Abstract
Our goal was to explore the factors influencing the visualization of anterior peritoneal reflections (APRs) using rectal MRI. We evaluated the usefulness of rectal MRI in measuring the distance from the anal verge to the APR and determining the relationship between the APR and the rectal tumor.
Clinical and imaging data from 319 patients who underwent surgery after MRI examination between October 2010 and December 2013 were retrospectively analyzed. The distance from the anal verge to the APR and the relationship between the APR and the location of the rectal tumor was evaluated. analysis of variance, logistic regression, independent samples t tests, and Kappa tests were used for statistical analysis.
The APR was visible in 283 of 319 cases using rectal MRI. The APR was more readily observed in patients who were older than 58 years (P = 0.046), in patients whose subcutaneous fat thicknesses were >22.2 mm (P = 0.004), in patients with nondistended bladders (P = 0.001), and in those with an anteversion of the uterus (P = 0.001). There was a significant difference between the distance from the anal verge to the APR between females (10.4 ± 1.1 cm) and males (10.0 ± 1.2 cm; P = 0.014). The accuracy in predicting tumor location with respect to the APR was 70%, 50%, 98.2%, respectively for patients with tumors located above, at, and below the APR (compared with the location determined during surgery).
Most of the APRs were visible using rectal MRI, whereas certain internal factors influence visualization. Rectal MRI could be a useful tool for evaluating the distance from the anal verge to the APR and relationship between rectal tumors and the APR.
INTRODUCTION
The anterior wall of the upper rectum is covered by the peritoneum, whereas the middle and lower thirds lie below the peritoneal reflection and are completely encircled by mesorectum.1 The discrimination of rectal tumors that are located in the area that lies between above and below anterior peritoneal reflection has important implications for both oncologic and surgical practice. Benzni's study showed that the localization of the tumor, according to the border of the peritoneum, is one of the essential factors that influence the prognosis of rectal cancers to be treated by neoadjuvant chemoradiotherapy (CRT) combined with surgery.2 On clinical grounds, there is growing evidence that neoadjuvant chemoradiotherapy is not useful for intraperitoneal cancers.3–5 Some surgeons believe that locally advanced (T3/4 or N+) rectal cancers of the upper third should be considered colon cancer and treated with primary resection, rather than considered rectal cancer and treated with preoperative radio(chemo)therapy.6 Accordingly, we considered that the anterior peritoneal reflection might be a useful landmark to determine the location of rectal subdivisions for rectal cancer treatment. Unfortunately, in our daily practice, not all the APRs can be visualized with MRI. APRs are visible in some, but not all, patients. Given this assumption, we decided to verify that the factors may influence the appearance of the APR on a rectal MRI. Having a reliable distance from the anal verge to the APR and understanding the relationship between the rectal tumor and the APR represent useful information for surgery. Previous studies have used rigid endoscopy or intraoperative proctoscopy to measure the distance from the anal verge to the APR.7–8 However, the results are highly variable, and the relationship between the rectal tumor and the APR cannot always be evaluated. If accurate measurement of the distance and relationship between the rectal tumor and the APR could be evaluated using rectal MRI, this would improve the confidence of practitioners during clinical operation and improve surgical outcomes.
In this study, we evaluated factors influencing visualization of the APR on rectal MRI. We measured the distance from the anal verge to the APR using rectal MRI, while evaluating the accuracy of rectal MRI in determining the tumor location with respect to the APR.
MATERIALS AND METHODS
Patients
We first obtained ethics committee approval from Fudan University Shanghai Cancer Center Institutional Review Board (Shanghai, China). Between October 2010 and December 2013, 319 consecutive patients diagnosed as having primary rectal cancer and then treated in the Fudan University Cancer Center were selected as samples in this retrospective study. Selection criteria included the following: a histologically (biopsy) proven primary rectal carcinoma, treatment by surgical resection, a description of the tumor location at the time of operation, and an initial rectal MRI. There were 704 patients matching these conditions. Exclusion criteria included the following: patients who received palliative treatment (21 patients), patients who received neoadjuvant treatment (316 patients), patients who experienced a long interval between rectal MRI and surgery >4 weeks (15 patients), patients who had received transanal resection (12 patients), and patients with MRIs with poor image quality (12 patients) or motion artifacts (9 patients) (Figure 1).
