 6.4 ± 1.1) mmol/L, P < 0.12.
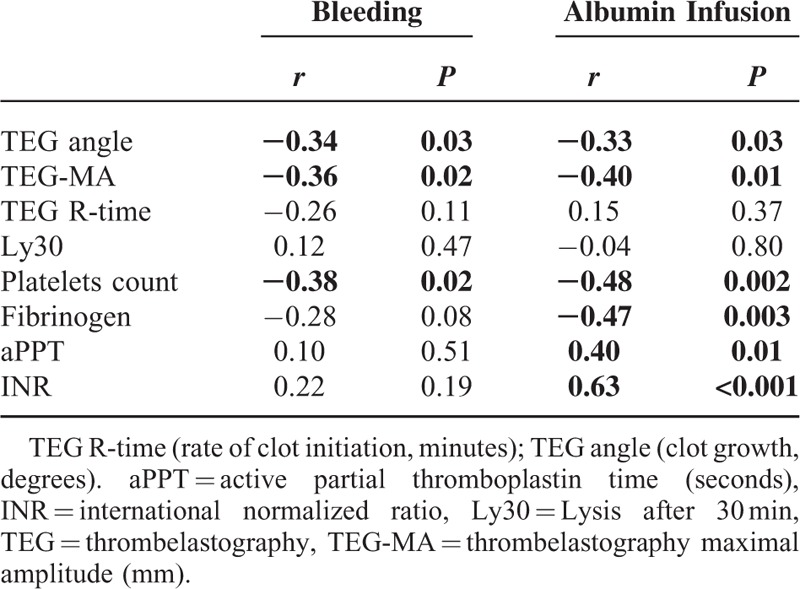
Correlations
Correlation between blood loss and albumin infusion during anesthesia versus coagulation variables is shown in Table 3. The changes in TEG-angle and TEG-MA from T1 to T3 were correlated to administration of albumin as was plasma coagulation variables. The perioperative blood loss was related to TEG-angle, TEG-MA, and platelets, whereas R-time, Ly30, aPPT, and INR demonstrated poor correlations.
TABLE 3.
Spearman Correlation of Changing in (TEG Values and Plasma Coagulation Tests (from T1 to T3) Related to Perioperative Bleeding and Albumin Administration in the Randomized Trial
Multivariate Linear Regression Analysis of the Association between Hemorrhage, Albumin Administration Versus Coagulation Competence
Independent association between bleeding and albumin infusion compared to reduced coagulation competence was evaluated by multivariate linear regression, including only univariate significant variables. The blood loss was independently associated with reduced TEG-MA, while both reduced TEG-MA and the biochemistry variables: aPPT and INR were independently associated with albumin infusion (Table 4, Figure 2).
TABLE 4.
Multivariate Linear Regression Analyses of Association Between Perioperative Bleeding and Administration of Albumin Evaluated by TEG and Conventional Plasma Coagulation Variables (Changes from T1 to T3)
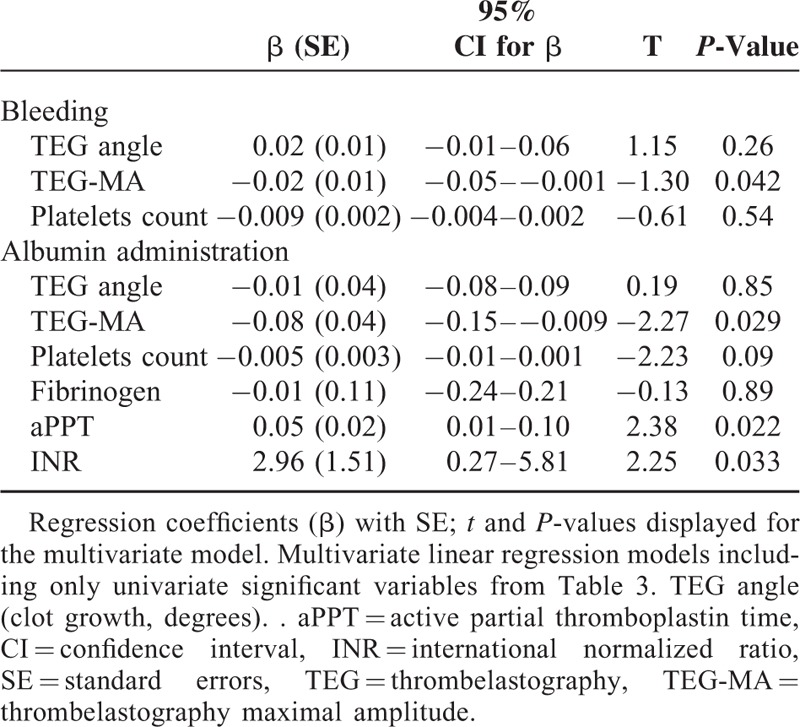
FIGURE 2.
