Abstract
Follicular lymphoma (FL) is the 2nd most common type of lymphoma diagnosed in the Western World. Bone marrow (BM) involvement is an adverse prognostic factor in FL, routinely assessed by an arbitrary biopsy of the iliac crest. This study was aimed to investigate the role of positron emission tomography/computed tomography (PET/CT) in identifying BM involvement by FL.
In this retrospective, single-center study we reviewed the records of consecutive patients with FL at diagnosis or relapse who underwent staging/restaging workup visual assessment of BM uptake was categorized as either normal, diffusely increased, or focally increased. Quantitative BM fluorine-18-fluro-deoxyglucose (FDG) uptake was measured using mean standardized uptake value (BM-SUVmean). The diagnosis of BM involvement was based on either BM histological findings or disappearance of increased uptake at end-treatment PET/CT in patients who responded to treatment.
Sixty eight cases with FL were included. Sixteen (23.5%) had BM involvement, 13 (19.1%) had a biopsy proven involvement, and 3 (4.4%) had a negative BM biopsy, but increased medullary uptake that normalized post-treatment. BM FDG uptake in these patients was diffuse in 8 (50%) and focal in 8 (50%). Focal increased uptake was indicative of BM involvement; however, diffuse uptake was associated with 17 false positive cases (32.7%). Overall, visual assessment of BM involvement had a negative predictive value (NPV) of 100% and a positive predictive value (PPV) of 48.5%. On a quantitative assessment, BM-SUVmean was significantly higher in patients with BM involvement (SUVmean of 3.7 [1.7–6] vs 1.4 [0.4–2.65], P < 0.001). On receiver operator curve (ROC) analysis, BM-SUVmean > 2.7 had a PPV of 100% for BM involvement (sensitivity of 68%), while BM-SUVmean < 1.7 had an NPV of 100% (specificity of 73%).
Visual assessment of PET/CT is appropriate for ruling out BM involvement by FL. Although focal increased uptake indicates marrow involvement, diffuse uptake is nonspecific. SUV measurement improves PET/CT diagnostic accuracy, identifying additional 19% of patients with BM involvement that would have been otherwise missed.
INTRODUCTION
Follicular lymphoma (FL) is the 2nd most common non-Hodgkin lymphoma diagnosed in adults. Overall, it accounts for about 20% of all non-Hodgkin lymphoma and 70% of indolent lymphomas worldwide. The clinical evolution of FL is highly variable in terms of presentation, clinical course, and response to treatment. Although some patients enjoy prolonged uneventful observation periods (watchful waiting), and some achieve long-term remissions, others experience recurrent disease relapses and histologic transformation to aggressive lymphomas which occurs at a rate of 3% per year. Therefore, identifying patients at risk of aggressive clinical course and adjusting treatment strategies accordingly is of major interest in FL. Disease extension is an adverse prognostic factor, which is included in prognostic indexes, such as the FL International Prognostic Index (FLIPI), and is relevant to therapeutic approach.
Bone marrow involvement (BMI) can be detected at presentation in up to 70% of patients with FL, whereas involvement of other extranodal sites is infrequent. Determining BMI is important in the staging of lymphoma as it signifies advanced disease. A bone marrow biopsy (BMB) taken arbitrary from the iliac crest is the routinely used procedure for determining BMI and is still an integral part of FL clinical staging, at both diagnosis and relapse. However, BMB has poor sensitivity due to sample size and sample error as BMI may be patchy,1 and the procedure might be complicated by pain, bleeding, or infection.2
Fluorine-18-fluro-deoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) has recently emerged as a powerful imaging tool for initial staging, response assessment, and follow-up in patients with diffuse large B-cell lymphoma and Hodgkin lymphoma.3,4
PET/CT efficiency in staging low-grade lymphoma varies depending on histology.5–8 Its sensitivity seems high in FL9 and it can identify nodal and extranodal sites of disease overlooked by standard CT.10,11 PET/CT is also a useful prognostic tool in patients with FL, patients with a negative post-treatment PET/CT were reported to have better outcome than patients with FDG-avid residual disease.11–14 Despite FDG avidity of FL, it was only recently that PET/CT was introduced to routine staging of FL.4
The role of PET/CT in detecting BMI and its ability to replace BMB in the staging of patients with lymphoma are still not fully determined (reviewed in Adams et al15). El Galaly et al16 have reported that in PET/CT-staged patients with Hodgkin lymphoma, of whom 6% had BMI, BMB up-staged 5 out of 454 patients. However, BMB results have not changed the allocated treatment in none of the patients, probably as all patients with positive BMB had advanced disease, irrespective of the BMB results. In patients with diffuse large B cell lymphoma, PET/CT was reported to detect all cases of BMI while BMB did not up-staged any of the patients.17,18 Yet, other studies suggest that the sensitivity of PET/CT in detecting BMI is low (68.8%) and that only a biopsy-proven BMI has prognostic value.19–21 Le Dortz et al11 studied the usefulness of PET/CT in staging and prognosis evaluation in 45 patients with untreated FL and reported that PET/CT was far more sensitive than CT for detecting BMI. In other types of lymphoma, it was suggested that the value of PET/CT in detection of BMI vary according to the type of lymphoma.22
Here, we specifically evaluated the diagnostic accuracy of PET/CT for detecting BMI in patients with FL, at diagnosis and at relapse.
