Abstract
Risk factors were evaluated for surgical bed soft tissue necrosis (STN) in head and neck cancer patients treated with postoperative radiation therapy (PORT) after transoral robotic surgery (TORS) or wide excision with primary closure. Sixty-seven patients were evaluated. STN was defined as ulceration and necrosis of the surgical bed or persistently unhealed high-grade acute mucositis with pain after PORT. The median RT dose of primary site was 63.6 Gy (range, 45–67.15 Gy) with 2 Gy/fx (range 1.8–2.2 Gy/fx). Total 41 patients (61.2%) were treated with concurrent chemoradiotherapy. The median follow-up period was 26 months. STN was diagnosed in 13 patients (19.4%). Most of the patients were treated with oral steroids, antibiotics, and analgesics and the lesions were eventually improved (median of 6 months after PORT). STN did not influence local control. A depth of invasion (DOI > 1.4 cm, odds ratio [OR] 14.04, p = 0.004) and maximum dose/fraction (CTVpmax/fx > 2.3 Gy, OR 6.344, p = 0.043) and grade 3 acute mucositis (OR 6.090, p = 0.054) were related to STN. The 12 (23.5%) of 51 oropharyngeal cancer patients presented STN, and the risk factors were DOI > 1.2 cm (OR 21.499, P = 0.005), CTVpmax/fx > 2.3 Gy (OR 12.972, P = 0.021) and grade 3 acute mucositis (OR 10.537, P = 0.052). Patients treated with TORS or WE with primary closure followed by PORT had a high risk of surgical bed STN. STN risk factors included DOI (>1.2–1.4 cm) and CTVpmax/fx (>2.3 Gy). Radiation therapy after TORS must be carefully designed to prevent STN.
INTRODUCTION
With advances in surgical and radiotherapy (RT) techniques, the oncologic and functional outcomes of head and neck cancer patients are improving. Organ and function preservation with a lesser treatment morbidity is an important goal of treatment plan. Several studies have shown the feasibility and safety and promising oncologic results of using transoral robotic surgery (TORS) since its introduction as a minimally invasive technique by Drs. Weinstein and O’Malley in 2006.1 TORS has been increasingly used to preserve patient functions2–4 and the most commonly applied anatomic site is oropharynx. With the favorable outcomes reported, the indication of TORS has been extended to laryngeal and hypopharyngeal cancer.5 Swallowing and speech functions have improved and surgical morbidities or cosmetic deformities have decreased in patients treated with TORS. According to surgical pathology, adjuvant RT or concurrent chemoradiotherapy (CCRT) after TORS is recommended to improve local control and overall survival. However, the treatment outcome and toxicity of adjuvant RT or CCRT after TORS are not well described.6 There is growing concern related to the complications after TORS followed by RT and the morbidities are not well documented. Several studies have reported unexpected delays in wound healing and STN related to adjuvant RT, and suggested contraindications related to TORS.7–9
Radiation-induced mucositis is resolved through the regeneration process, which requires proliferative stem cells and vascular and connective supplies.10 Disruptions to the lymphovascular supply and injuries to connective tissue along with the reduced repopulation of stem cells in surgical beds might be induced by TORS or wide excision (WE) without reconstructive surgery. This could result in unexpected complications after postoperative radiation therapy (PORT).
We evaluated the frequency and risk factors for surgical bed soft tissue necrosis (STN) in head and neck cancer patients treated with PORT after TORS or WE with primary closure. We attempted to determine quantitative values related to STN, including tumor and dosimetric factors.
PATIENTS AND METHODS
We retrospectively reviewed patients treated with TORS or WE and PORT for head and neck cancer between 2008 and 2014 at Seoul St. Mary's hospital. Adjuvant treatment was determined by our head and neck tumor board. Adjuvant RT was prescribed to patients with multiple lymph node (LN) metastases, the presence of extra-capsular extension (ECE) of LNs, positive or close margins, deep depth of invasion (DOI), lymphovascular invasion, perineural invasion.11 Patients with flap reconstruction of primary surgical site were excluded. Patients who were followed for at least 3 months after RT were included. A total of 67 patients were included in this study.
