Abstract
Asthma has been described as an “acephalic migraine” and “pulmonary migraine.” However, no study has investigated the temporal frequency of migraine development in patients with asthma, and the results of previous studies may be difficult to generalize.
We investigated the effect of asthma on the subsequent development of migraine by using a population-based data set in Taiwan.
We retrieved our study sample from the National Health Insurance Research Database. Specifically, 25,560 patients aged 12 years and older with newly diagnosed asthma were identified as the asthma group, and 102,238 sex and age-matched patients without asthma were identified as the nonasthma group. Cox proportional-hazards regression models were employed to measure the risk of migraine for the asthmatic group compared with that for the nonasthmatic group.
The risk of migraine in the asthmatic group was 1.45-fold higher (95% confidence interval 1.33–1.59) than that in the nonasthmatic group after adjustment for sex, age, the Charlson comorbidity index, common medications prescribed for patients with asthma, and annual outpatient department visits. An additional stratified analysis revealed that the risk of migraine remained significantly higher in both sexes and all age groups older than 20 years.
Asthma could be an independent predisposing risk factor for migraine development in adults.
INTRODUCTION
Asthma is a chronic syndrome characterized by reversible airway obstruction, airway inflammation, and airway hyperresponsiveness; estimates have indicated that 8% (18.7 million) of adults in the United States currently have asthma, and more than 300 million people are affected by asthma worldwide.1,2 Moreover, its prevalence has increased considerably in the past 20 years.3,4 Patients with asthma may experience recurrent episodes of chest tightness, shortness of breath, coughing, and wheezing; consequently, people with this condition are at reportedly higher risks of nonrespiratory disorders such as depression, anxiety disorder, and dementia development compared with people without asthma.5,6 The goal of current asthma management is to focus on managing rather than curing the disease.
Migraine is a highly prevalent and disabling neurological disorder characterized by episodic unilateral headache attacks that are often accompanied by photophobia, phonophobia, nausea, and vomiting,7 and the estimated cumulative lifetime incidence of migraine is 43% in women and 18% in men.8 Although various factors have been identified to be associated with migraine attacks, such as stress, auditory hypersensitivity, and hormone imbalance,9 the pathogenesis of migraine is only partially understood.10
Asthma has been described as an “acephalic migraine” and “pulmonary migraine,”11,12 and several epidemiologic studies have reported an association between migraine and respiratory disorders.13–16 However, some of these studies have used a relatively small sample,15,16 and all of them have adopted a case-control design. No study has investigated the temporal frequency of migraine development in patients with asthma, and the results of previous studies may be difficult to generalize. Therefore, we conducted this retrospective nationwide cohort study by using data retrieved from Taiwan's National Health Insurance Research Database (NHIRD) to test the hypothesis that patients with asthma have a higher risk of migraine.
METHODS
Data Sources
The NHIRD was created by the National Health Research Institutes (NHRI) and contains claims data from the Taiwan National Health Insurance (NHI) program. The NHI program, implemented in 1995, is a compulsory single-payer health care system with over 99.9% coverage of the population of Taiwan at the end of 2014. This study used the Longitudinal Health Insurance Database 2000 (LHID2000), which contains data for 1 million enrollees derived from the medical claims records of the NHI program between 1996 and 2011. The NHIRD contains beneficiary demographics, clinical visit dates, prescription details, and diagnostic codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The NHRI manages the claims data and provides scrambled random identification numbers for insured patients to secure patient privacy. This study was approved by the Institutional Review Board of China Medical University (CMUH-104-REC2-115).
Study Population
We conducted a nationwide retrospective population-based cohort study involving 2 groups: an asthma group and a nonasthma group. From outpatient and inpatient care dates in the LHID2000, we identified 41,011 patients newly diagnosed with asthma (ICD-9-CM 493) between January 1, 2000 and December 31, 2005. We excluded patients from the analyses if they were younger than 12 years (n = 13,813), were previously diagnosed with migraine (ICD-9-CM-346) (n = 1635), or had missing information on age or sex (n = 3). The date of asthma diagnosis was considered the index date. To ensure the accuracy of the asthma diagnosis, we selected patients who had received treatment involving inhaled corticosteroids, systemic (oral or intravenous) corticosteroids, or inhaled beta-2 agonists (short-acting beta-2 agonists [SABAs] or long-acting beta-2 agonists [LABAs]) as the asthma group. For each patient with asthma, 4 insured persons without a diagnosis of asthma were selected from the LHID2000 as the nonasthma group and were frequency-matched according to sex, age (every 5-year span), and index year by using the same inclusion criteria as those of the asthma cohort.