METHODS
In this retrospective, single-center study we reviewed the records of consecutive patients with FL at diagnosis or relapse who underwent staging/restaging workup at the hematology institute during January 2005 to April 2013. Most patients with newly diagnosed FL and all patients with intent-to-treat FL underwent FDG-PET/CT and a BMB. The inclusion criteria were patients older than 18 years with FL diagnosed on biopsy of a nodal or extranodal lesion, and for whom a BMB and F18-FDG PET/CT results were available. Exclusion criteria were patients with known concomitant malignancy and those for whom more than 14 weeks had passed between BMB and FDG-PET/CT (12 cases were excluded, Figure 1). The study was approved by the local institutional ethics committee and written informed consent was waved.
FIGURE 1.
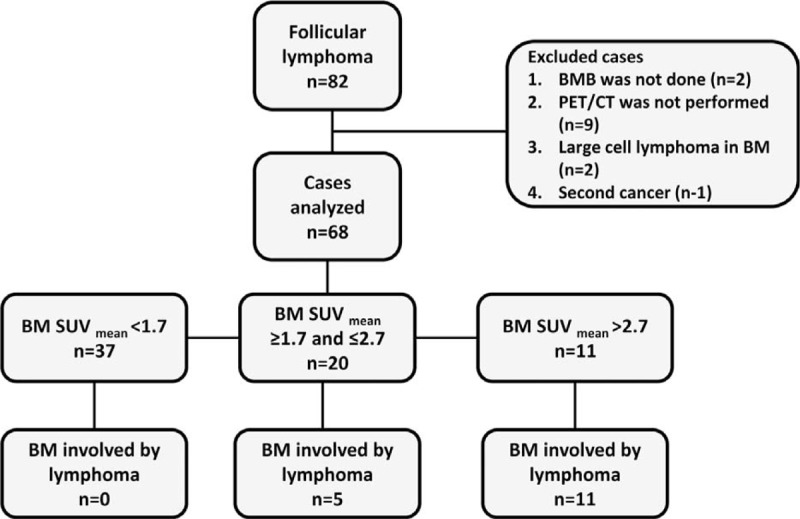
Cohort selection flow diagram.
Bone Marrow Biopsy
BMBs were performed by a unilateral puncture of the posterior iliac crest using a T-lock bone marrow biopsy needle (Angiotech, FL). The BM biopsies were evaluated by a single, experienced hematopathologist (JB). The diagnosis of indolent FL (grade 1, 2, 3a) was according to the WHO 2008 criteria.23
FDG-PET/CT Acquisition
Patients were asked to fast for at least 4 hours before undergoing the examination. All patients had glucose levels below 150 mg/dL. The patients received an intravenous injection of 370 to 666 MBq (10–18 mCi) of 18F-FDG. Data acquisitions were performed 60 to 120 minutes after injection using an integrated in-line PET/CT system (Discovery 690; GE Medical Systems, Milwaukee). Data acquisition was as follows: CT scanning was performed first followed by PET scan with acquisition time of 4 minutes per each table position. PET image datasets were reconstructed iteratively by using CT data for attenuation correction, and coregistered images were displayed on a workstation (Xeleris, GE, Haifa, Israel).