The patients’ age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, co-morbidity, smoking status, stage, pathologic tumor size, DOI, and adjuvant treatment scheme (CCRT and RT alone) were reviewed. Human papilloma virus (HPV) status was reviewed for oropharyngeal cancer patients. HPV-positive was defined as a positive status according to an in situ hybridization technique, positive immunohistochemical staining of p16 expression or HPV DNA detection by polymerase chain reaction. RT planning data was collected. The time interval between surgery and RT (from surgery to RT commencement), RT duration (from RT start to RT completion), total treatment duration (from surgery to RT completion), and RT modalities were recorded. Radiation doses to the clinical target volume of primary tumor bed (CTVp) were reviewed, including the mean dose of CTVp (CTVpmean), fraction size of CTVpmean (CTVpmean/fx), maximum total dose of CTVp (CTVpmax), and fraction size of CTVpmax (CTVpmax/fx). Radiation was delivered using 3-dimentional conformal RT in 17.9% of patients and intensity modulated radiotherapy (IMRT) with simultaneous integrated boost in 82.1% of patients. A total of 60 Gy was prescribed to the high risk lesion of primary tumor bed or positive LN area and 54 to 58 Gy to the low risk area of neck. Patients with ECE of the nodal region or positive margin were treated with up to 66 Gy. Because of variations in the fractional scheme and total doses, the doses were converted into biological equivalent doses, using α/β = 10 (BED10) because the analyzed mucosal events were acute. BED10 was calculated for the CTVpmean (CTVpmean BED [10]) and CTVpmax (CTVpmax BED [10]). One patient was treated in other radiation oncology institution and dosimetric data were available for 66 patients.
The acute mucositis grade and local recurrence data were collected. The mucositis grade was defined according to the Common Terminology Criteria for Adverse Events version 4.0. STN is defined as ulceration and necrosis of the surgical bed that developed after recovery of acute mucositis or persistent nonhealing high-grade acute mucositis with pain after completion of RT. Surgical bed necrosis was identified by nasopharyngolaryngoscopy (NPL) and the imaging study after completion of PORT.
Fisher exact test and the Mann–Whitney U test were used to compare variables between patients with and without STN. Statistically significant factors in the univariate analysis (P-value <0.10) were included in the multivariate logistic regression model. A receiver operator characteristic (ROC) curve was generated to present the best cut-off value, and Youden index was calculated.12 The local control rate (LCR) was calculated as the period from the completion of RT to the date of disease recurrence in the irradiated volume. The overall survival rate (OSR) was calculated as the period from the completion of RT to the date of death due to any cause. The LCR and OSR were analyzed using the Kaplan–Meier method. All of the tests were performed at the 0.05 significance level. Statistical analyses were performed using SPSS version 20 software (SPSS, Inc., Chicago, IL). This study was performed with the approval of our hospital's Institutional Review Board.
RESULTS
The median follow-up period was 26 months (range, 4–74 months), and the median age was 59 years old (range, 35–89 years old). Surgery types were as follows: TORS (30 patients) and WE with primary closure without reconstructive surgery (37 patients). Patients that were undergoing flap reconstruction were not included in this study. A total of 41 patients (61.2%) were treated with CCRT, and the chemotherapy regimens consisted of fluorouracil/cisplatin (34 of 41) and cisplatin (7 of 41). RT was intentionally delivered to primary tumor bed in 57 patients, and another 10 patients received radiation on the regional neck lymphatics only. However, the adjacent mucosa was indirectly irradiated. The median RT dose of the primary site was 63.6 Gy (range, 45–67.15 Gy) with 2 Gy/fx (range 1.8–2.2 Gy/fx). Indications for adjuvant RT were extracapsular extension (41.8%), positive margin (26.9%), close margin (4.5%), perineural invasion (6%), lymphovascular invasion (50.7%), and stage pN2b, pN2c, pN3 (76.1%) and pT3–4 (14.9%).