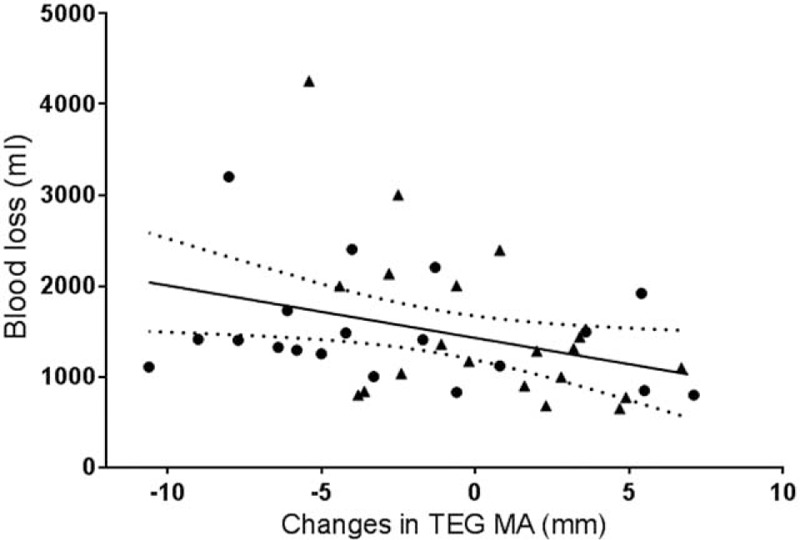
Correlation between TEG-MA and blood loss. Change in plasma TEG-MA from start (T1) to end of anesthesia (T3) in patients receiving lactated Ringer solution (▴) or albumin (•), r = −0.36 (95% CI −0.62 to −0.10), P = 0.02 Regression lines shown with 95% confidence interval. CI = confidence interval, TEG-MA = thrombelastography maximal amplitude.
Outcome
The postoperative course and number of treated complications were similar in the 2 groups of patients (Table 1).
DISCUSSION
This randomized controlled study used either HA or LR to support the circulation during cystectomy according to individualized goal-directed principles without demonstrating significant difference in perioperative bleeding or on outcome. Yet, perioperative hemorrhage was related to the TEG variable MA, but not to plasma coagulation variables.
Even though albumin is the most abundant protein in plasma and administration to patients therefore might be considered to be safe, there has been concern about the use of albumin to support the circulation during surgery. HA has been used without reports of viral infections,17 but in a systematic review including 1419 critically ill patients in 30 studies, the use of albumin seemed to increase mortality.18 Yet, the use of albumin was not mentioned in the comments from European Medicine Agency on the use of colloids and there has been no suggestion of banning albumin solutions.19 Albumin does not affect outcome, including mortality, in intensive care when compared to the use of saline.20 In support, blood loss during cardiac surgery is similar when administering albumin and LR.11 In contrast to the experience from cardiac surgery, the present study did not demonstrate increased need for administration of blood products with the use of albumin.
In this randomized controlled study comparing administration of LR versus albumin, both TEG-angle, TEG-MA and plasma coagulation variables were correlated to albumin infusion (Table 3), and these variables were used in the Spearman correlation analysis to design a multivariable regression analysis. Changes in TEG-MA and the biochemistry variables aPPT and INR were the only independent variables associated with infusion of albumin in line with the data from Skhirtladze et al11 who showed maximal clot firmness lower in the albumin compared to LR group when assessed in a postoperative evaluation.