Covariates and Outcomes
The considered demographic factors were sex and age (12–19, 20–44, and ≥45 years). Comorbidities were evaluated using the Charlson comorbidity index (CCI) and were considered confounding factors.17,18 According to each subject's inpatient diagnosis, we calculated the CCI scores as the sum of the weighted score of 17 comorbid conditions. A weight was assigned to each indicated diagnosis and added together to provide a total CCI score. For the CCI score, 16 types of comorbidities were classified into different categories. Comorbidities such as myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, and diabetes mellitus were weighted 1; moderate to severe diabetes with chronic complications, hemiplegia or paraplegia, renal disease, leukemia, tumor of any type, and malignant lymphoma were weighted 2; moderate-to-severe liver diseases were weighted 3; and AIDS and metastatic solid tumor were weighted 6.
Medications that could affect the progression of migraine, such as inhaled corticosteroids, systemic corticosteroids, and beta-2 agonists (including SABAs and LABAs), were included as analysis variables. Prescribed medications were defined as those prescribed for 30 successive days within 1 year of the index date. Moreover, the number of annual outpatient department (OPD) visits was also included as an analysis variable. The outcome measure of interest, which was determined at least thrice by outpatient services or inpatient hospitalization claims, was based on the ICD-9-CM diagnosis code for migraine (ICD-9-CM 346). Both groups were observed from the index date until the date of migraine diagnosis, withdrawal from the NHI program, or the end of 2011.
Statistical Analysis
The continuous variables are expressed as the mean and standard deviation (SD), whereas the categorical variables are expressed as frequencies and percentages. This study used the Student t test for continuous variables and the Pearson chi-square test for categorical variables to compare the differences in the demographic factors, CCI scores, medications used, and annual OPD visits between the asthma and nonasthma groups. The sex, age, and CCI score-specific incidence density rates (per 1000 person-years) of migraine were calculated using the number of migraine incidents divided by the person-years at risk in both groups. The cumulative