A total of 13 patients (19.4%) had a diagnosis of STN. In 7 patients, STN developed after recovery from acute mucositis, and the median time interval from RT completion was 2.7 months (range, 1.7–5.3 month). The remaining 6 patients showed sustained high-grade mucositis in the resection bed of the primary lesion. The NPL showed the ulceration or necrosis of the mucosa in the resection bed of the primary lesion (Figure 1). Magnetic resonance imaging (MRI) showed that 7 patients had mucosal thickening and enhancement in the surgical bed that requiring differentiation between RT related changes and tumor recurrence. The remaining 6 patients did not present abnormal enhancement. The positron emission tomography/computed tomography (PET/CT) were evaluated showing the localized increased fluorodeoxyglucose uptake. The maximum standard uptake value was median 4.1 (range, 2.3–8.6), and the uptake was regressed in the follow-up PET/CT.
FIGURE 1.

Soft tissue necrosis of surgical bed in tonsillar cancer patient. A 72-year-old male patient was treated with TORS for tonsillar ca (pT2N2b). A radiation dose of 60 Gy/30 fx was prescribed to the tonsillar surgical bed, Rt. retropharyngeal lymphatics and Rt. neck IB-II lymphatics and a dose of 54 Gy/30 fx was prescribed to the Rt. level III–V lymphatics because of a positive resection margin and multiple lymph node metastases. The patient developed pain and necrosis of the Rt. tonsillar bed area 11 weeks after RT completion. In the computed tomography, mucosal thickening with necrotic change was noted. Mild FDG uptake (mSUV3.1) was observed along the Rt. pharyngeal wall in the PET/CT evaluation, and the uptake regressed in the follow-up PET/CT. The lesion healed after 7 weeks with antibiotics and steroid treatments. The patient had multiple risk factors for STN, including a primary tumor size of 2.8 cm and depth of invasion of 2 cm. The maximum fraction size delivered to the primary surgical bed was 2.4 Gy/fx. FDG = fluorodeoxyglucose, mSUV = maximum standard uptake value, PET/CT = Positron emission tomography/computed tomography, TORS = transoral robotic surgery.
Two patients were evaluated through a biopsy of the ulcerative lesion to differentiate the tumor recurrence; however, necrotic tissue and several atypical cells with reactive atypia were found. Most of the patients were treated with oral steroids, antibiotics, and analgesics. Eight patients complained of severe pain at the primary surgical bed, and narcotic analgesics were required for pain control. One patient had to be treated with debridement of the tumor bed, and 1 patient was treated with steroid injection into the necrosis bed. STN lesions were eventually resolved at a median of 6 months (range, 2.5–14 months) after PORT. Local recurrence was not observed in STN patients. The 3-year LCR was 92.7%, 100%, and 91.0% for all patients, STN patients, and non-STN patients, respectively. The 3-year OSR was 74.9% for all patients (Figure 2).
FIGURE 2.

Local control rate and overall survival rate for all patients. LCR = local control rate, STN = soft tissue necrosis.
Risk Factors for STN for All Patients
The frequency of STN according to the primary lesion was as follows: oropharynx (12 of 51 patients, 23.5%), hypopharynx (1 of 5, 20%), larynx (0 of 9, 0%), and oral cavity (0 of 2, 0%). The patient and treatment characteristics are shown in Table 1. Comorbidities (P = 0.013), DOI (P = 0.003), and grade 3 acute mucositis (P = 0.007) were significantly associated with STN occurrence. The comorbidities were as follows: hypertension (HTN) in 26 patients, diabetes in 8 patients, other cancer in 8 patients, liver disease in 7 patients, lung disease in 2 patients, coronary artery disease in 2 patients, cerebrovascular disease in 1 patient, kidney disease in 1 patient, and thyroid disease in 1 patient. Sex, age, smoking status, pathologic T stage, tumor size, and concurrent chemoradiotherapy did not present significant differences between the STN and non-STN patients. As for the dosimetric factors, a higher maximum dose/fraction (CTVpmax/fx) was significantly associated with STN occurrence (STN vs non-STN, 2.3 Gy vs 2.19, P = 0.038). The time interval between surgery and RT commencement, RT duration, total treatment duration, RT modality, CTVp volume, CTVpmean, CTVpmax, CTVpmean BED (10), and CTVpmax BED (10) had no influence on the occurrence of STN.