Evaluation of coagulation competence remains debated.21,22 For trauma patients it is recommended that TEG rather than conventional plasma coagulation tests is used because TEG allows for a fast result23 and here the blood loss was correlated to TEG-MA rather than to plasma coagulation variables.
It may seem an advantage to administer albumin rather than LR as the postoperative fluid balance was 54% higher (1095 vs 1687) mL, P < 0.001 in the LR group. A positive postoperative fluid balance has been related to gut edema and may contribute to intestinal dysfunction, postoperative complications and therefore extended hospital stay.24,25 On the other hand, the volume balance that maintains the central blood volume during and after surgery has not been determined. We suggest that such an evaluation is made, for example, by echocardiographic determination of ventricular filling or determination of which volume load that would maintain plasma atrial natriuretic peptide as an indication of a stable central blood volume.26
There are limitations to this investigation. The volume used for GDT influences the estimate of the deficit. We used 200 mL, reflecting the volume used in most RCT studies, but the volume may be related to the weight, body mass index, age, and health of the patient.27 Besides, the study has a short observation period and a larger cohort may be needed to evaluate an effect of albumin versus LR on complications and survival.
CONCLUSION
The results suggest that the perioperative use of 5% HA compared to LR to support the circulation during cystectomy reduces the postoperative volume surplus but affects coagulation competence, but not to an extent that albumin affects bleeding or outcome measures. The results suggest that the perioperative use of 5% HA compared to LR only affects coagulation competence and blood loss little during major surgery. Also the use of HA reduced the postoperative volume surplus, but not the incidence of postoperative complications, or hospital stay.
Footnotes
Abbreviations: GDT = goal directed fluid therapy, HA = human albumin, LR = lactated Ringer solution, Ly30 = Lysis after 30 min, TEG = thrombelastography, TEG-MA = thrombelastography maximum amplitude.
KCR contributed to conception and design of the study, acquisition, analysis, and interpretation of presented data, drafting the article, and final approval of the version to be published. MH contributed to acquisition, and interpretation of presented data, helped draft the manuscript. PIJ contributed to conception and design of the study. IK contributed to acquisition, and interpretation of presented data. TK contributed to acquisition, analysis, and interpretation of presented data, helped draft the manuscript. LS participated in interpretation of presented data, helped draft the manuscript. HBN contributed to interpretation of presented data, helped draft the manuscript. BR contributed to conception, acquisition, interpretation of presented data, helped draft the manuscript. TP contributed to statistical analysis, interpretation of presented data, helped draft the manuscript. NHS contributed to conception and design of the study, critical revision of the article for important intellectual content. All authors approved the final version to be published.
Trial registration: ClinTrials.gov (NCT02270723) and EudraCT 2013–005350–29.
This study was supported by AP Møller's (Maersk) Fond, The Augustinus Fond, Holger Hesses's Fond, Aase and Ejnar Danielsen's Fond, Ove and Edith Olesen's Legat, and by T & A Frimodt's Fond.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Zaar M, Lauritzen B, Secher NH, et al. Initial administration of hydroxyethyl starch vs. lactated Ringer after liver trauma in the pig. Br J Anaesth 2009; 102:221–226. [DOI] [PubMed] [Google Scholar]
- 2.Johansson PI, Stensballe J. Hemostatic resuscitation for massive bleeding: the paradigm of plasma and platelets – a review of the current literature. Tranfusion 2010; 50:701–710. [DOI] [PubMed] [Google Scholar]
- 3.Gruen RL, Brohi K, Schreiber M, et al. Hemorrhage control in severely injured patients. Lancet 2012; 380:1099–1108. [DOI] [PubMed] [Google Scholar]