FDG-PET/CT Uptake Analysis
An experienced reader (HLS), who was blinded to BMB results, patients’ clinical parameters and outcome, evaluated all FDG-PET/CT images for the presence or absence of BMI. BM uptake was categorized as: no increased uptake, diffusely increased uptake, and focally increased uptake. BM-mean standardized uptake value (SUVmean) and maximum standardized uptake value (SUVmax) were measured in all study patients at the posterior aspect of the iliac bones bilaterally. In patients with focally increased uptake, the latter was also measured at that specific location. BM-SUV at the iliac bone or focal site of uptake was obtained 3 times and the mean value of these measurements was recorded. SUV values of nodal sites of disease were also obtained.
Statistical Analysis
We compared continuous variables between groups using the student t-test and analysis of variance. We used the Fisher exact test and the Chi-square test to compare categorical variables. To identify the utility of uptake intensity measurement for predicting BMI we used the receiver operator curve (ROC) method. We report the area under the curve (AUC) and the cut-off values that maximize the positive predictive value (PPV) for ruling-in BMI and the cut-off values for maximizing the negative predictive values (NPVs) for ruling it out. We performed survival analysis using the Kaplan–Meier method for binary variables and the Cox proportional hazard method for continuous variables. To derive optimal uptake intensity cut-off values for predicting survival we used the survival ROC method as implemented in R. Cut-off values with optimal PPV were chosen. Overall survival was defined as the time from diagnosis until death or censoring. Time to treatment was defined as the time from diagnosis until treatment, death, or censoring. Statistical analysis was performed using SPSS (IBM, NY) and R (R Foundation for Statistical Computing, Austria).
RESULTS
Patient Characteristics
We analyzed 68 PET/CT studies performed concomitantly with BMB in 64 patients with FL (Table 1). Fifty six patients were evaluated at 1st presentation (1st staging), 4 at relapse (2nd staging), and 4 at both 1st presentation and relapse. Median age of the study population was 60 years (range; 22–83 years).
TABLE 1.
Patients Data
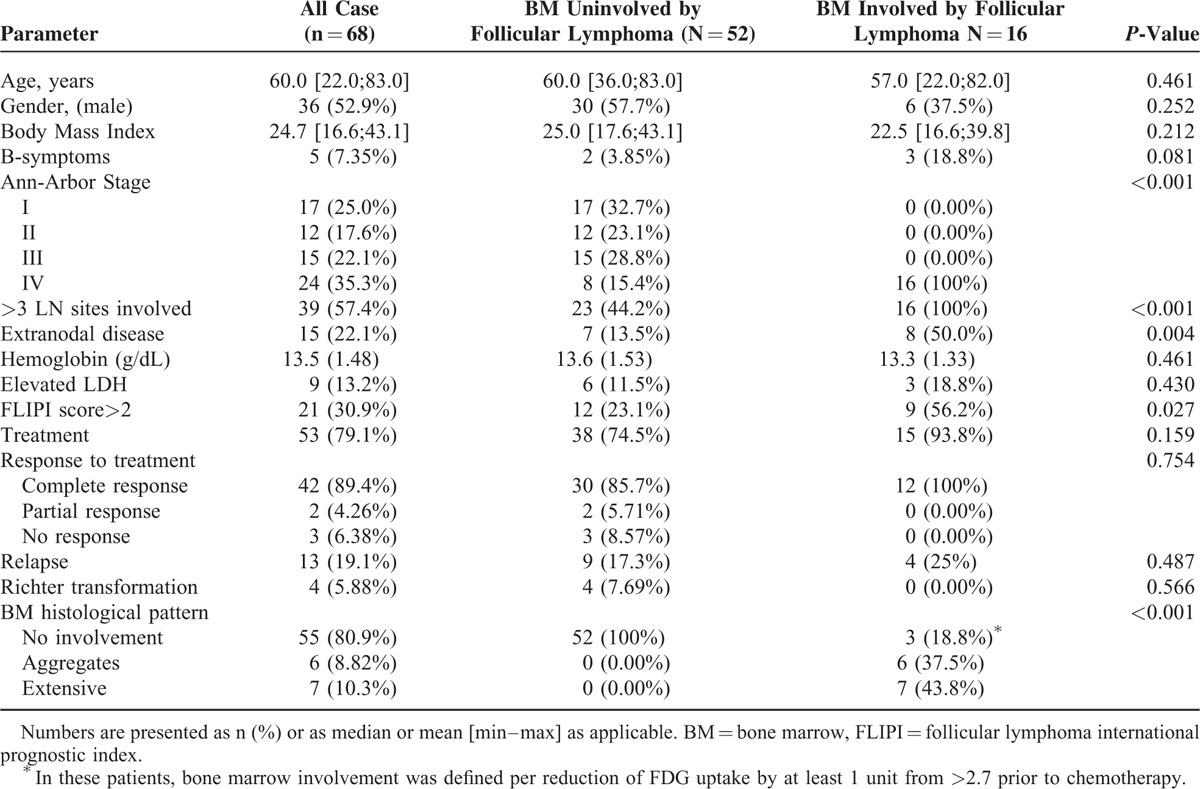