TABLE 1.
Patient and Treatment Characteristics of All Patients

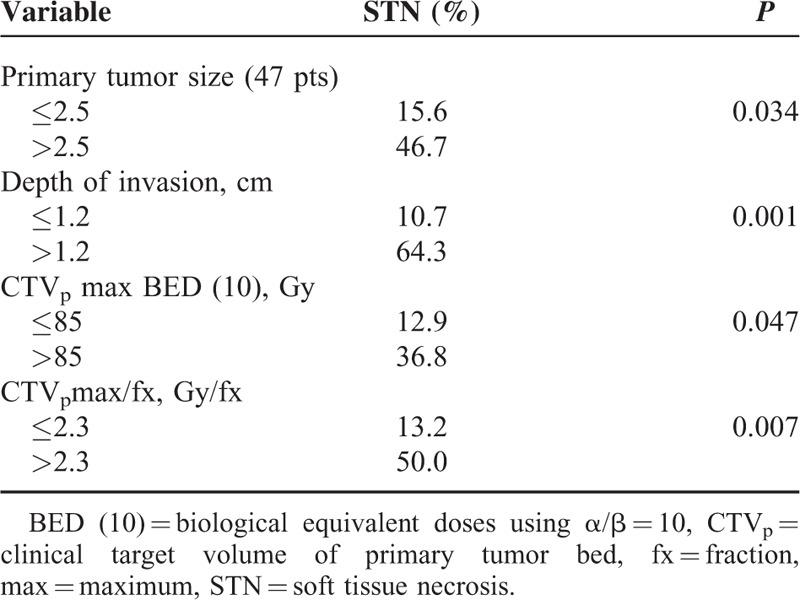
To determine the cut-off values for the DOI and CTVpmax/fx, an ROC curve was generated and Youden index was calculated. For the DOI, the AUC value of the ROC curve was 0.773 (95% confidence interval [CI] = 0.641–0.874, P = 0.0021) and the cut-off value according to Youden index was the 1.4 cm (sensitivity 61.54%, specificity 93.02%). For the CTVpmax/fx, the AUC values was 0.718 (95% CI = 0.594–0.822, P = 0.0122) and cut-off value was 2.3 Gy/fx (sensitivity 50.0%, specificity 87.04%). ROC curves of DOI and CTVpmax/fx are described in Figure 3A. In addition, DOI values >1.4 cm, and CTVpmax/fx values >2.3 Gy/fx had significantly associated with STN occurrence in the univariate analysis (Table 2).
FIGURE 3.

A receiver operator characteristic curves for (A) all patients and (B) oropharyngeal cancer patients, DOI = depth of invasion, CTVp max/fx = fraction size of maximum total dose for primary tumor bed, BED (10) = biological equivalent doses using α/β = 10.
TABLE 2.
Cut-Off Values as a Risk Factor of STN

Significant factors (P-value of <0.10) in the univariate analysis and the cut-off values from the ROC curve were included in the multivariate analysis. Comorbidities, acute grade 3 mucositis, stage pT2–3, DOI, DOI > 1.4 cm, CTVpmax/fx and CTVpmax/fx > 2.3 Gy/fx were included in the multivariate logistic regression analysis. DOI > 1.4 cm (odds ratio [OR] 14.04, P = 0.004) and CTVpmax/fx > 2.3 Gy/fx (OR 6.344, P = 0.043) were predictors for STN occurrence in all patients. Acute grade 3 mucositis presented a tendency of significance (OR 6.090, P = 0.054). The results of the multivariate analysis are shown in Table 3.
TABLE 3.
