Abstract
Taiwan has the highest prevalence of chronic kidney disease (CKD) worldwide. CKD, a manifestation of vascular diseases, is associated with a high risk of dementia. Here, we estimated the association between influenza vaccination and dementia risk in patients with CKD.
Data from the National Health Insurance Research Database of Taiwan were used in this study. The study cohort included all patients diagnosed with CKD (according to International Classification of Disease, Ninth Revision, Clinical Modification codes) at healthcare facilities in Taiwan (n = 32,844) from January 1, 2000, to December 31, 2007. Each patient was followed up to assess dementia risk or protective factors: demographic characteristics of age and sex; comorbidities of diabetes, hypertension, dyslipidemia, cerebrovascular diseases, parkinsonism, epilepsy, substance and alcohol use disorders, mood disorder, anxiety disorder, psychotic disorder, and sleep disorder; urbanization level; monthly income; and statin, metformin, aspirin, and angiotensin-converting enzyme inhibitor (ACEI) use. A propensity score was derived using a logistic regression model for estimating the effect of vaccination by accounting for covariates that predict receiving the intervention (vaccine). A time-dependent Cox proportional hazard model was used to calculate the hazard ratios (HRs) of dementia among vaccinated and unvaccinated CKD patients.
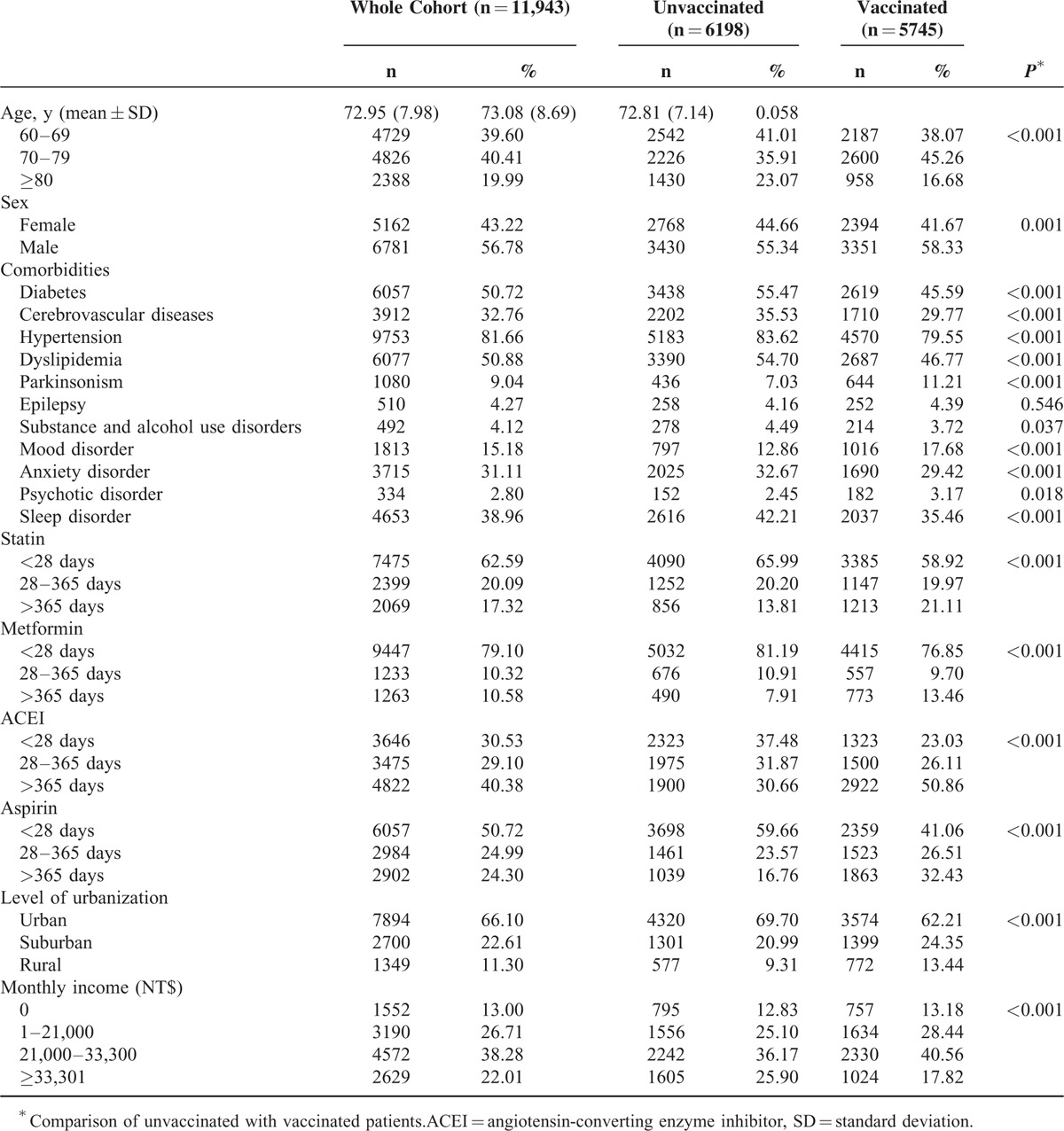
The study population comprised 11,943 eligible patients with CKD; 5745 (48%) received influenza vaccination and the remaining 6198 (52%) did not. The adjusted HRs (aHRs) of dementia decreased in vaccinated patients compared with those in unvaccinated patients (influenza season, noninfluenza season, and all seasons: aHRs = 0.68, 0.58, and 0.64; P < 0.0001, P < 0.0001, and P < 0.0001, respectively). In the sensitivity analysis, adjustments were made to estimate the association of age and sex; diabetes, dyslipidemia, hypertension, cerebrovascular diseases, anxiety disorder; and statin, metformin, ACEI, and aspirin use with the incidence of dementia in various models. A stronger protective effect against dementia risk was demonstrated during the noninfluenza season. Regardless of comorbidities or drug use, influenza vaccination was an independent protective factor and dose-dependently reduced the risk of dementia in CKD patients.
Influenza vaccination exerts dose–response and synergistic protective effects against dementia in CKD patients with dementia risk factors by reducing the incidence of dementia.