According to the FLIPI, 21 cases (30.9%) had a FLIPI score of above 2. Twenty four cases (35.3%) had an Ann Arbor stage IV disease and 15 (22.1%) had extranodal involvement other than in the BM (Table 1).
Bone Marrow Involvement by Bone Marrow Biopsy and PET
Overall, 16 cases (23.5%) were considered to have BMI. Thirteen subjects had a biopsy-proven involvement with histological patterns of either focal aggregates in 6 cases or extensive involvement in 7 cases (Table 1). Additional 3 cases had a negative iliac crest biopsy, but were diagnosed with BMI based on increased BM SUVmean uptake that had normalized following treatment (from pretreatment BM SUVmean of 4.1 [range 2.8–6] to 0.7 [range 0.4–0.9] post treatment). There was no difference in patients or disease characteristics between biopsy proven or PET-FDG-based identification of BMI (Table 2). Notably, all 3 cases had additional extranodal sites of disease and were designated as stage IV disease irrespective of BMI.
TABLE 2.
Assessment of Bone Marrow Involvement Using PET-CT
Two patients with a nodal biopsy of FL were excluded from this study after BMB revealed involvement with large cells consistent with transformation to diffuse large B cell lymphoma. Mean BM uptake intensity in these patients were 6.0 and 8.5, while the maximal value observed in our cohort was 6.
Visual Assessment
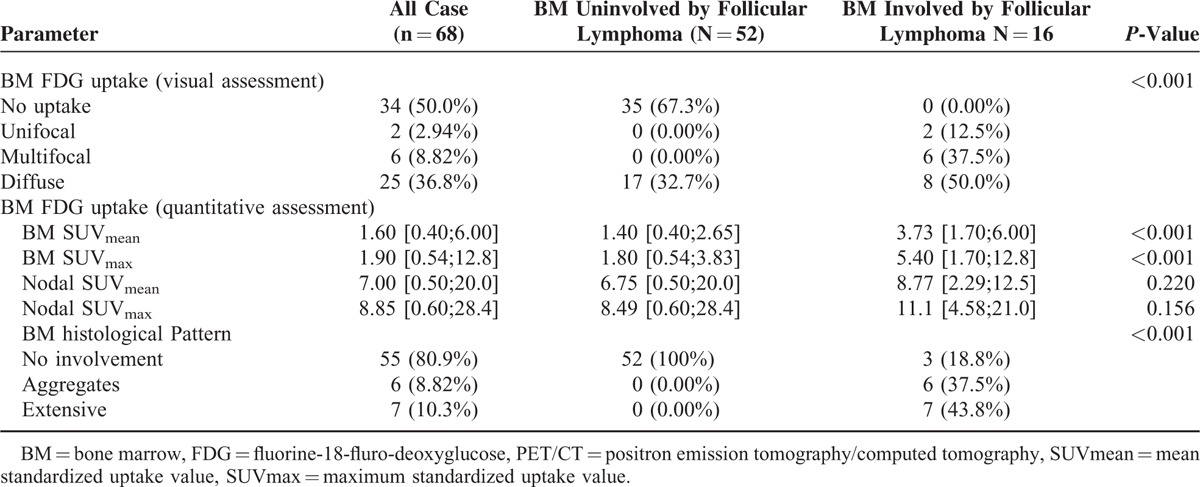
Visual assessment of increased BM FDG uptake was detected in 33 cases. Of these, BM uptake was diffuse in 25 cases (36.8%), multifocal in 6 cases (8.8%), and unifocal in 2 cases (2.9%). Figure 2 illustrates focal and diffuse pattern of uptake associated with marrow involvement. All 13 patients with histologically confirmed BMI had BM disease identified by PET/CT, consisting of a diffuse (61.5%, n = 8), multifocal (30.8%, n = 4), or unifocal (7.7%, n = 1) uptake pattern. Of the 3 patients with BMI based on increased FDG uptake but negative BM biopsy: 2 had a multifocal and 1 had a unifocal uptake pattern. Additional 17 patients had falsely increased diffuse BM uptake, with no BMI (either histological or decreased posttreatment uptake). Thirty five cases had normal BM uptake and none of these were found to have BMI. Accordingly, visual assessment of BMI by PET/CT in patients with FL had an NPV of 100% and a PPV of 48.5%.