incidence curves of migraine in the asthma and nonasthma groups were estimated using Kaplan–Meier analysis, and the difference between the groups was compared using the log-rank test. Univariate and multivariate Cox proportional-hazards regression models were used to assess the risk of migraine and migraine-associated risk factors. The multivariate model was adjusted for sex, age, the CCI score, medications used, and annual OPD visits. We also compared the hazard ratio (HR) of migraine between the asthma and nonasthma groups after stratification by sex, age, and the CCI score. A P value less than 0.05 was considered statistically significant, and SAS Version 9.3 software (SAS Institute, Inc., Cary, NC) was used to perform all the statistical analyses.
RESULTS
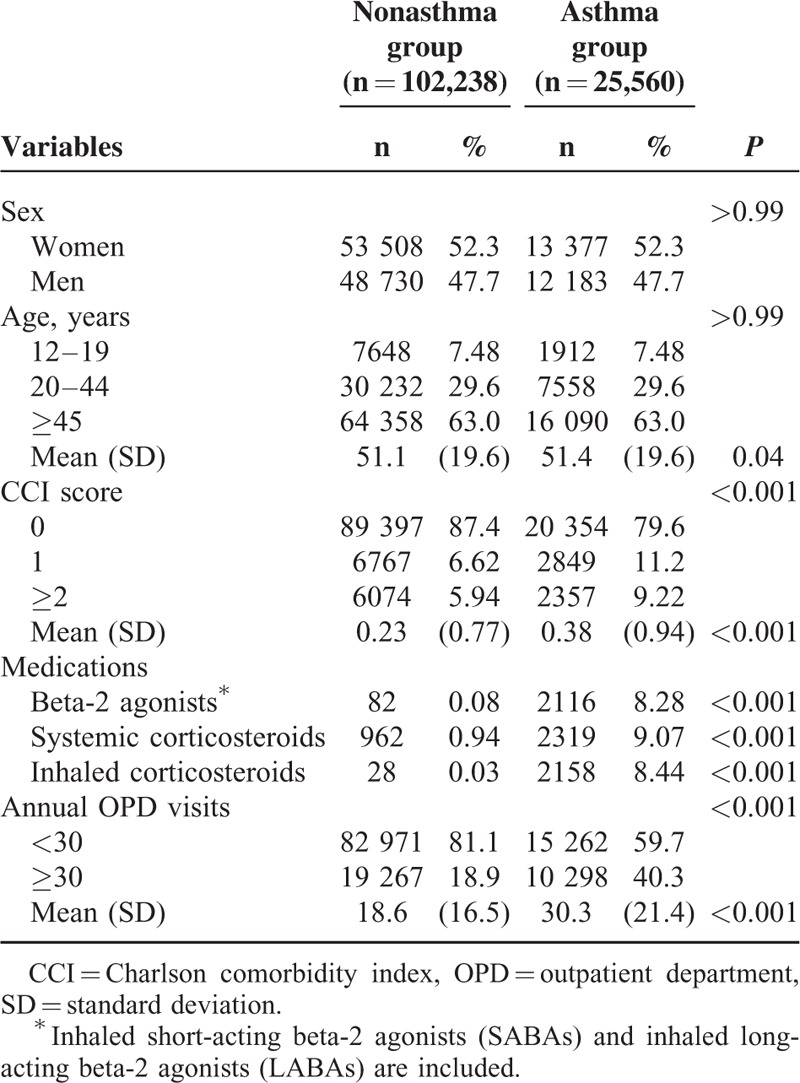
Our study population comprised 25,560 and 102,238 participants in the asthma and nonasthma groups, respectively. Table 1 presents the demographic characteristics, CCI scores, medications used, and annual OPD visits in both groups. The study groups were predominantly female (52.3%), and 63.0% of the study participants were older than 45 years. No significant differences in the distributions of sex and age were observed between the asthma and nonasthma groups. The mean ages of the asthma and nonasthma groups were 51.4 (SD = 19.6) and 51.1 (SD = 19.6) years, respectively. A higher CCI score was found in the asthma group than in the nonasthma group (0.38 ± 0.94 vs 0.23 ± 0.77). The asthma group exhibited a higher prevalence of using beta-2 agonists, systemic corticosteroids, inhaled corticosteroids, and OPD visits compared with the nonasthma group.
TABLE 1.
Baseline Demographic Factors and Comorbidity of Study Participants According to Asthma Status
The mean follow-ups were 8.40 years (SD = 2.79 years) for the asthma group and 8.42 years (SD = 2.72 years) for the nonasthma group. Figure 1 shows the cumulative incidence curves of migraine according to asthma status. We used the log-rank test to examine the cumulative incidence of migraine between the groups with and without asthma. We found that the cumulative incidence of migraine was significantly higher in the asthma group than in the nonasthma group (P < 0.001).
FIGURE 1.
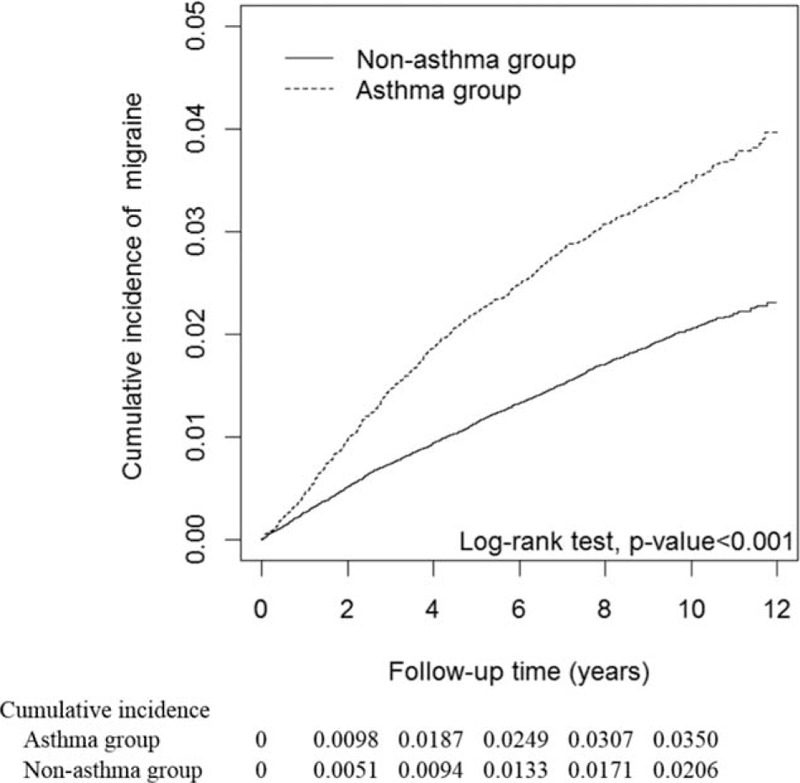
Cumulative incidence curves of migraine for groups with and without asthma.