INTRODUCTION
Chronic kidney disease (CKD) is associated with a high risk of cardiovascular diseases, end-stage renal disease (ESRD), infection, and malignancy and high mortality.1 Taiwan has the highest prevalence of ESRD worldwide.2 A large Taiwanese cohort study demonstrated a high prevalence of CKD (11.9%) in adults and an even higher prevalence (37.2%) among the elderly population.3 Wen et al demonstrated that patients with CKD had 83% higher mortality for all causes and 100% higher mortality for cardiovascular diseases.3 A moderate reduction in the glomerular filtration rate (GFR), defined as a serum creatinine level ≥1.5 mg/dL (133 μmol/L) for men and ≥1.3 mg/dL (115 μmol/L) for women, is associated with accelerated cognitive decline and increased dementia risk in elderly people.4–6 Renal impairment is associated with vascular dementia in individual with good to excellent health. Moreover, faster GFR decline is associated with more significant cognitive decline.7 These observations are consistent with accumulated data showing an increase in the risk of coronary heart disease in patients with mild to moderate CKD. Hence, CKD is considered a manifestation of vascular diseases.
Patients with CKD have a 100% higher risk of cardiovascular diseases, and many studies have shown that cardiovascular diseases are associated with dementia.8–11 The incidence of dementia among Taiwanese people aged 65 years and older increased from 4.1% in 1980 to 10.2% in 2007. A study of the population of Taiwan revealed that the prevalence of dementia was ∼1.7% to 4.3% among elderly people.12 In Taiwan, the high prevalence of CKD may be correlated with dementia. Establishing health policies for reducing dementia risk in patients with CKD is crucial for lowering the societal burden and the care cost.
The influenza vaccination policy in Taiwan has gradually expanded from high-risk populations to key spreaders to further reduce the number of influenza cases and deaths. Patients with CKD are considered a high-risk population for influenza. Hence, the government announces the priority groups for vaccinations every year on the basis of the recommendations of the Advisory Committee on Immunization Practices (ACIP).13 Here, we estimated the association between influenza vaccination and dementia risk in patients with CKD to determine whether influenza vaccination reduces dementia risk in such patients.
Patients And Methods
The National Health Insurance (NHI) program, which was established in 1995, currently provides comprehensive health insurance coverage to 98% of >23 million people. In this study, data from the National Health Insurance Research Database (NHIRD) were used. No statistically significant differences were observed in age, sex, or healthcare costs between the sample group and all enrollees. According to the Taiwan Center for Disease Control, the influenza season is defined as the interval from October to March. Data in the NHIRD that could be used to identify patients or care providers, including medical institutions and physicians, are scrambled before being sent to the National Health Research Institutes for database construction and are further scrambled before being released to researchers. Theoretically, querying the data alone to identify people at any level by using this database is impractical. All researchers using the NHIRD and the data subsets must sign a written agreement declaring that they have no intention of attempting to obtain information that could potentially violate the privacy of patients or care providers.14
The study cohort comprised all patients diagnosed with CKD (according to International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes) at healthcare facilities in Taiwan (n = 32,844) from January 1, 2000, to December 31, 2007. We excluded all patients without a subsequent outpatient visit, emergency department visit, or inpatient hospitalization for CKD within 12 months of the first presentation (n = 9535), because they were considered to not have CKD (Figure 1). We also excluded 27,276 patients who were <60 years (n = 8557) and had a history of any inpatient or outpatient diagnosis related to cancer before the enrollment date (n = 1605) or those vaccinated within 6 months before the enrollment date (n = 1386).
FIGURE 1.
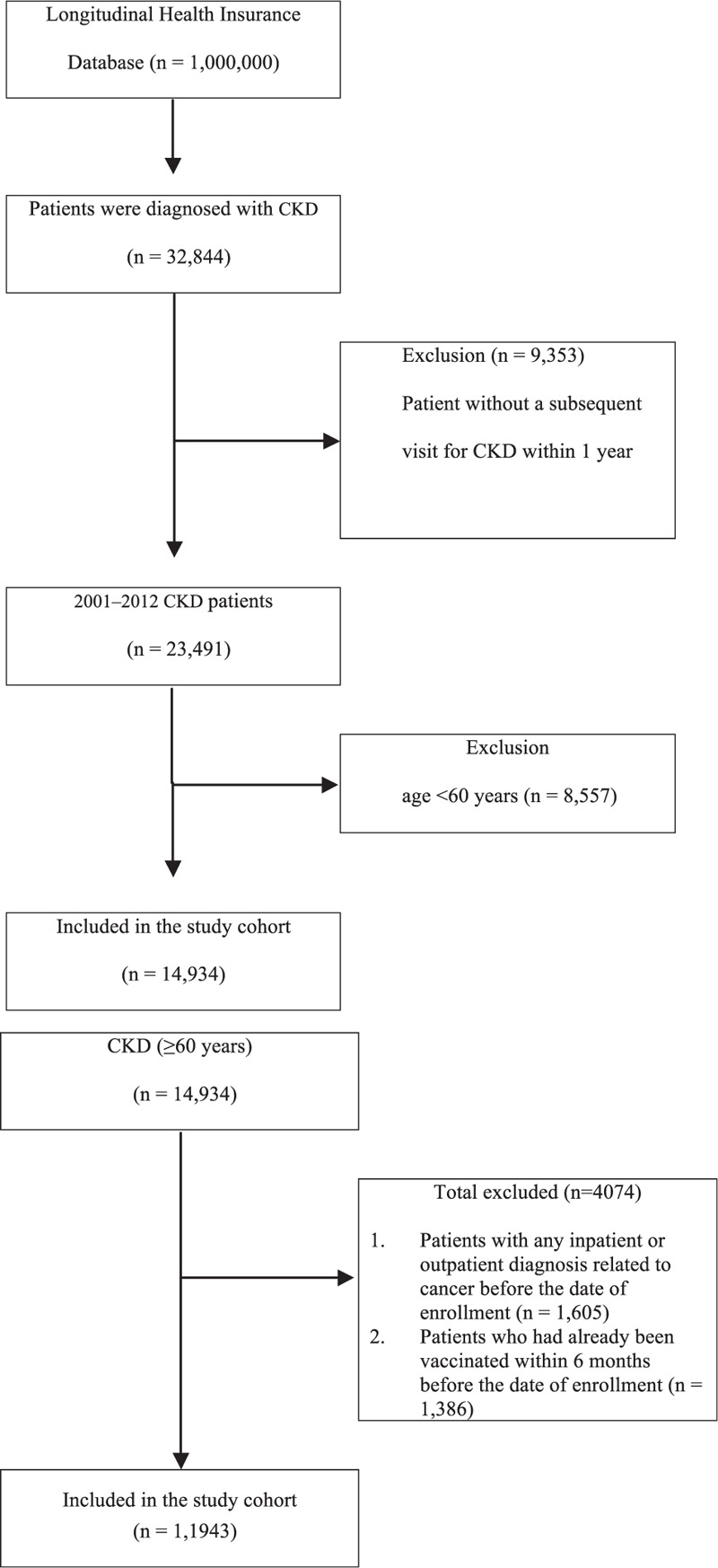
Patient selection flowchart.