FIGURE 2.
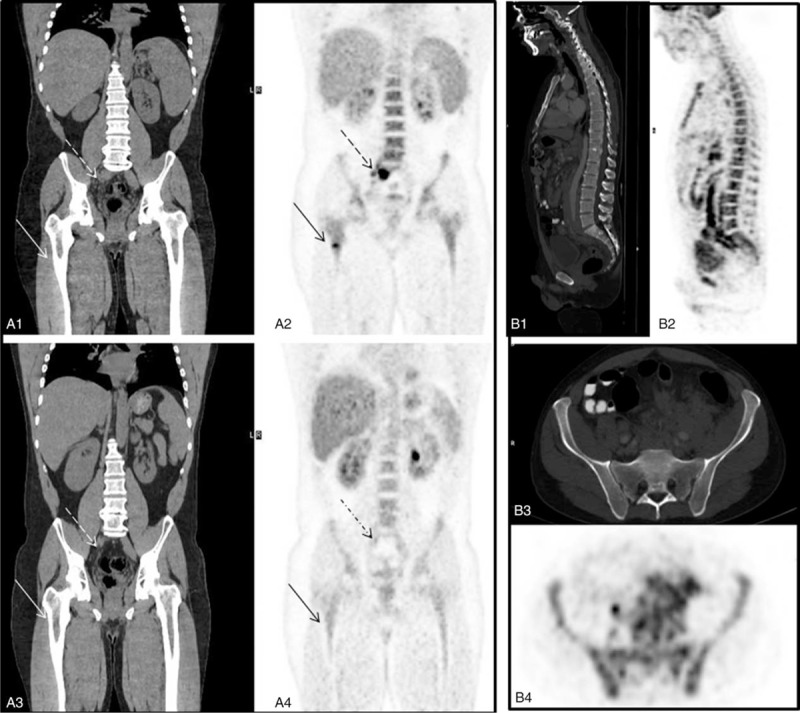
FDG-PET/CT identifies focal BM involvement by follicular lymphomas. (A) Focal BM involvement: Shown are staging CT coronal data (A1) and FDG-PET (A2) demonstrating focal increased FDG uptake in the bone marrow at the right femur (arrow) and in an enlarged presacral lymph node (dashed arrow). Follow-up FDG-PET/CT post successful treatment (A3–4): The presacral lymph node presented in A1–2 decreased in size on coronal CT (dashed arrow, A3) and shows no FDG uptake on FDG-PET (dashed arrow, A4), as well as the bone marrow at the right femur (arrow, A4). (B) Diffuse bone marrow involvement: Sagittal images of the spine on CT (B1) and FDG-PET (B2) showing an enhanced FDG uptake in the spine. B3: An axial CT (soft tissue window), demonstrating a dense bone marrow tissue; B4: FDG-PET images demonstrating diffuse medullary FDG uptake with a mean SUV of 4.3. BM = bone marrow, FDG = fluorine-18-fluro-deoxyglucose, PET/CT = positron emission tomography/computed tomography, SUVmean = mean standardized uptake value, SUV = standardized uptake value.