FIGURE 1.

Selection criteria and exclusion criteria.
Clinical and imaging data were retrieved from the patient database after we obtained ethics committee approval from the Fudan University Shanghai Cancer Center Institutional Review Board (Shanghai, China). The imaging data collected included the following: the distance from the anal verge to the APR, the relationship between the tumor location and the APR, tumor size, thickness of the subcutaneous fat measured at the level of the superior margin of the pubic symphysis, the degree of bladder filling, and the orientation of the uterus (in females). The clinical data included patient age, height, and operation date.
Rectal MRI Protocol
Rectal MRI was performed on a 3.0 Tesla (T) MR magnet (Signa Horizon, GE Medical Systems, Milwaukee, WI) using a phased-array body coil. A sagittal T2-weighted (T2W) fast spin echo (repetition time/echo time [TR/TE]: 2540/100 ms, echo train length: 16, field of view [FOV]: 16 cm, section thickness: 3 mm, interspace: 0.5 mm, number of slices: 16 slices, number of excitations (NEX):1, matrix: 224 × 320) and an oblique axial thin-section T2W (repetition time/echo time [TR/TE]: 3420/110 ms, flip angle: 90 degree, echo train length: 20, field of view [FOV]: 20 cm, section thickness: 3 mm, interspace: 1 mm, number of slices: 20 slices, number of excitations (NEX): 2, matrix: 384 × 224) were used for this investigation. Patients did not receive bowel preparation.
Radiologist Revaluation Strategy and Anatomic Measurements
Rectal MR images were reviewed by 2 radiologists on a PACS (Picture Archiving and Communication Systems) monitor independently. Axial and midsagittal T2W images were used for identification of the APR. Prior reports depicted the APR on axial images with the appearance of a midline in-folding fascial reflection, called the V-shaped configuration. In the midsagittal plane, the peritoneum was identified as a thin hypointense linear structure noted along the superior bladder (men) or uterus (women), which extended inferiorly and posteriorly to the cul-de-sac in women and approximately to the tip of the seminal vesicles in men, after which the posterior extension attached to the rectal wall anteriorly.9–10 The APR specifically was recorded as the insertion site into the colon. Identification of the APR was rated subjectively as a 3-point confidence scale according to the method of Gollub9 as follows: definitely not visible, probably visible, and definitely visible. Under the consensus decision, these 2 radiologists determined the confidence scale for the identification of the APR. Then, for all the cases rated with a probably visible or definitely visible APR, lengths were measured as a line from the anal verge to the APR along the direction of rectum (Figure 2). Finally, under a consensus decision, 2 radiologists determined the spatial relationships between the rectal tumor and the APR, and assigned tumor locations to the following categories: above the APR, at the level of the APR, and below the APR. The definition of a distended bladder and the orientation of the uterus were as follows: a bladder wall showing no folds on both sagittal and oblique axial thin-section T2WI was defined as a distended bladder. Uterine orientation was described according to the angle between the axis of the uterus and the axis of the axial plane on the sagittal T2WI and categorized as follows: absent uterus, anteversion, perpendicular, or retroversion. The directions of the tumor were divided into the following 5 categories: circumferential, mainly anterior, mainly posterior, mainly left, and mainly right lateral. The thickness of the subcutaneous fat was measured at the level of the superior margin of the pubic symphysis, and the tumor size was measured along the direction of tumor. Operative findings of the tumor location with respect to the APR were recorded by colorectal surgeons. Intraoperative tumor location was also placed into the following categories: at, above, or below the APR.
FIGURE 2.

Magnetic resonance imaging measurements: Distance from anal verge (yellow line) to APR (yellow arrow), measuring along the direction of rectum (double yellow arrows), units of measure is mm (A) Curvature of rectum is small, total distance = L1 + L2. (B) Curvature of rectum is large, total distance = L1 + L2 + L3; if curvature of rectum is larger, total distance = L1 + L2 + L3 +……+ Ln.
Statistical Analysis
Age, height, thickness of subcutaneous fat, tumor size, and distance from the anal verge to the APR were reported as mean ± standard deviation.
Analysis of variance was used to test the differences in visualization of the APR between the following groups: female versus male, distended bladder versus not non-distended bladder and with different orientations of uterus (female), different locations of the tumor, and the measurement data. Then, the odds ratio (OR) between these potential influencing factors and the APR was calculated by logistic regression.