In Taiwan, influenza vaccination has been free of charge and recommended for high-risk adults aged ≥50 years (i.e., those with type 2 diabetes, chronic liver infection or liver cirrhosis, cardiovascular diseases, or chronic pulmonary diseases) since 1998 and for all adults aged >65 years since 2001. The vaccination status was identified using the ICD-9-CM code V048 and/or the use of vaccine (confirmed by drug codes).15 Our final study cohort contained 11,943 patients diagnosed with CKD in Taiwan >8-year period; 5745 were vaccinated against influenza and 6198 were not. Each patient was followed up to assess the risk of dementia or protective factors: the demographic characteristics of age and sex; the comorbidities of diabetes, hypertension, dyslipidemia, cerebrovascular diseases, parkinsonism, epilepsy, substance- and alcohol-use disorders, mood disorder, anxiety disorder, psychotic disorder, and sleep disorder; urbanization level; monthly income; and statin, metformin, aspirin, and angiotensin-converting enzyme inhibitor (ACEI) use. A propensity score (PS) was derived using a logistic regression model to estimate the effect of vaccination by accounting for the covariates predicting receiving the intervention (vaccine). This method is used in observational studies to reduce selection bias.16 The covariates in the main model were adjusted for PSs of age, sex, comorbidities, urbanization level (urban, suburban, and rural), monthly income (0; NT$1–NT$21,000; NT$21,000–NT$33,300; and ≥NT$33,301; NT$ represents new Taiwan dollars) (Table 2). The endpoint was the incidence of dementia (ICD-9-CM codes 331.0, 290.4, and 290.0–290.3) in vaccinated or unvaccinated patients with a subsequent outpatient visit, emergency department visit, or inpatient hospitalization for dementia within 12 months, and unvaccinated patients served as the reference arm. Because the protective effect of vaccination is specific to the influenza season, evaluating the noninfluenza season could indicate the possible contribution of bias to the estimates observed during the influenza season. The relationship between the seasonal effect of vaccination and dementia risk was also analyzed. The cumulative incidence of dementia in vaccinated and unvaccinated CKD patients was estimated using the Kaplan–Meier method. To examine the effect of the total number of vaccinations on the cumulative incidence of dementia, we categorized patients into 4 groups according to the vaccination status: unvaccinated and 1, 2 to 3, and ≥4 total vaccinations.
TABLE 2.
Risk of Dementia Among Unvaccinated and Vaccinated Patients in the Study Cohort
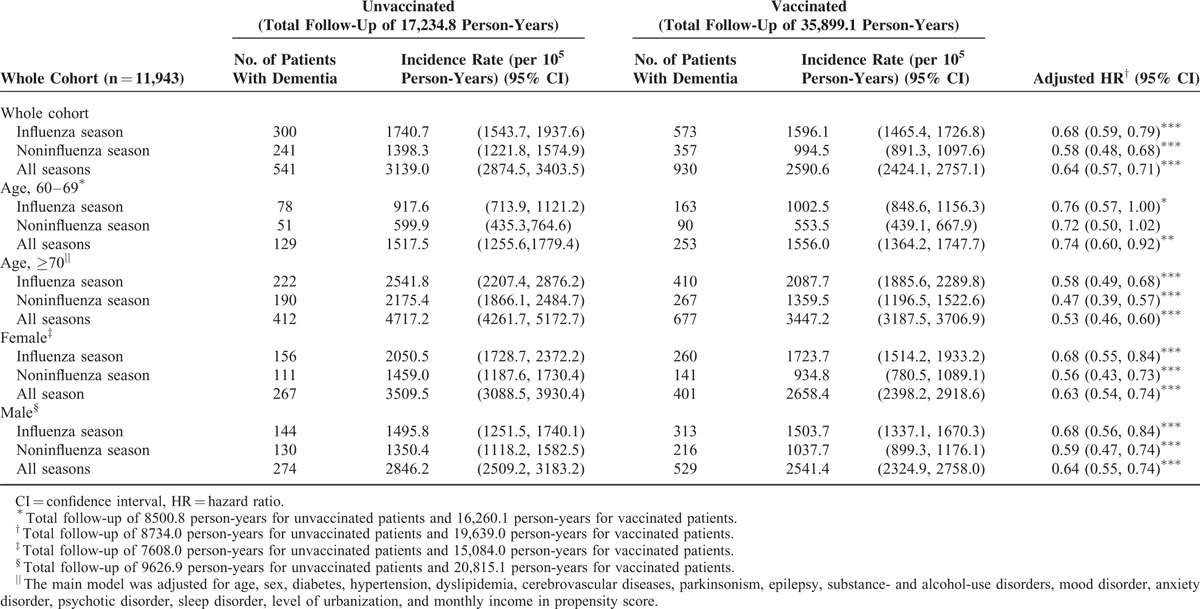
A time-dependent Cox proportional hazard model was used to calculate the hazard ratios (HRs) of dementia among vaccinated and unvaccinated patients with CKD. The HRs were adjusted for age, sex, baseline comorbidities, and metformin, statin, aspirin, and ACEI use in the multivariate analysis. A stratified analysis was conducted to evaluate the effect of vaccination on age and sex (Table 2). All analyses were conducted using SAS software, Version 9.3 (SAS, Cary, NC); 2-tailed P < 0.05 was considered significant. In sensitivity analyses, external adjustments improve the understanding of the effects of drugs and other covariates in epidemiologic database studies.17 Hence, in the sensitivity analysis, adjustments were made to estimate the association of age and sex; diabetes, dyslipidemia, hypertension, cerebrovascular diseases, and anxiety disorder; and statin, metformin, ACEI, and aspirin use with the incidence of dementia in different models. The models stratified by different seasons were adjusted for covariates in the main model and each additional covariate (Tables 3–5).