Bone Marrow FDG Uptake Intensity
In this cohort, the median values of the mean and maximal SUV measured in the BM were 1.60 (0.4–6) and 1.9 (0.5–12.8), respectively, compared to SUVmean of 7.4 (0.5–20) and SUVmax of 8.8 (0.6–28.4) in nodal areas. BM uptake intensity was significantly higher in patients with BMI as compared to patients with no BMI (SUVmean of 3.7 [1.7–6] vs 1.4 [0.4–2.65, respectively, P < 0.001, Figure 3A). No association was noted between nodal uptake intensity and BMI (nodal SUVmax of 11.1 [4.58–21] vs 8.49 [0.6 ± 28.4], respectively P = 0.156). Mean BM uptake intensity had an AUC of 0.967 for identifying BMI, while BM SUVmax had a lower AUC of 0.944, mainly due to inferiority in ruling out BMI. Using an ROC curve analysis, SUVmean greater than 2.7 had a PPV of 100% (11/11) for BMI and a sensitivity of 68% (11 of 16 cases). Reducing the threshold to 2.5 had a PPV of 93% (13/14) and a sensitivity of 81% (13 of 16 cases). SUVmean lower than 1.7 had an NPV of 100% and specificity of 73% for cases without BMI. Namely, BM SUVmean greater that 2.7 was synonymous with BMI, while mean SUV of less than 1.7 ruled out BMI by FL.
FIGURE 3.
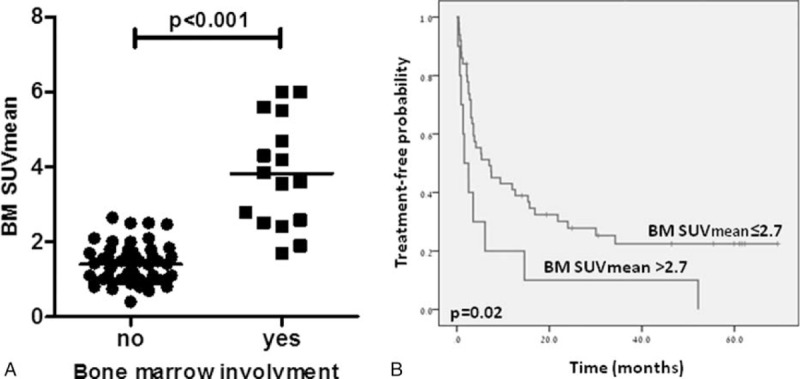
(A) Dot-plot of bone marrow mean standardized uptake value (SUVmean) uptake in follicular lymphoma patients with involved and uninvolved bone marrow. (B) Kaplan–Meier curves for time to 1st treated in patients with follicular lymphoma by bone marrow SUVmean uptake.
Next, we performed that analysis including the 3 discordant cases with negative BMB and positive BMI per PET/CT as “false positives.” In this analysis, an SUV uptake greater than 2.7 resulted in a PPV of 82%, a sensitivity of 69%, and an AUC of 0.93, while an SUV uptake of less than 1.7 retained its 100% NPV for BMI. In addition, reanalysis after excluding the 3 cases altogether resulted in similar findings to those reported for the entire cohort (data not shown).
Correlation Between Bone Marrow FDG Uptake Intensity and Clinical Outcome
Sixty patients were evaluated at presentation and were included in the survival analysis. Median follow-up time for this cohort was 45.8 (range 0.2–120 months). Forty seven patients were treated for their lymphoma. Median time to treatment in patients with BM uptake intensity greater than 2.7 was 1.6 versus 7 months in patients with BM uptake intensity of 2.7 or lower (P = 0.02) (Figure 3B). No association was found between FDG uptake intensity and the risk of relapse.