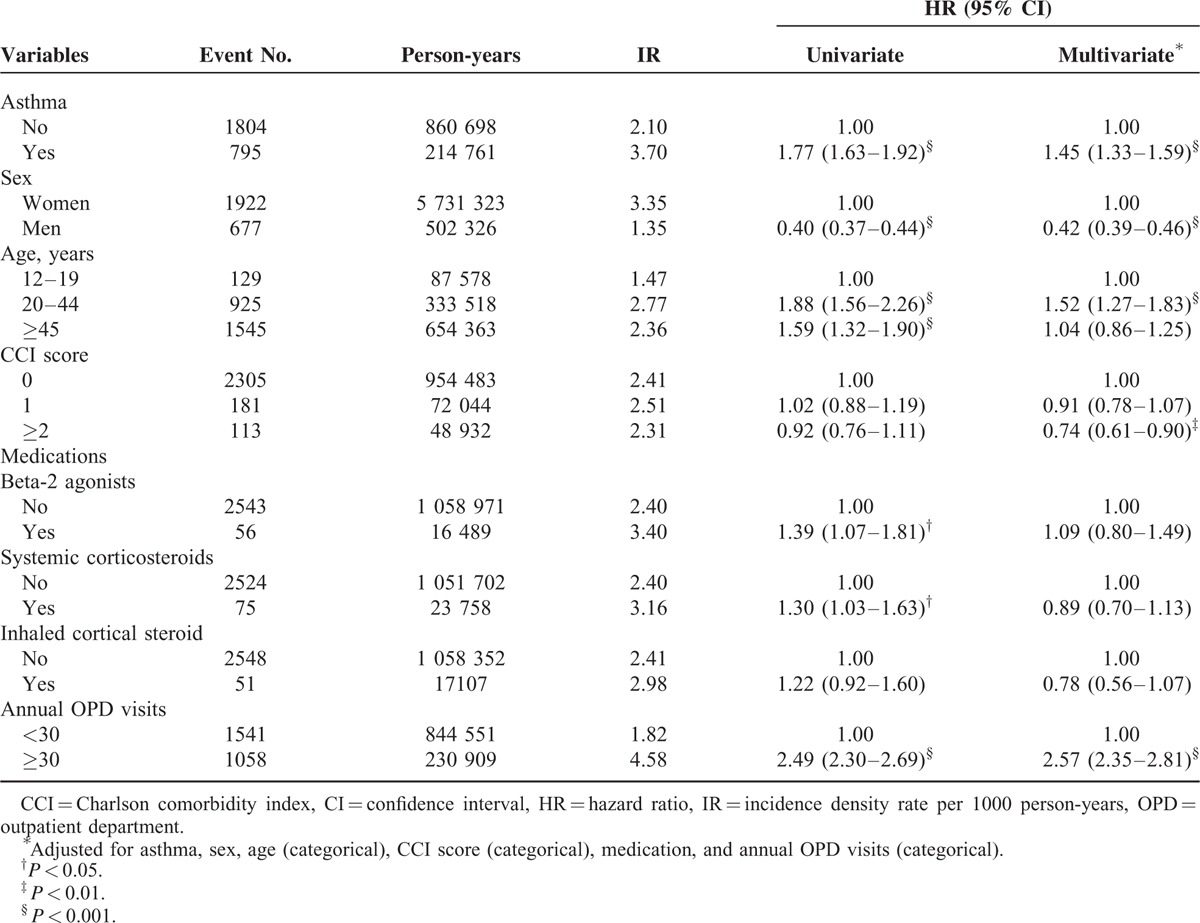
The incidence density rate of migraine was 3.70 per 1000 person-years in the asthma group, 1.77-fold higher than that in the nonasthma group (2.10 per 1000 person-years), with an adjusted HR of 1.45 (95% confidence interval [CI] 1.33–1.59) (Table 2). Multivariate analysis showed that men (adjusted HR 0.42, 95% CI 0.39–0.46) and CCI scores of 2 and higher (adjusted HR 0.74, 95% CI 0.61–0.90) were significantly associated with a lower risk of migraine. By contrast, the results showed that patients aged 20–44 years and those with more than 30 annual OPD visits had an increased risk of migraine (adjusted HR 1.52, 95% CI 1.27–1.83; adjusted HR 2.57, 95% CI 2.35–2.81, respectively).