To evaluate the differences in the distance from the anal verge to the APR in Chinese females and males, an independent sample t test was used.
The value of rectal MRI in the diagnosis of the location of the rectal tumor with respect to the APR in visible anterior peritoneal reflections was evaluated using the consistency check of a diagnostic test (Kappa statistics). A Kappa value of <0.20 indicated poor agreement; a Kappa value of 0.21 to 0.40 indicated fair agreement; a Kappa value of 0.41 to 0.60 indicated moderate agreement; a Kappa value of 0.61 to 0.80 indicated good agreement, and a Kappa value of >0.81 indicated excellent agreement.
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, version 17.0). All P values <0.05 were considered statistically significant.
RESULTS
Clinical, Histopathological, and MRI Findings
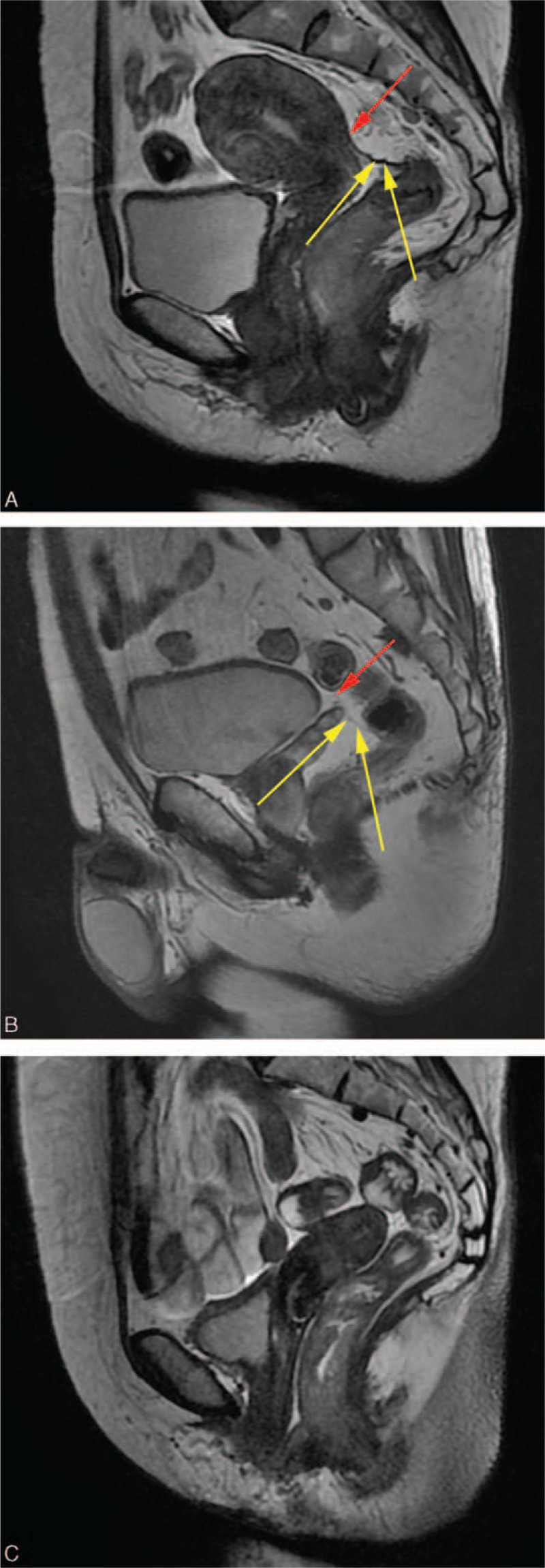
The final study population consisted of 319 patients. The characteristics of these patients are listed in Table 1. Of these, 199 patients received anterior resection, 114 patients underwent abdominopefineal resection, and 6 patients underwent a Hartmann operation. Furthermore, 110 patients were females, and 209 patients were male. The mean age of females and males was 59.6 ± 12.8 (range, 27–89 years), 56.6 ± 11.3 years (range, 27–85 years), respectively. Sixty-eight patients had filling bladders, and 251 patients did not have a filling bladder upon rectal MRI. One female patient had a resected uterus. Uterine orientations were as follows: anteversion (n = 56), perpendicular (n = 27), or retroversion (n = 26). There were 239 patients with tumors that were circumferential. As far as orientation, 37, 26, 6, and 11 were mainly anterior, mainly posterior, mainly left, and mainly right lateral, respectively. The mean height for whole patients was 165.5 ± 7.5 cm. The mean thickness of the subcutaneous fat was 21.7 ± 7.9 mm, and tumor size was 43.8 ± 17.5 mm.