Multivariate Analysis for STN

Risk Factors for STN in Oropharyngeal Cancer Patients
Oropharyngeal cancer patients (51 patients) were analyzed separately. A total of 11 patients (21.6%) developed STN. The significant predictors for STN from the univariate analysis were comorbidities (P = 0.034), DOI (P = 0.002), acute grade 3 mucositis (P = 0.044), and CTVpmax/fx (P = 0.028). HPV status was not associated with STN occurrence (Table 4). For the DOI, the AUC value in the ROC curve was 0.810 (95% CI = 0.659–0.914, P = 0.0002) and the cut-off value according to Youden index was >1.2 cm (sensitivity 75%, specificity 85.33%). For the CTVpmax/fx, the AUC value was 0.735 (95% CI = 0.561–0.908, P = 0.023) and cut-off value was 2.3 Gy/fx (sensitivity 54.5%, specificity 86.7%). For the tumor size, the AUC value were 0.667 (95% CI = 0.514–0.797, P = 0.056) and cut-off value was >2.5 cm (sensitivity 58.33%, specificity 77.14%). For CTVpmax BED (10), the AUC value was 0.688 (95% CI = 0.500–0.876, P = 0.068) and cut-off value was >85 Gy (sensitivity 63.6%, specificity 73.3%). ROC curves for oropharyngeal cancer patients are described in Figure 3B. In the univariate analysis, the following values were significant; tumor size >2.5 cm, DOI > 1.2 cm, CTVpmax/fx > 2.3 Gy/fx, and CTVpmax BED (10) > 85 Gy in Table 5. A DOI > 1.2 cm (OR 21.499, P = 0.005) and CTVpmax/fx > 2.3 Gy/fx (OR 12.972, P = 0.021) remained significant predictors of STN in multivariate analysis. Acute grade 3 mucositis showed a correlation with STN occurrence (OR 10.537, P = 0.052) (Table 3).
TABLE 4.
Patient and Treatment Characteristics of Oropharyngeal Cancer Patients
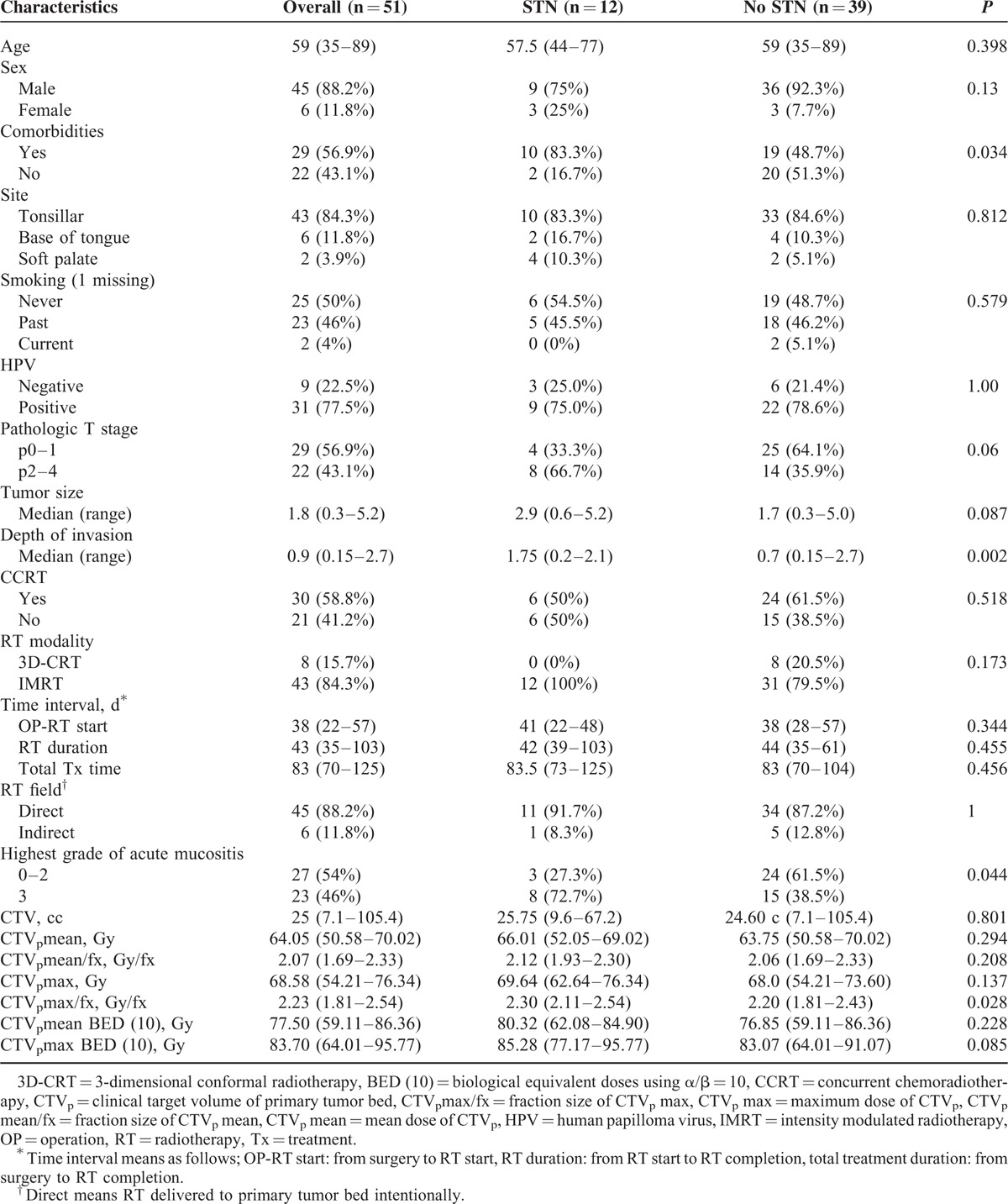
TABLE 5.
Cut-Off Values as a Risk Factor of STN for Oropharyngeal Cancer Patients
DISCUSSION