TABLE 3.
Sensitivity Analysis of Adjusted HRs of Vaccination in Risk Reduction of Dementia in Influenza Season
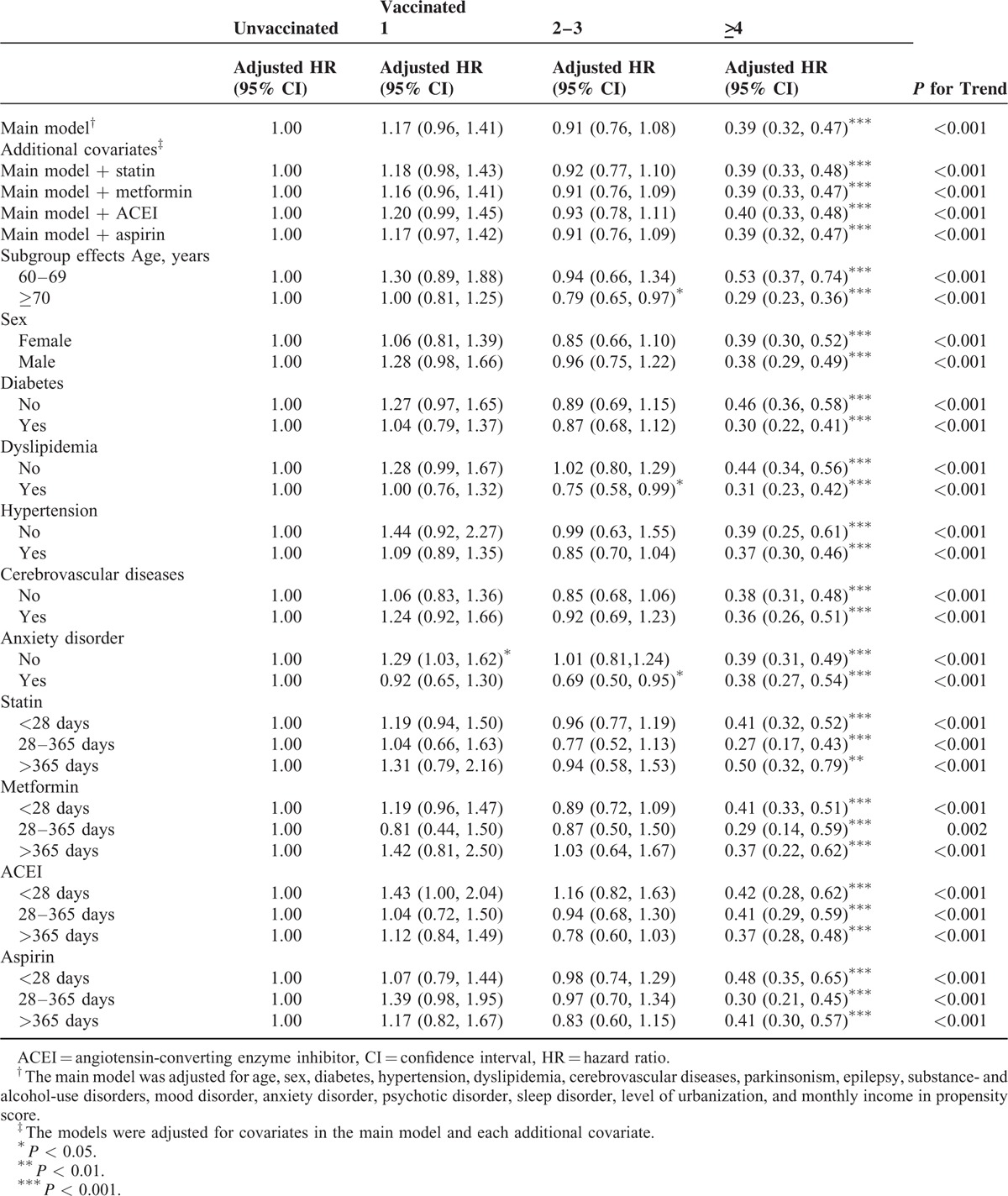
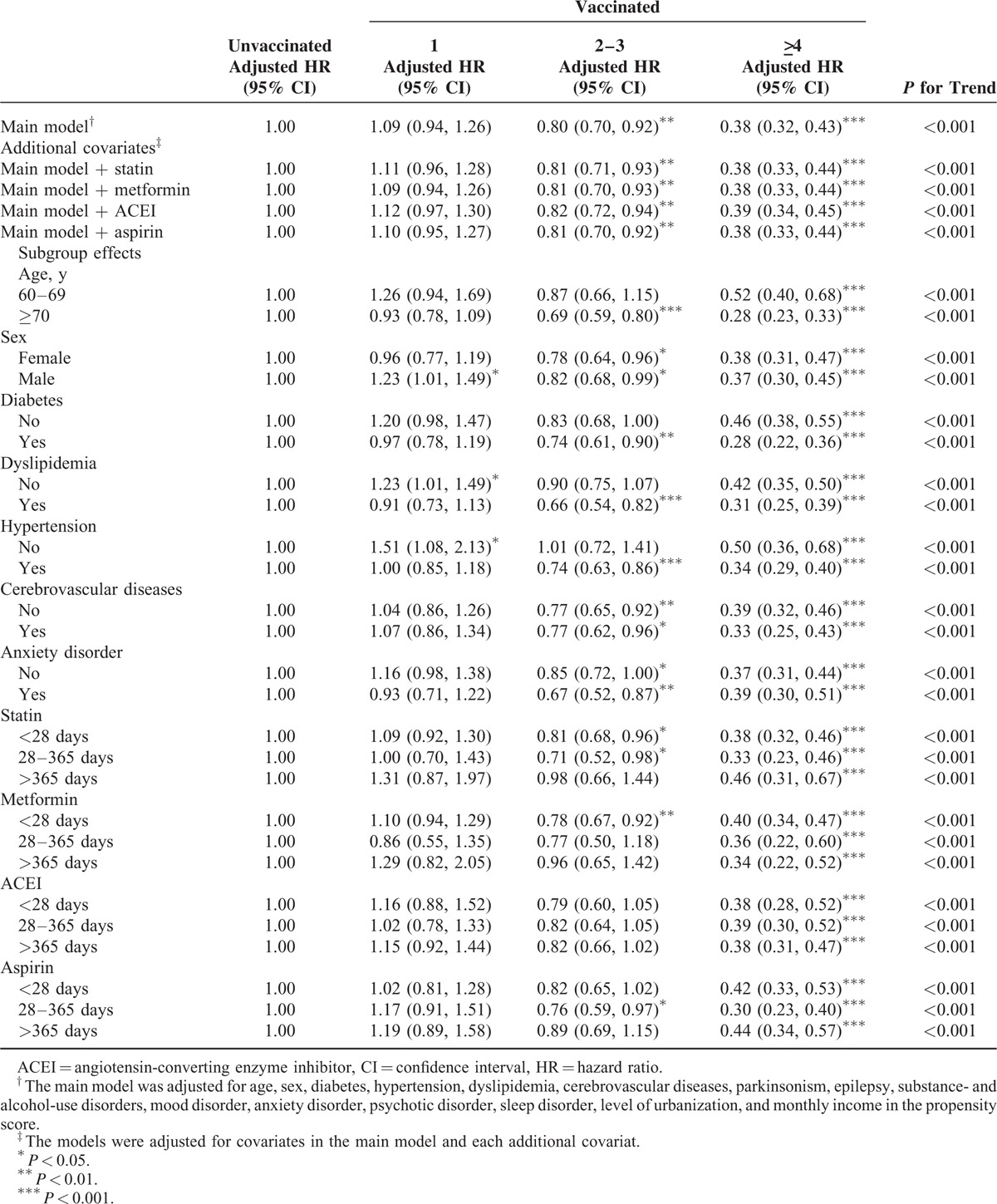
TABLE 5.
Sensitivity Analysis of Adjusted HRs of Vaccination in Risk Reduction of Dementia in All Seasons
RESULTS
The study cohort comprised 11,943 patients, of whom 5745 (48%) received influenza vaccination and the remaining 6198 (52%) did not (Table 1). The total follow-up duration was 17,234.8 and 35,899.1 person-years for unvaccinated and vaccinated patients, respectively. Compared with vaccinated patients, unvaccinated patients exhibited a higher prevalence of preexisting medical comorbidities including diabetes (P < 0.001), cerebrovascular diseases (P < 0.001), hypertension (P < 0.001), dyslipidemia (P < 0.001), substance- and alcohol-use disorders (P = 0.037), anxiety disorder (P < 0.001), and sleep disorder (P < 0.001). By contrast, vaccinated patients exhibited a higher prevalence of comorbidities such as parkinsonism (P < 0.001), mood disorder (P < 0.001), and psychotic disorder (P = 0.018). In addition, significant differences were observed in the distributions of age, monthly income, and urbanization level as well as statin, aspirin, ACEI, and metformin use between vaccinated and unvaccinated patients (Table 1). A higher proportion of unvaccinated patients used statin, metformin, ACEI, and aspirin for < 365 days; however, most vaccinated patients used these drugs for >365 days. A lower proportion of vaccinated patients had a monthly income of ≥NT$33,301 and resided in urban areas. Table 2 shows the risk of dementia among the unvaccinated and vaccinated patients in the study cohort. After adjustment for age, sex, comorbidities, urbanization level, and monthly income, we analyzed the PS. The adjusted HRs (aHRs) of dementia decreased in vaccinated