TABLE 2.
Cox Model Measured Hazard Ratios and 95% Confidence Intervals of Migraine Associated With Asthma and Covariates
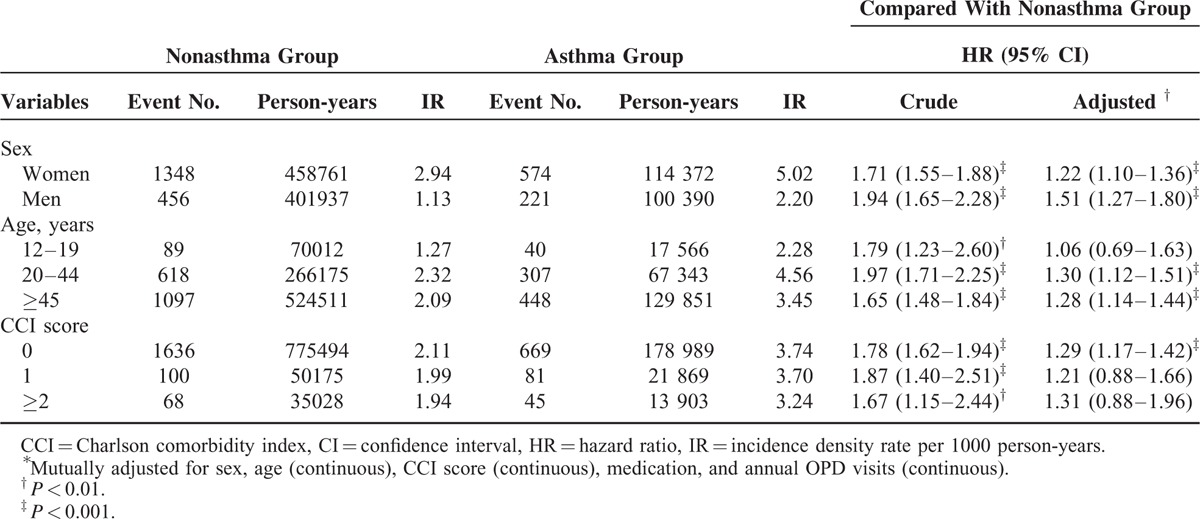
In the analysis stratified by sex, the risk of migraine in the asthma patients was significantly higher in both sexes compared with the patients without asthma, with adjusted HRs of 1.22 (95% CI 1.10–1.36) and 1.51 (95% CI 1.27–1.80) for women and men, respectively. Stratified by age group, the patients with asthma had a significantly higher risk of migraine compared with those without asthma in all age groups, except for the 12 to 19-year-old age group. The adjusted HRs of migraine were 1.30 (95% CI 1.12–1.51) among the 20 to 44-year-olds and 1.28 (95% CI 1.14–1.44) among the ≥45-year-olds. In study participants with a CCI score of 0, patients with asthma had a higher risk of migraine than that of the patients without asthma (adjusted HR 1.29, 95% CI 1.17–1.42) (Table 3).
TABLE 3.
Incidence Density Rates and Hazard Ratios of Migraine According to Asthma Status Stratified By Sex, Age, and CCI Score
DISCUSSION
In this large nationwide cohort study, after adjusting for sex, age, the CCI score, medications used, and annual OPD visits, we observed that adult patients with asthma were 1.45-fold more likely to develop migraine than those without asthma. Although the mean CCI score, prevalence of beta-2 agonists and corticosteroids used, and number of annual OPD visits were significantly higher in the asthmatic patients than in the participants without asthma, the risk of migraine remained significantly higher after adjustment for these confounding factors. An additional stratified analysis revealed that the risk of migraine in asthmatic patients remained significantly higher between both sexes and among all age groups older than 20 years.