- 4.Lunde J, Stensballe J, Wikkelsø A, et al. Fibrinogen concentrate for bleeding – a systematic review. Acta Anaesthesiol Scand 2014; 58:1061–1074. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen KC, Johansson PI, Hoejskov M, et al. Hydroxyethyl starch reduces coagulation competence and increase blood loss during major surgery. Results from a randomized controlled trial. Ann Surg 2014; 2:249–254. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen KC, Hoejskov M, Johansson PI, et al. Coagulation competence for predicting perioperative haemorrhage in patients treated with lactated Ringer's vs Dextran – a randomized controlled trial. BMC Anesthesiol 2015; 15:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob M, Fellahi J, Chappell D, et al. The impact of hydroxyethyl starches in cardiac surgery: a meta-analysis. Crit Care 2014; 18:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell JA, Navickis RJ, Wilkes MM. Albumin versus crystalloid for pump priming in cardiac surgery: meta-analysis of controlled trials. J Cardiothorac Vasc Anesth 2004; 18:429–437. [DOI] [PubMed] [Google Scholar]
- 9.Adrian K, Mellgren K, Skogby M, et al. The effect of albumin priming solution on platelet activation during experimental long-term perfusion. Perfusion 1998; 13:187–191. [DOI] [PubMed] [Google Scholar]
- 10.Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg 2012; 144:223–230. [DOI] [PubMed] [Google Scholar]
- 11.Skhirtladze K, Base EM, Lassnigg A, et al. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer's lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth 2013; 112:255–264. [DOI] [PubMed] [Google Scholar]
- 12.ICH Steering Committee. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: ICH Harmonised Tripartite Guideline for Good Clinical Practice. 3rd edLondon, UK: Brookwood Med Pub; 1998. [Google Scholar]
- 13.Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133:299S–339S. [DOI] [PubMed] [Google Scholar]
- 14.de Vaal JB, de Wilde RB, van den Berg PC, et al. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth 2005; 95:326–331. [DOI] [PubMed] [Google Scholar]
- 15.Bundgaard-Nielsen M, Holte K, Secher NH, et al. Monitoring of intraoperative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand 2007; 51:331–340. [DOI] [PubMed] [Google Scholar]
- 16.Johansson PI, Svendsen MS, Salado J, et al. Investigation of the thrombin-generating capacity, evaluated by thrombogram and clot formation evaluated by thrombelastography of platelets stored in the blood bank for up to 7 days. Vox Sang 2008; 94:113–118. [DOI] [PubMed] [Google Scholar]
- 17.Niemi TT, Miyashita R, Yamakage M. Colloid solutions: a clinical update. J Anesth 2010; 24:913–925. [DOI] [PubMed] [Google Scholar]
- 18.The Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998; 317:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arunachalam L, MacFie J. Colloid versus crystalloid fluid therapy in surgical patients. BJS 2015; 102:145–147. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S, Bellomo R, Boyce N, et al. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256. [DOI] [PubMed] [Google Scholar]
- 21.Ågren A, Wikman AT, Holmström M, et al. Thromboelastography (TEG®) compared to conventional coagulation tests in surgical patients – a laboratory evaluation. Scand J Clin Lab Invest 2013; 73:214–220. [DOI] [PubMed] [Google Scholar]
- 22.Pekelharing J, Furck A, Banya W, et al. Comparison between thromboelastography and conventional coagulation tests after cardiopulmonary bypass surgery in the paediatric intensive care unit. Int J Lab Hematol 2014; 36:465–471. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg 2012; 256:476–486. [DOI] [PubMed] [Google Scholar]
- 24.Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal‘ vs. ‘restrictive‘ intraoperative fluid therapy: a critical assessment of the evidence. Acta Anaesthesiol Scand 2009; 53:843–851. [DOI] [PubMed] [Google Scholar]
- 25.Corcoran T, Rhodes JE, Clarke S, et al. Intraoperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 2012; 114:640–651. [DOI] [PubMed] [Google Scholar]
- 26.Matzen S, Perko G, Groth S, et al. Blood volume distribution during head-up tilt induced central hypovolaemia in man. Clin Physiol 1991; 1:411–422. [DOI] [PubMed] [Google Scholar]
- 27.Bundgaard-Nielsen M, Jørgensen CC, Secher NH, et al. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand 2010; 54:464–469. [DOI] [PubMed] [Google Scholar]