patients compared with those in unvaccinated patients (influenza season, noninfluenza season, and all seasons: aHRs = 0.68, 0.58, and 0.64; P < 0.0001, P < 0.0001, and P < 0.0001, respectively). The stratified analysis showed that the aHRs still significantly decreased in vaccinated patients, particularly in patients aged ≥70 years, regardless of sex. During the noninfluenza season, the aHRs decreased regardless of age or sex, except for the 60 to 69 age group, whose sample size was relatively small compared with that of the other subgroups (Table 2).
TABLE 1.
Characteristics of the Study Population
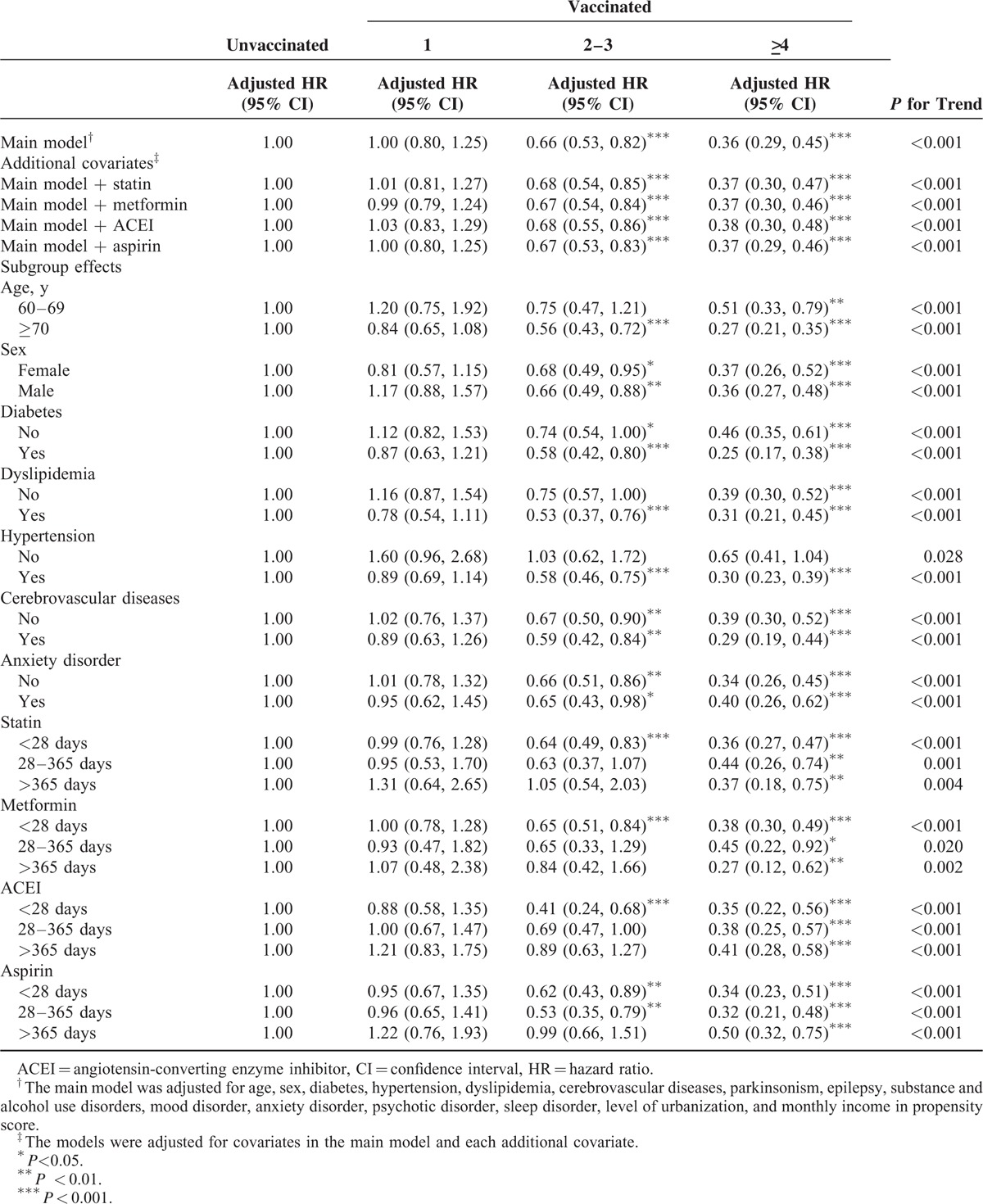
In the sensitivity analysis, adjustments were made to estimate the association of age and sex; diabetes, dyslipidemia, hypertension, cerebrovascular diseases, and anxiety disorder; and statin, metformin, ACEI, and aspirin use with the incidence of dementia in different models. Table 3 shows that the effects of vaccination remained significant in the subgroups of various covariates during the influenza season. Vaccination dose-dependently reduced the risk of dementia in all subgroups and the main model with additional covariates (statin, metformin, ACEI, or aspirin use). All aHRs indicated that vaccination induced >4-fold statistically significant reductions in the risk of dementia in all subgroups, regardless of comorbidities or drug use (P < 0.001). Our data revealed that the vaccination frequency reflected the protective effect against dementia during the influenza season. The protective effect was more predominant in patients aged ≥70 years (≥4 vaccinations: aHR = 0.29, 95% confidence interval [CI]: 0.23, 0.33) and in those with diabetes (≥4 vaccinations: aHR = 0.30, 95% CI: 0.22, 0.41) and dyslipidemia (≥4 vaccinations: aHR = 0.31, 95% CI: 0.23, 0.42). As shown in Table 4, we analyzed the sensitivity analysis