TABLE 1.
Features of 319 Rectal Tumor Patients
Factors Influencing the Visualization of the APR Upon Rectal MRI
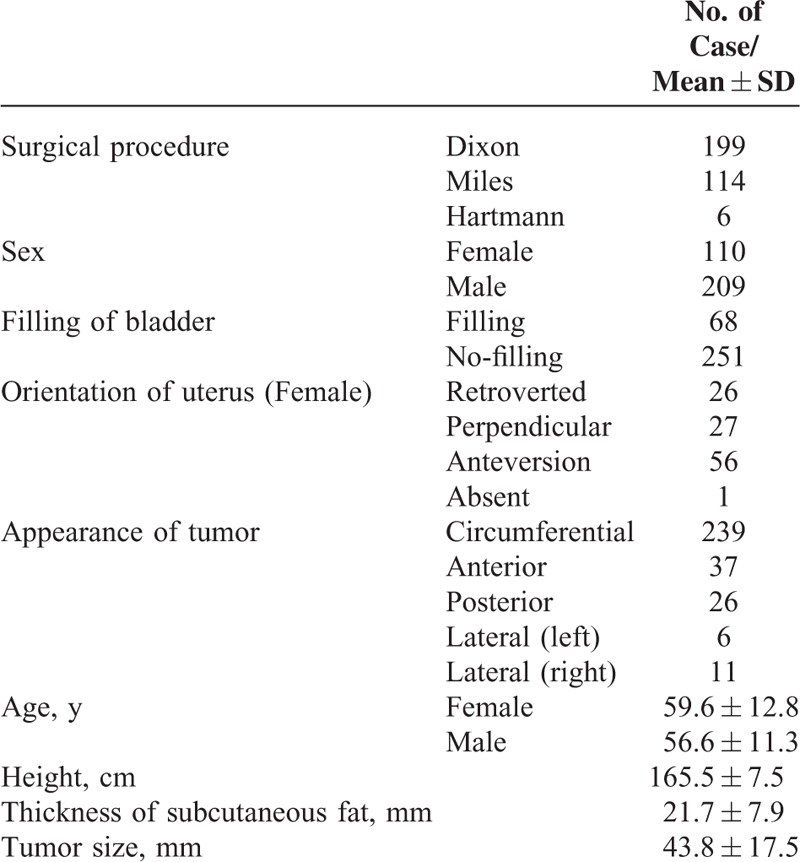
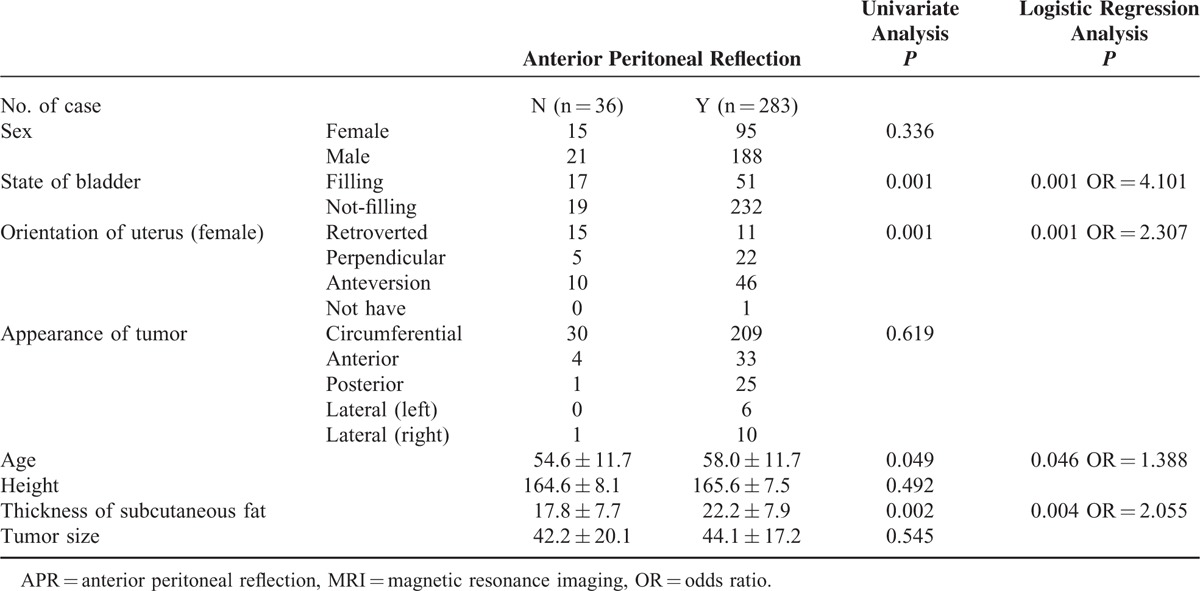
Both radiologists indicated that the APR was “probably” or “definitely” visible (Figure 3) in 283 of 319 cases (88.7%) upon rectal MRI. Factors influencing the visualization of the APR were age (P = 0.049), the thickness of the subcutaneous fat (P = 0.002), filling of the bladder (P = 0.001), and the uterine position in females (P = 0.001), as determined by univariate analysis. Other factors, such as sex, height, the direction of the tumor, and the tumor size did not influence the visualization of the APR. Further logistic regression analysis showed that factors influencing the visualization of the APR were patient age (OR = 1.388, P = 0.046), the thickness of the subcutaneous fat (OR = 2.055, P = 0.004), a non-filing bladder (OR = 4.101, P = 0.001), and an anteversion (OR = 2.307, P = 0.001). The above results are shown in Table 2.
FIGURE 3.
Sagittal T2-weighted rectal MRI showing APR. (A) Anterior peritoneal reflection (yellow arrow), which is definitely visible and is seen inserting into rectum. Note also peritoneal lining (red arrow) seen along superior uterine border. (B) Anterior peritoneal reflection (yellow arrow), which is probably visible and is seen inserting into rectum, with reflection also seen along the superior seminal vesicle and bladder margins (red arrow). (C) Anterior peritoneal reflection is not definitely visible.