In this study, we evaluated STN in head and neck cancer patients treated with PORT after TORS and WE with primary closure. A total of 19% of the patients developed STN, and the soft tissue lesions and related pain were eventually improved by oral steroid and opioids. Deeper DOI (>1.2–1.4 cm) and higher CTVpmax/fx (>2.3 Gy/fx) were related to the occurrence of STN, which did not have an effect on local recurrence. Consistent risk factors for STN were observed in a subset analysis of the oropharyngeal cancer patients.
Lukens et al8 first described the significance of STN in patients treated with TORS and PORT, and they reported that DOI, total radiation dose to the resection bed and grade 3 acute mucositis were risk factors for developing STN. These authors were able to reduce the occurrence of STN after limiting the mucosal dose (negative margin: 60 Gy/30 fx; close: 63 Gy/30 fx; and positive: 66 Gy/30 fx), and although they indicated that careful radiation planning is required in patients treated with TORS, clear quantitative relationships were not reported between the mucosal dose and STN occurrence. Though the frequency of STN in our study was lower than the 28% in Lukens report, a similar maximal radiation dose per fraction and greater DOI were found to be important for the development of STN. Furthermore, we provide suggestions for the critical dosimetric and pathologic values of STN patients. Higher CTVpmax/fx at >2.3 Gy/fx and greater DOI at >1.2 cm were significantly associated with STN. In oropharyngeal cancer patients, larger tumor of >2.5 cm also showed a significant effect on STN. The lower frequency of STN in our study may have been caused by a lower median total dose to the resection bed compared with that in Lukas’ study. In addition, the total dose calculated as BED (10) due to variable fractionation scheme was not related to the STN.