adjustments in the noninfluenza season. A stronger protective effect against dementia was demonstrated during the noninfluenza season. Less frequent vaccination statistically significantly reduced dementia. Vaccination at a frequency of 2 to 3 times conferred a protective effect to CKD patients. The protective effect was more predominant in patients aged ≥70 years (2–3 vaccinations: aHR = 0.56, 95% CI: 0.43, 0.72; ≥4 vaccinations: aHR = 0.27, 95% CI: 0.21, 0.35) and in those with diabetes (2–3 vaccinations: aHR = 0.58, 95% CI: 0.42, 0.80; ≥4 vaccinations: aHR = 0.25, 95% CI: 0.17, 0.38), dyslipidemia (2–3 vaccinations: aHR = 0.53, 95% CI: 0.37, 0.76; ≥4 vaccinations: aHR = 0.31, 95% CI: 0.21, 0.45), hypertension (2–3 vaccinations: aHR = 0.58, 95% CI: 0.46, 0.75; ≥4 vaccinations: aHR = 0.30, 95% CI: 0.23, 0.39), and cardiovascular diseases (2–3 vaccinations: aHR = 0.59, 95% CI: 0.42, 0.84; ≥4 vaccinations: aHR = 0.29, 95% CI: 0.19, 0.44). During all seasons (Table 5), the trend of dementia reduction still reflected the frequency of vaccination. The protective effect was more predominant in patients aged ≥70 years (2–3 vaccinations: aHR = 0.69, 95% CI: 0.59, 0.80; ≥4 vaccinations: aHR = 0.28, 95% CI: 0.23, 0.33) and in those with diabetes (2–3 vaccinations: aHR = 0.74, 95% CI: 0.61, 0.90; ≥4 vaccinations: aHR = 0.28, 95% CI: 0.22, 0.36), dyslipidemia (2–3 vaccinations: aHR = 0.66, 95% CI: 0.54, 0.82; ≥4 vaccinations: aHR = 0.31, 95% CI: 0.25, 0.39), hypertension (2–3 vaccinations: aHR = 0.74, 95% CI: 0.63, 0.86; ≥4 vaccinations: aHR = 0.34, 95% CI: 0.29, 0.40), and cardiovascular diseases (2–3 vaccinations: aHR = 0.77, 95% CI: 0.62, 0.96; ≥4 vaccinations: aHR = 0.29, 95% CI: 0.33, 0.44). Regardless of statin, metformin, ACEI, or aspirin use, vaccination was an independent protective factor and dose-dependently reduced dementia in patients with CKD.
TABLE 4.