TABLE 2.
Factors Influencing Visualization of the APR at Rectal MRI
The Distance from the Anal Verge to the APR Measured Upon Rectal MRI
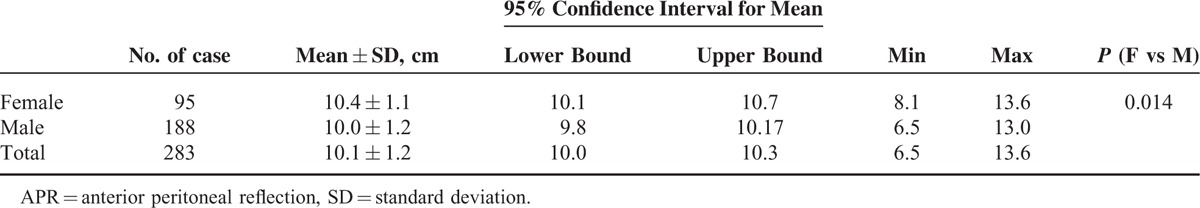
There were 283 patients whose APRs were definitely visible, including 95 females and 188 males. The distances between the APR and the anal verge are shown in Table 3. The mean distance was 10.4 ± 1.1 cm in females, 10.0 ± 1.2 cm in males, and 10.1 ± 1.2 cm in total. There was a significant difference in the distances from anal verge to APR between females and males (P = 0.014).
TABLE 3.
The Distance From Anal Verge to the APR
Location of the Rectal Tumor With Respect to the APR, Differences between Radiologic and Operative Findings
The accuracy of the estimated tumor location (determined via MRI) relative to the APR was 83.0%, which was evaluated during surgery. The accuracy of the rectal MRI was 70% in patients with a tumor located above the APR, 50% in patients with a tumor located at the level of the APR, and 98.2% in patients with a tumor located below the APR (Table 4, Figure 4). Measure of agreement between the Kappa value of location with respect to the anterior peritoneal reflection determined by radiologic and operative findings was 0.678 (P = 0.000).
TABLE 4.
Location of Rectal Cancer With Respect to the APR by Radiologic and Operative Findings
FIGURE 4.