DISCUSSION
The role of PET/CT in predicting BMI in FL and whether it is sufficiently accurate to render BMB unnecessary in the staging procedure is still under investigation. In the current study, PET/CT was found to be accurate in detecting BMI in patients with FL. Visual interpretation of PET/CT correctly identified all patients with BM disease. None of the patients with a negative PET/CT had a positive BMB, suggesting that the NPV of PET/CT for BMI with FL is excellent. Detection of focally increased uptake, either multifocal or at a single site, was indicative of marrow involvement. However, diffusely increased uptake identified visually was associated with a high false positive rate. Adding quantitative assessment of BM uptake intensity by measurement of BM-SUVmean, improved the diagnostic accuracy of PET/CT. Measuring BM-SUVmean value greater than 2.7 had a PPV of 100% for BMI with sensitivity of 68%. A lower threshold of >2.5 improved sensitivity, while BM-SUVmean value lower than 1.7 had an NPV of 100% and specificity of 73% for excluding BMI. Thus, combining visual assessment with quantitative uptake intensity enabled us to identify additional patients with BMI that would have been missed by an arbitrary BMB of the iliac crest. The improved accuracy of PET/CT in detecting BMI in patients with FL when utilizing quantitative FDG uptake analysis reported here is compatible with previous reports.24
High uptake intensity seemed to be associated with a more aggressive-natured disease. High mean BM uptake intensity was associated with significantly shorter time to treatment, compared to patients with lower BM uptake intensity. Two patients were excluded from this study after BMB revealed transformation to diffuse large B cell lymphoma. Mean BM uptake intensity in these patients reached or exceeded the maximal value observed in our cohort, suggesting that excessively high BM FDG uptake intensity in patients with FL should be suspected for large cell transformation and probably be further investigated by BMB.
Our study has 2 limitations that span beyond its retrospective nature. First, of the 68 subjects included in the analysis only 16 had BMI. However, previous studied that addressed PET/CT evaluation of BMI by FL also had to draw conclusions from a relatively low number of patients with BMI.11,25 The second limitation is that of the 16 cases of BMI, 3 cases were not confirmed by biopsy, but rather were deduced according to a considerable decline in FDG uptake following chemotherapy. It is known that when only BMB is used as reference standard for BMI, lesions located outside the iliac crest can be missed and result in erroneous interpretation of PET/CT as false positive.26 Previous studies have used follow-up PET scans trying to overcome this problem in patients with aggressive lymphoma, considering FDG-avid BM lesions at baseline as true-positive if these lesions disappeared after therapy.27
Interestingly, all 3 patients had extended disease with additional extranodal sites of involvement, already designating them as stage IV disease, thus, BMI identified by PET/CT did not up-staged any of these patients. This supports the notion that BMI detected by PET/CT in these patients was not false but probably missed by BMB.
In conclusion, the results of this retrospective study suggest that normal appearing marrow on visual assessment of PET/CT rules out BMI in patients with FL with a high degree of certainty. Focal increased marrow uptake accurately identifies BMI while diffusely increased uptake is nonspecific with a relatively high false positive rate. Quantitation of FDG uptake intensity improves PET/CT diagnostic accuracy: BM SUVmean below 1.7 may spare the need for BMB while BM SUVmean of above 2.7 should be considered compatible with BMI, though BMB may still be recommended to exclude large cell transformation.
Footnotes
Abbreviations: AUCarea = under the curve, BM = bone marrow, BMB = bone marrow biopsy, BMI = bone marrow involvement, FDG = fluorine-18-fluro-deoxyglucose, FL = follicular lymphoma, FLIPI = Follicular Lymphoma International Prognostic Index, NPV = negative predictive value, PET/CT = positron emission tomography/computed tomography, PPV = positive predictive value, ROC = receiver operator curve, SUVmax = maximum standardized uptake value, SUVmean = mean standardized uptake value.
CP and HL contributed equally to this work.
EES and YH contributed equally to this work and are co-senior authors.