Sensitivity Analysis of Adjusted HRs of Vaccination in Risk Reduction of Dementia in Noninfluenza Season
DISCUSSION
To date, few studies have investigated the association between dementia risk and influenza vaccination. Nichol et al demonstrated that the influenza vaccination of elderly patients is associated with reduced risks of heart and cerebrovascular diseases.18 Possible mechanisms of the increased risk of cerebrovascular and cardiovascular events after upper respiratory tract illnesses (e.g., influenza) include alterations in circulating clotting factors, inflammatory response protein levels, and cytokine levels and platelet aggregation and lysis. These changes might enhance thrombotic tendencies, impair vasodilation, or cause endothelial injury. Clinical manifestations of cardiovascular diseases and markers of atherosclerotic disease burden may also be used to identify people at risk for cognitive decline and dementia.19–22 Verreault et al showed that previous influenza vaccination may protect against Alzheimer disease (AD) development.23 Cerebrovascular diseases and AD frequently coexist. Hypertension is the primary risk factor for vascular diseases in the brain. Cerebrovascular diseases are associated with deteriorated cognitive performance in AD patients, and clinicopathologic studies have suggested that cerebrovascular diseases lower the threshold for clinical dementia in patients with a neuropathologic diagnosis of AD.24,25 Our finding that influenza vaccination reduced dementia risk might reflect the response of heart disease, cerebrovascular diseases, and AD decreasing. Based on our literature review, this is the first study to demonstrate the association of dementia risk with influenza vaccination in patients with CKD.
A moderate reduction in GFR is associated with accelerated cognitive decline and increased dementia risk in elderly patients.5,6 Among people with good to excellent health, renal impairment is associated with vascular dementia, but not AD. Faster GFR decline is also associated with more significant cognitive decline.7 Our observations are consistent with accumulated data showing an increase in the risk of coronary heart disease in patients with mild to moderate CKD; hence, CKD is considered a manifestation of vascular diseases.26–28 Vascular risk factors such as hypertension, dyslipidemia, and diabetes are associated with cognitive decline and all-cause dementia and AD and vascular dementia individually. Influenza vaccination reduces the risk of cerebrovascular and cardiovascular diseases.18 Vaccination predominately reduced dementia in patients aged ≥70 years (2–3 vaccinations: aHR = 0.69, 95% CI: 0.59, 0.80; ≥4 vaccinations: aHR = 0.28, 95% CI: 0.23, 0.33) and in those with diabetes (2–3 vaccinations: aHR = 0.74, 95% CI: 0.61, 0.90; ≥4 vaccinations: aHR = 0.28, 95% CI: 0.22, 0.36), dyslipidemia (2–3 vaccinations: aHR = 0.66, 95% CI: 0.54, 0.82; ≥4 vaccinations: aHR = 0.31, 95% CI: 0.25, 0.39), hypertension (2–3 vaccinations: aHR = 0.74, 95% CI: 0.63, 0.86; ≥4 vaccinations: aHR = 0.34, 95% CI: 0.29, 0.40), and cardiovascular diseases (2–3 vaccinations: aHR = 0.77, 95% CI: 0.62, 0.96; ≥4 vaccinations: aHR = 0.29, 95% CI: 0.33, 0.44). Our results suggest that influenza vaccination reduces dementia risk in patients with CKD.
Patients with CKD exhibit an increased risk of infection.29,30 The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that patients with all stages of CKD should be annually vaccinated against influenza, unless contraindicated.31 However, whether influenza vaccination reduces the morbidity or mortality of CKD patients is unclear. Studies have suggested that vaccination is associated with a diminished incidence of influenza and all-cause hospitalization, improving the survival of such patients.32,33 However, these results may have been confounded by unmeasured prognostic variables. In addition, a study revealed that the antibody response to the influenza vaccine in CKD patients was lower than that in the general population.34 This response, in contrast to that to other vaccines, was not significantly different from that in controls, although the number of CKD patients investigated was relatively low. In our study, the largest current series of CKD patients were analyzed, and the protective effect against dementia was obvious. The influenza vaccination of patients with CKD reduced not only mortality (controversial)33 but also dementia.
In clinical practice, we suggest that CKD patients with high dementia risk be vaccinated.5–7 Whitmer et al identified that hypertension, high cholesterol, and diabetes at midlife were associated with a 20% to 40% increase in dementia risk; multiple risk factors were dose-dependently associated with a higher risk of dementia.35 In our study, dementia risk reduction and the protective effect were more predominant in patients with diabetes, dyslipidemia, hypertension, and cerebrovascular diseases compared with other patients. This is because the presence of additional dementia risk factors could proportionally increase the incidence of dementia in patients with CKD; hence, the protective effect of influenza vaccination was stronger. CKD is considered a manifestation of vascular diseases.26–28 CKD patients with additional risk factors are more susceptible to dementia. Our findings demonstrate that influenza vaccination had dose-dependent protective effects on elderly CKD patients, particularly those with diabetes, dyslipidemia, hypertension, and cerebrovascular diseases (Tables 3–5).
In this study, the magnitude of the bias demonstrated by the associations observed during the noninfluenza season was sufficient to completely account for the associations observed during the influenza season. A competing risk factor exists between influenza and noninfluenza seasons. Studies have demonstrated an increased risk of hospital admission and a high mortality rate among elderly men during the influenza season.33,36,37 The higher mortality risk during the influenza season might mask the incidence of dementia episodes, and CKD patients might die before dementia development. This is why influenza vaccination had a stronger protective effect against dementia during the noninfluenza season (Tables 3 and 4).
Based on our research, this is the first study to demonstrate the association of influenza vaccination with the risk of dementia in CKD patients. Previous studies showed that patients with CKD had a high risk of influenza-related mortality and high risk of dementia; however, we do not have sufficient evidence to resolve the problem in patients with CKD. Our findings demonstrate that the protective effect against dementia risk in patients with CKD was dose dependent. The major solution might be the regular influenza vaccination of CKD patients, particularly those with dementia risk factors such as hypertension, dyslipidemia, diabetes, and cerebrovascular diseases. Tables 3–5 show the sensitivity analysis of the aHRs of age, sex, comorbidities, urbanization level, and monthly income in the PS. The models were adjusted for covariates in the main model and each additional covariate to estimate the reduction in dementia risk during the follow-up period. The dose-dependent protective effect of influenza vaccination was observed regardless of age and sex; diabetes, hypertension, dyslipidemia, cerebrovascular diseases, and anxiety disorder; or ACEI, metformin, statin, and aspirin use, with stratification by various frequencies of influenza vaccination. The dose-dependent protective effect of influenza vaccination was observed for different conditional statuses. In addition, this is the first study to evaluate the dose–response effect of influenza vaccination on dementia risk. Our results show that influenza vaccination was less effective in reducing dementia only once. Influenza vaccines should be administered at least once or >2 to 3 times for achieving a significant protective effect in CKD patients with dementia risk factors during the noninfluenza season (Table 4). The higher the frequency of influenza vaccination is, the more significant the protective effect against dementia in CKD patients becomes. The strength of the present study is its large sample size. The results suggest that the incidence of dementia was reduced in CKD patients through the preventive strategy of influenza vaccination. This is the first study to demonstrate that influenza vaccination exerts dose–response and synergistic protective effects against dementia in CKD patients with dementia risk factors by reducing the incidence of dementia.