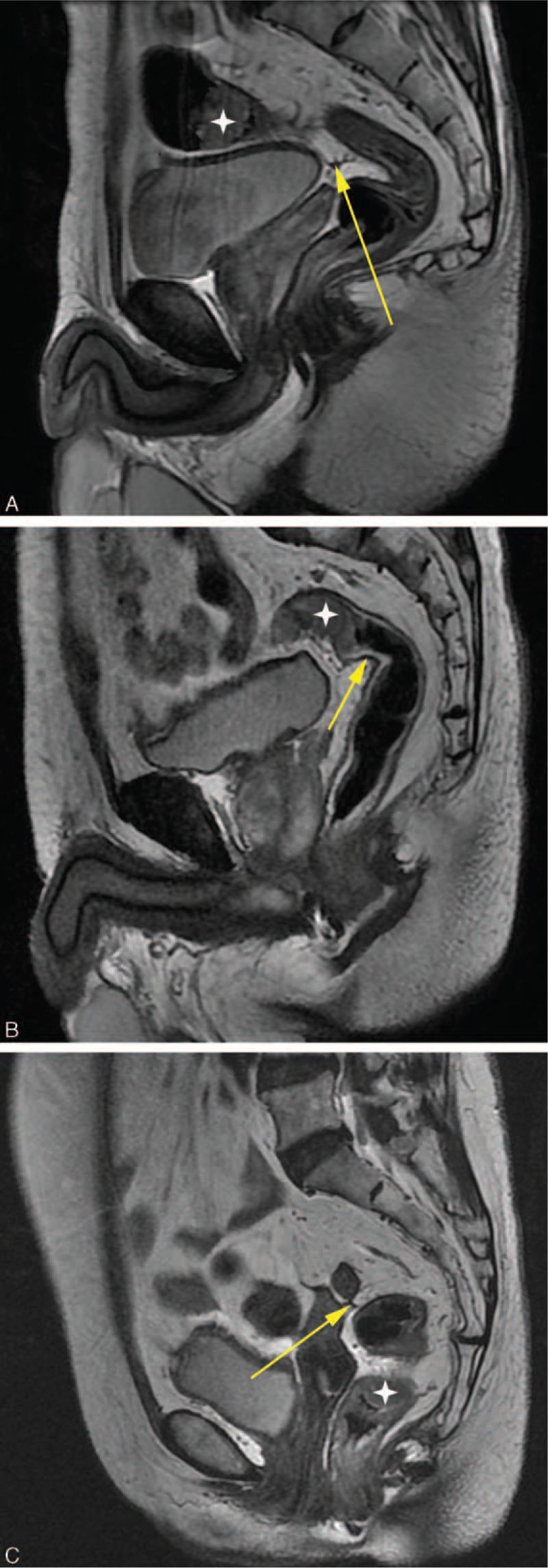
Tumor location with respect to anterior peritoneal reflection (yellow arrow), white star means tumor, A: Tumor above the APR, B: Tumor at the level of the APR, C: Tumor below the APR.
DISCUSSION
Recent studies have already shown that the location of rectal tumors with respect to the APR has important treatment implications, AS above and below the APR, cancers are characterized by peculiar routes of lymphatic spread.4 Therefore, an accurate preoperative identification of the APR is useful in choosing the optimal treatment for each patient to avoid under- or overtreatment. Rectal MRI scans not only have clinical usefulness in terms of preoperatively evaluating the TNM stage, mesorectal fascia involvement, and adjacent organ involvement, but can also identify the APR.9,11–16 However, not all APRs are visible, and certain factors may influence visualization. Given this assumption, we decided to verify which factors influence visualization. The height of the rectal tumors, the distance from the APR to the anal verge, and the relationship between the rectal tumor and APR are critical factors that enable the clinician to decide which treatment strategies are most appropriate. There is at least one article describing the MRI-defined height of rectal tumors.17 However, few reports have described the clinical usefulness of rectal MRI in determining the distance from the anal verge to the APR and the relationship between rectal tumors and the APR.