Radiation-induced mucosal injuries are healed during the regeneration process through surviving stem cells within the exposed area or migrating stem cells from untreated areas. Patients treated with TORS or WE without flap reconstruction may have fewer stem cells in the primary surgical bed. Furthermore, in cases with larger tumor and greater DOI, fewer stem cells may remain. In our study, greater DOI and larger tumor size correlated with STN. In addition, tumor size >2.5 cm and stage pT2–4 were significant in oropharyngeal cancer patients. We suggest that RT planning should be performed carefully for patients with larger tumors (>2.5 cm) and greater DOI (>1.2 cm) treated with TORS or WE with primary closure.
Comorbidities are risk factors for radiation-induced normal tissue injury. Hypertension and diabetes are highly correlated to radiation damage because they impair vascularity.13,14 In our study, the comorbidities were related to STN, with an STN incidence of 34.5% in patients with comorbidities and 9% in patients without comorbidities. HTN and diabetes were the most common comorbidities, and this result indirectly shows the relationship between STN and impaired vascularity.
STN is considered a consequential late effect (CLE), and the complexity and aggressiveness of treatment may have increase the CLE.13 The relationship of acute mucositis and CLE has been previously described in accelerated RT studies.15,16 To improve local control, accelerated RT schedule have been prescribed for the head and neck cancer patients. Acute mucositis were significantly higher in patients treated with accelerated RT compared with conventional RT.16 As prolonged severe acute mucosal reactions progressed to CLE, radiation necrosis developed in 22% of the accelerated RT group; however, after reducing the fraction size from 2 to 1.8 Gy/fx, further necrosis did not occur. In the PORT studies of patients with accelerated schedules, the authors also reported that at 1.8 Gy/fx, mucosal necrosis did not develop.17 According to these studies, the RT fraction size was important risk factor for developing STN. Similarly, our study showed that higher fraction size could increase STN occurrence even with conventional RT schedule.
Acute mucositis and CLE are known to be related to treatment time.18 However, in our study, treatment time did not affect STN. Significant differences were not observed for the OP to RT interval, RT duration, and overall treatment duration (OP plus RT) between patients with and without STN. RT was usually planned 4 to 6 weeks after surgery, and the total RT treatment lasted approximately 6 to 7 weeks. Therefore the time effect on the STN could not be discerned with this RT schedule. As in Lukas’ study, the treatment time could not be differentiated according to the STN occurrence.8
An increasing number of studies have focused on complications related to TORS. Several studies have suggested that the contraindications of TORS and treatment outcomes of PORT after TORS are under evaluated.7,9 Disruptions of lymphovascular supply and injuries to the connective tissue, and reductions in stem cells in the surgical bed could result in unexpected complications after PORT. The surgical bed of TORS or WE with primary closure is poorly vascularized, and PORT induces further vasculature damage through the occlusion or loss of small vasculature and thrombi formation,10,19 and we speculate that such damage may occur in the time interval between completion of RT and STN occurrence. To improve the therapeutic ratio, appropriate patients must be selected for TORS and risk factors of STN should be carefully reviewed in the planning of PORT.
Our study contained certain limitations. The direct comparison of STN occurrence results between patients with TORS or WE with primary closure and patients with reconstructive surgery were not provided. Additionally, biopsy specimens were not collected in all STN cases, which limited our ability to explain the pathophysiologic mechanisms of STN occurrence.
As minimal invasive treatment, TORS has showed the promising treatment result and its indication has been extended. STN related TORS and PORT is a complication to be manageable, but the morbidity of treatment could be worse. The physicians should be cautious when they decide to do TORS and prescribe radiation therapy to prevent STN.
In conclusion, patients treated with TORS or WE with primary closure followed by PORT had a high risk of surgical bed STN. The risk factors of STN determined from the multivariate analysis included greater DOI (>1.2–1.4 cm) and higher CTVpmax/fx (>2.3 Gy/fx). STN was not related to local recurrence. RT plans after TORS must be carefully designed to prevent STN, and maximal fractional dose to the surgical bed above 2.3 Gy should be avoided. The surgical bed must be contoured, even when only irradiating the neck lymphatics and the actual RT dose delivered to the surgical bed should be evaluated.