CP wrote the paper, HL analyzed PET/CT scans, EJ analyzed data, MK analyzed data, JBE analyzed the bone marrow biopsies, and EES supervised and wrote the paper. YH initiated the study, collected the data, and wrote the paper.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Brunning RD, Bloomfield CD, McKenna RW, et al. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med 1975; 82:365–366. [DOI] [PubMed] [Google Scholar]
- 2.Bain BJ. Bone marrow biopsy morbidity and mortality. Br J Haematol 2003; 121:949–951. [DOI] [PubMed] [Google Scholar]
- 3.Burton C, Ell P, Linch D. The role of PET imaging in lymphoma. Br J Haematol 2004; 126:772–784. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol 2005; 23:4643–4651. [DOI] [PubMed] [Google Scholar]
- 6.Elstrom R, Guan L, Baker G, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood 2003; 101:3875–3876. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto N, Kojima M, Hasegawa M, et al. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer 2007; 110:652–659. [DOI] [PubMed] [Google Scholar]
- 8.Perry C, Herishanu Y, Metzer U, et al. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur J Haematol 2007; 79:205–209. [DOI] [PubMed] [Google Scholar]
- 9.Karam M, Novak L, Cyriac J, et al. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer 2006; 107:175–183. [DOI] [PubMed] [Google Scholar]
- 10.Luminari S, Biasoli I, Arcaini L, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol 2013; 24:2108–2112. [DOI] [PubMed] [Google Scholar]
- 11.Le Dortz L, De Guibert S, Bayat S, et al. Diagnostic and prognostic impact of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imaging 2010; 37:2307–2314. [DOI] [PubMed] [Google Scholar]
- 12.Tychyj-Pinel C, Ricard F, Fulham M, et al. PET/CT assessment in follicular lymphoma using standardized criteria: central review in the PRIMA study. Eur J Nucl Med Mol Imaging 2014; 41:408–415. [DOI] [PubMed] [Google Scholar]
- 13.Alcantara M, Dupuis J, Mareschal S, et al. PET/CT before autologous stem cell transplantation predicts outcome in refractory/relapsed follicular lymphoma. Eur J Nucl Med Mol Imaging 2015; 42:215–221. [DOI] [PubMed] [Google Scholar]
- 14.Novelli S, Briones J, Flotats A, et al. PET/CT assessment of follicular lymphoma and high grade B cell lymphoma – good correlation with clinical and histological features at diagnosis. Adv Clin Exp Med 2015; 24:325–330. [DOI] [PubMed] [Google Scholar]
- 15.Adams HJ, Nievelstein RA, Kwee TC. Opportunities and limitations of bone marrow biopsy and bone marrow FDG-PET in lymphoma. Blood Rev 2015; 29:417–425. [DOI] [PubMed] [Google Scholar]
- 16.El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012; 30:4508–4514. [DOI] [PubMed] [Google Scholar]
- 17.Khan AB, Barrington SF, Mikhaeel NG, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 2013; 122:61–67. [DOI] [PubMed] [Google Scholar]
- 18.Berthet L, Cochet A, Kanoun S, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med 2013; 54:1244–1250. [DOI] [PubMed] [Google Scholar]
- 19.Adams HJ, Kwee TC. Critical considerations on the utility of 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography for posttreatment restaging of the bone marrow in diffuse large B-cell lymphoma. Am J Hematol 2014; 89:935. [DOI] [PubMed] [Google Scholar]
- 20.Adams HJ, Kwee TC. Do not abandon the bone marrow biopsy yet in diffuse large B-cell lymphoma. J Clin Oncol 2015; 33:1217. [DOI] [PubMed] [Google Scholar]
- 21.Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011; 29:1452–1457. [DOI] [PubMed] [Google Scholar]
- 22.Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med 2005; 46:958–963. [PubMed] [Google Scholar]
- 23.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program 2009; 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams HJ, Kwee TC, Fijnheer R, et al. Utility of quantitative FDG-PET/CT for the detection of bone marrow involvement in follicular lymphoma: a histopathological correlation study. Skeletal Radiol 2014; 43:1231–1236. [DOI] [PubMed] [Google Scholar]
- 25.Wohrer S, Jaeger U, Kletter K, et al. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) visualizes follicular lymphoma irrespective of grading. Ann Oncol 2006; 17:780–784. [DOI] [PubMed] [Google Scholar]
- 26.Adams HJ, Kwee TC, Nievelstein RA. Influence of imperfect reference standard bias on the diagnostic performance of MRI in the detection of lymphomatous bone marrow involvement. Clin Radiol 2013; 68:750–751. [DOI] [PubMed] [Google Scholar]
- 27.Adams HJ, Kwee TC, de Keizer B, et al. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2014; 41:565–574. [DOI] [PubMed] [Google Scholar]