Of the 319 rectal cancer patients in this study, there were 283 (88.7%) patients whose APRs were not visible upon rectal MRI. Filling bladder, the orientation of the uterus, thickness of the subcutaneous fat, and age were factors influencing the visualization of the APR at rectal MRI. We found that with less urine in the bladder and a closer distance between the uterus and the ventral abdominal wall, it was easier to observe the APR. The wider the space surrounding the APR, the easier it was to observe the APR. The APR was also more easily observed in patients with thicker abdominal subcutaneous fat layers and in the elderly. The peritoneum was mainly composed of connective tissue, and the thickness of abdominal subcutaneous fat could reflect the abdominal content of the connective tissue indirectly and partly. The greater the volume of peritoneal connective tissue, the easier it was to observe the APR.
The distance from the anal verge to the visible APR can be measured during rectal MRI by a radiologist. This generates important information for the surgeon. Females on average had longer distances from the anal verge to the APR than males. The female pelvis, especially in women who gave birth, was bigger than males; childbirth may be one factor affecting this distance. In a Korean report, the estimated length to the anal verge in females and males was 8.1 ± 1.7 and 8.8 ± 2.2 cm, respectively, determined with a conventional rigid sigmoidoscope. Furthermore, Najarian et al's8 results showed the distance in females and males was 9 and 9.7 cm, respectively (also determined by rigid sigmoidoscope). The distance estimated by rigid sigmoidoscope was shorter than our reported distance estimated by rectal MRI. A rigid sigmoidoscope is straight and rigid, making it difficult to measure the distance along the direction of rectum. Under these circumstances, the distance is measured along a straight line, whereas MRI can measure along the curve. Memon et al18 also showed the mean distance from the anal verge to the APR was 11.9 cm (male) and 10 cm (female) using a rigid sigmoidoscope in an open peritoneal cavity. Under these circumstances, the normal conditions of the rectum could not be simulated. The distance estimated in our study could provide more information in vivo. In earlier articles,19,20 researchers reported that the distance from the anal verge to the APR was 5.5 to 12 cm, as estimated by cadaveric dissections. However, the fixed and dehydrated cadaveric measurements might not be applicable in vivo.
In our study, we also found that rectal MRI can be used to evaluate the relationships between rectal tumors and the APR. The accuracy of predicting the rectal tumor location with respect to the peritoneal reflection was close to 85%, and the accuracy was 98.2% in patients with a tumor located below the peritoneal reflection. However, the accuracy was 50% in patients with a tumor located at the level of the peritoneal reflection. The reason might be that the level of the peritoneal reflection is merely a point, not a range, like the areas above or below the peritoneal reflection. Therefore, the location of the tumor at the level of the peritoneal reflection was harder to evaluate than others. Gerades et al also used transendorectal ultrasound (TRUS)21 to demonstrate that TRUS was able to determine the location of a rectal tumor with regard to the peritoneal reflection. However, their study had 2 limitations. The APR could not be found in the absence of bowel peristalsis or fluid collection. However, TRUS was a practitioner-dependent subjective procedure. We believe that the location of a rectal tumor with respect to the peritoneal reflection as determined by rectal MRI is more objective and applicable in vivo.
There were some limitations in our study. First, the possible influencing factors (such as weight and body mass index (BMI)) could not be analyzed because of incomplete clinical data. Second, we did not include healthy adults of all ages to measure the distance from the anal verge to the APR to determine the average distance. Third, with respect to the factor of age, there may be a selection bias because most rectal tumor patients enrolled in this study were elderly.
CONCLUSION
Most of the APRs are visible with rectal MRI, whereas many internal factors influence visualization. Therefore, rectal MRI could be a useful tool for evaluating the distance from the anal verge to the APR and the relationship between rectal tumors and the APR. The more information obtained from a rectal MRI, the greater benefits in designing a treatment strategy.
Acknowledgements
The authors thank Duoease Scientific Service Center for excellent language editing service and suggestions for figure revision.
Footnotes
Abbreviations: APR = anterior peritoneal reflection, BMI = body mass index, CRT = chemoradiotherapy, FOV = field of view, MRI = magnetic resonance imaging, N = Nodal stage, NEX = number of excitations, OR = odd ratio, PACS = Picture Archiving and Communication Systems, SD = standard deviation, T = Tumor stage, T2W = T2-weighted, T2WI = T2-weighted image, TE = echo time, TR = repetition time, TRUS = trans-endorectal ultrasound.
GY is the first co-corresponding author.
SY, TT, LF contributed equally to this work.