Footnotes
Abbreviations: CCRT = concurrent chemoradiotherapy, CLE = consequential late effect, CTVp = clinical target volume of primary tumor bed, CTVpmax = maximum total dose of CTVp , CTVpmax/fx = fraction size of CTVpmax, CTVpmean = mean dose of CTVp , CTVpmean/fx = fraction size of CTVpmean, DOI = depth of invasion, IMRT = intensity modulated radiotherapy, PORT = postoperative radiation therapy, RT = radiotherapy, STN = soft tissue necrosis, TORS = transoral robotic surgery, WE = wide excision.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Weinstein GS, O’Malley BW, Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 2012; 122:1701–1707. [DOI] [PubMed] [Google Scholar]
- 2.Kelly K, Johnson-Obaseki S, Lumingu J, et al. Oncologic, functional and surgical outcomes of primary Transoral Robotic Surgery for early squamous cell cancer of the oropharynx: a systematic review. Oral Oncol 2014; 50:696–703. [DOI] [PubMed] [Google Scholar]
- 3.Lorincz BB, Mockelmann N, Busch CJ, et al. Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: single-institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck 2015; 37:1618–1624. [DOI] [PubMed] [Google Scholar]
- 4.Dziegielewski PT, Teknos TN, Durmus K, et al. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg 2013; 139:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorincz BB, Busch CJ, Mockelmann N, et al. Feasibility and safety of transoral robotic surgery (TORS) for early hypopharyngeal cancer: a subset analysis of the Hamburg University TORS-trial. Eur Arch Otorhinolaryngol 2015; 272:2993–2998. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter TJ, Kann B, Buckstein MH, et al. Tolerability, toxicity, and temporal implications of transoral robotic surgery (TORS) on adjuvant radiation therapy in carcinoma of the head and neck. Ann Otol Rhinol Laryngol 2014; 123:791–797. [DOI] [PubMed] [Google Scholar]
- 7.Huang SH, Hansen A, Rathod S, et al. Primary surgery versus (chemo)radiotherapy in oropharyngeal cancer: the radiation oncologist's and medical oncologist's perspectives. Curr Opin Otolaryngol Head Neck Surg 2015; 23:139–147. [DOI] [PubMed] [Google Scholar]
- 8.Lukens JN, Lin A, Gamerman V, et al. Late consequential surgical bed soft tissue necrosis in advanced oropharyngeal squamous cell carcinomas treated with transoral robotic surgery and postoperative radiation therapy. Int J Radiat Oncol Biol Phys 2014; 89:981–988. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein GS, O’Malley BW, Jr, Rinaldo A, et al. Understanding contraindications for transoral robotic surgery (TORS) for oropharyngeal cancer. Eur Arch Otorhinolaryngol 2015; 272:1551–1552. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J 2014; 32:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005; 27:843–850. [DOI] [PubMed] [Google Scholar]
- 12.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 13.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 2003; 4:529–536. [DOI] [PubMed] [Google Scholar]
- 14.Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist 2002; 7:136–143. [DOI] [PubMed] [Google Scholar]
- 15.Denham JW, Peters LJ, Johansen J, et al. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiother Oncol 1999; 52:157–164. [DOI] [PubMed] [Google Scholar]
- 16.Skladowski K, Maciejewski B, Golen M, et al. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: long-term results of phase III clinical trial. Int J Radiat Oncol Biol Phys 2006; 66:706–713. [DOI] [PubMed] [Google Scholar]
- 17.Suwinski R, Bankowska-Wozniak M, Majewski W, et al. Randomized clinical trial on continuous 7-days-a-week postoperative radiotherapy for high-risk squamous cell head-and-neck cancer: a report on acute normal tissue reactions. Radiother Oncol 2005; 77:58–64. [DOI] [PubMed] [Google Scholar]
- 18.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol 2001; 61:223–231. [DOI] [PubMed] [Google Scholar]
- 19.Dorr W. Radiobiology of tissue reactions. Ann ICRP 2015; 44:58–68. [DOI] [PubMed] [Google Scholar]