This study has potential limitations. Evidence from observational studies suggests that lifestyle factors, particularly social, mental, and physical activity, are inversely associated with the risk of cognitive decline and dementia. However, methodologic concerns may obscure the precise relationship between these factors and dementia risk. One theory states that higher educational levels and cognitive activity produce a cognitive reserve that reduces the impact of neurodegeneration on cognitive function. In our study, we used the PS to match age, sex, comorbidities, urbanization level, and monthly income. The urbanization level and monthly income are unvalidated alternatives to lifestyle factors and the educational level. To obtain such information, a large randomized trial should apply a suitable regimen to appropriately selected patients for comparing standard approaches. Moreover, the diagnoses of dementia and all other comorbidities were completely dependent on the ICD codes. Nevertheless, the National Health Insurance Administration randomly reviews medical records and interviews patients to validate diagnoses. Hospitals with outlier diagnoses and practices may be audited and subsequently heavily penalized if malpractice and discrepancies are discovered. Another limitation is that information on several unmeasured confounders, including body mass index, smoking, alcohol intake, and use of other over-the-counter drugs (which are associated with dementia), is not available in the NHIRD. However, considering the magnitude and significance of the observed effects, it is unlikely that these limitations compromised the results. Finally, our study is not a prospective randomized blinded study; hence, a cause–effect relationship could not be established. The findings of this study suggest that influenza vaccination exerts a significant protective effect against dementia in CKD patients with dementia risk factors by reducing the incidence of dementia. Additional randomized studies are required to verify these findings.
CONCLUSIONS
Influenza vaccination exerts dose–response and synergistic protective effects against dementia in CKD patients with dementia risk factors by reducing the incidence of dementia.
Footnotes
Abbreviations: PS = propensity score, ACEI = angiotensin-converting enzyme inhibitor, AD = Alzheimer disease, CKD = chronic kidney disease, ESRD = end-stage renal disease, GFR = glomerular filtration rate, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database.
J-CL and Y-PH have contributed equally to this study (joint first authors).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton) 2010; 15 Suppl 2:3–9. [DOI] [PubMed] [Google Scholar]
- 3.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 4.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004; 15:1904–1911. [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Tanne D, Boyle PA, et al. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009; 73:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miwa K, Tanaka M, Okazaki S, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 2014; 82:1051–1057. [DOI] [PubMed] [Google Scholar]
- 7.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011; 77:2043–2051. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012; 2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obisesan TO, Gillum RF, Johnson S, et al. Neuroprotection and neurodegeneration in Alzheimer's disease: role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in african americans. Int J Alzheimers Dis 2012; 568382:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligthart SA, Moll van Charante EP, Van Gool WA, et al. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag 2010; 6:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Mosley TH, Jr, Gottesman RF, et al. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009; 80:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuh JL, Wang SJ. Dementia in Taiwan: past, present, and future. Acta Neurol Taiwan 2008; 17:153–161. [PubMed] [Google Scholar]
- 13.Chan TC, Fu YC, Wang DW, et al. Determinants of receiving the pandemic (H1N1) 2009 vaccine and intention to receive the seasonal influenza vaccine in Taiwan. PLoS One 2014; 9:e101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CI, Kuan CF, Fang YA, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore) 2015; 94:e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung LC, Chen CI, Fang YA, et al. Influenza vaccination reduces hospitalization for acute coronary syndrome in elderly patients with chronic obstructive pulmonary disease: a population-based cohort study. Vaccine 2014; 32:3843–3849. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006; 15:291–303. [DOI] [PubMed] [Google Scholar]
- 18.Nichol KL, Nordin J, Mullooly J, et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003; 348:1322–1332. [DOI] [PubMed] [Google Scholar]
- 19.Wendell CR, Waldstein SR, Ferrucci L, et al. Carotid atherosclerosis and prospective risk of dementia. Stroke 2012; 43:3319–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis JP, Launer LJ, Terry JG, et al. Subclinical atherosclerotic calcification and cognitive functioning in middle-aged adults: the CARDIA study. Atherosclerosis 2013; 231:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan H, Crain B, Troncoso J, et al. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol 2010; 68:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thacker EL, McKnight B, Psaty BM, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013; 81:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verreault R, Laurin D, Lindsay J, et al. Past exposure to vaccines and subsequent risk of Alzheimer's disease. CMAJ 2001; 165:1495–1498. [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett DA, Wilson RS, Arvanitakis Z, et al. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2013; 33 Suppl 1:S397–S403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer's disease pathology. Ann Neurol 2006; 60:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004; 140:167–174. [DOI] [PubMed] [Google Scholar]
- 28.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002; 13:745–753. [DOI] [PubMed] [Google Scholar]
- 29.James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009; 54:24–32. [DOI] [PubMed] [Google Scholar]
- 30.Dalrymple LS, Katz R, Kestenbaum B, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis 2012; 59:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrassy KM. Comments on ’KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 2013; 84:622–623. [DOI] [PubMed] [Google Scholar]
- 32.Wang CS, Wang ST, Lai CT, et al. Impact of influenza vaccination on major cause-specific mortality. Vaccine 2007; 25:1196–1203. [DOI] [PubMed] [Google Scholar]
- 33.Liu IF, Huang CC, Chan WL, et al. Effects of annual influenza vaccination on mortality and hospitalization in elderly patients with ischemic heart disease: a nationwide population-based study. Prev Med 2012; 54:431–433. [DOI] [PubMed] [Google Scholar]
- 34.Remschmidt C, Wichmann O, Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med 2014; 12:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005; 64:277–281. [DOI] [PubMed] [Google Scholar]
- 36.Lau D, Eurich DT, Majumdar SR, et al. Effectiveness of influenza vaccination in working-age adults with diabetes: a population-based cohort study. Thorax 2013; 68:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 2007; 176:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]