Information whether submission contains previously published material
This submission does not contain contains previously published material.
The authors declare that there are no conflicts of interest regarding the publication of this article.
This study was supported by the National Natural Science Foundation of China (Grant No. 81501437). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Authors’ contributions: GY and TT conceived, designed, and drafted the research protocol; LF, XY, and CS designed and provided guidance for the drafting of the article; XC aided in the acquisition of data.
REFERENCES
- 1.Nougaret S, Reinhold C, Mikhael HW, et al. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology 2013; 268:330–344. [DOI] [PubMed] [Google Scholar]
- 2.Benzoni E, Terrosu G, Bresadola V, et al. Analysis of clinical outcomes and prognostic factors of neoadjuvant chemoradiotherapy combined with surgery: intraperitoneal versus extraperitoneal rectal cancer. Eur J Cancer Care (Engl) 2006; 15:286–292. [DOI] [PubMed] [Google Scholar]
- 3.Myerson RJ, Michalski JM, King ML, et al. Adjuvant radiation therapy for rectal carcinoma: predictors of outcome. Int J Radiat Oncol Biol Phys 1995; 32:41–50. [DOI] [PubMed] [Google Scholar]
- 4.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23:5644–5650. [DOI] [PubMed] [Google Scholar]
- 5.Nijkamp J, Kusters M, Beets-Tan RG, et al. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys 2011; 80:103–110. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg R, Maak M, Schuster T, et al. Does a rectal cancer of the upper third behave more like a colon or a rectal cancer? Dis Colon Rectum 2010; 53:761–770. [DOI] [PubMed] [Google Scholar]
- 7.Yun HR, Chun HK, Lee WS, et al. Intra-operative measurement of surgical lengths of the rectum and the peritoneal reflection in Korean. J Korean Med Sci 2008; 23:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najarian MM, Belzer GE, Cogbill TH, et al. Determination of the peritoneal reflection using intraoperative proctoscopy. Dis Colon Rectum 2004; 47:2080–2085. [DOI] [PubMed] [Google Scholar]
- 9.Gollub MJ, Maas M, Weiser M, et al. Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol 2013; 200:97–101. [DOI] [PubMed] [Google Scholar]
- 10.Brown G, Kirkham A, Williams GT, et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol 2004; 182:431–439. [DOI] [PubMed] [Google Scholar]
- 11.Beets-Tan RG, Lambregts DM, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013; 23:2522–2531. [DOI] [PubMed] [Google Scholar]
- 12.Uçar A, Obuz F, Sökmen S, et al. Efficacy of high resolution magnetic resonance imaging in preoperative local staging of rectal cancer. Mol Imaging Radionucl Ther 2013; 22:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chand M, Swift RI, Tekkis PP, et al. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer 2014; 110:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bipat S, Glas AS, Slors FJ<T-AL>. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging–a meta-analysis. Radiology 2004; 232:773–783. [DOI] [PubMed] [Google Scholar]
- 15.Kim NK, Kim MJ, Park JK, et al. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol 2000; 7:732–737. [DOI] [PubMed] [Google Scholar]
- 16.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferentialmargin involvement in the local recurrence of rectal cancer. Lancet 1994; 344:707–771. [DOI] [PubMed] [Google Scholar]
- 17.Keller DS, Paspulati R, Kjellmo A, et al. MRI-defined height of rectal tumours. Br J Surg 2014; 101:127–132. [DOI] [PubMed] [Google Scholar]
- 18.Memon S, Keating JP, Cooke HS, et al. A study into external rectal anatomy: improving patient selection for radiotherapy for rectal cancer. Dis Colon Rectum 2009; 52:87–90. [DOI] [PubMed] [Google Scholar]
- 19.Buess G, Mentges B, Manncke K, et al. Technique and results of transanal endoscopic microsurgery in early rectal cancer. Am J Surg 1992; 163:63–69. [DOI] [PubMed] [Google Scholar]
- 20.Bannister LH, Gray H. Gray's anatomy. 38th ed1995; New York: Churchill livingstone, 1779. [Google Scholar]
- 21.Gerdes B, Langer P, Kopp I, et al. Localization of the peritonealreflection in the pelvis by endorectal ultrasound. Surg Endosc 1998; 12:1401–1404. [DOI] [PubMed] [Google Scholar]