Previous studies19–21 have explored the association between general headaches and asthma. Recently, Davey et al13 reported that the relative risk of asthma in migraineurs was 1.59 compared with nonmigraineurs in a case-control study that used 64,678 case-control pairs from the British General Practice Research Database. Ozge et al15 reported in a clinical study that 41.4% of migraineurs have at least 1 atopic disorder, including asthma, which was higher than in the general population. A large questionnaire-based study by Aamodt et al14 reported that both migraine and nonmigrainous headaches were approximately 1.5-fold more likely in people with asthma than in those without asthma. Moreover, Kaleagasi et al16 reported that a positive bronchial provocation test, a key feature of asthma, was more prevalent for migraineurs than for controls. Our data are consistent with these studies, indicating an association between asthma and migraine. However, the definitions of asthma and migraine in the study by Aamodt et al14 were based on participant-report questionnaires, which might not be as valid as our data source. In addition, the relatively small samples in the studies by Ozge et al and Kaleagasi et al might render the study results difficult to generalize.15,16 Finally, the temporal relationship between asthma and migraine risk has been poorly defined in all of these studies. On the basis of our research, the present study is the first large population-based nationwide cohort study demonstrating that adult patients with asthma have a significantly higher subsequent risk of migraine than those without asthma do.
The specific pathophysiology underlying the association between asthma and migraine is unknown. However, several lines of evidence from previous studies have suggested that asthma and migraine have a shared pathophysiology. First, a nonselective cation channel expressed in the cell membranes of afferent sensory fibers, named transient receptor potential vanilloid subfamily member 1 (TRPV1), has been shown to play a significant role in the generation and pathophysiology of both asthma and migraine.22–24 TRPV1 in the airway C-fiber sensory nerves activated by an endogenous or inhaled irritant can result in the release of various neuropeptides, which are believed to contribute to the manifestation of pathophysiological features of asthma such as bronchoconstriction, hypersecretion, and coughing.22,24,25 Similarly, activation of TPRV1 by various chemical substances, low pH, and noxious temperature may result in the release of various neuropeptides at the peripheral termini of the trigeminal nociceptors, and these neuropeptides can exert a vasodilatory effect and initiate neurogenic inflammation, both of which are crucial in the generation of migraine headache.23,26,27 Second, although it is well established that mast cells are involved in the pathogenesis of asthma by infiltrating the airway smooth muscle and inducing airway remodeling by releasing various inflammatory mediators,28–30 emerging evidence has shown that meningeal and brain mast cells are closely associated with neurons—particularly in the dura—and these mast cells are believed to be the potent modulators of meningeal nociceptor activity and the genesis of migraine headache.31–33 Finally, production of the platelet activating factor, a proinflammatory mediator that has been implicated as being responsible for airway hyperresponsiveness and airway inflammation in asthma,34,35 has been reported to increase and potentially result in persistent platelet activation and hyperfunction in the cerebral circulation during a migraine attack.36
In our study, women displayed a significantly higher risk of migraine than men did, and people aged 20 to 44 years had a significantly higher risk of migraine compared with their younger and older counterparts, consistent with several studies indicating that the prevalence of migraine is generally higher in women than in men, and varies considerably with age, increasing from adolescence to approximately 40 to 45 years of age, and declining thereafter in both sexes.37–39 This was why we stratified adult asthmatic patients into 2 subgroups: aged 20 to 44 years and ≥45 years, to observe the influences of asthma on migraine risk. Although the prevalence of migraine is higher in women than in men in the general population, as indicated by both previous studies and ours, the HR of migraine among asthmatic patients seems to be no different between the sexes. Because this study was observational and, thus far, no comparative studies have explored the sex differences of migraine risk in patients with asthma, future study is warranted to explore this issue.
Because inhaled beta-2 agonists, and oral and systemic corticosteroids are common medications prescribed to patients with asthma, and because corticosteroids are also known to be prescribed either as monotherapy or as adjuvant therapy in aborting migraines when other acute care medications have failed,40 we evaluated whether these medications prescribed for 30 successive days after asthma diagnosis affect migraine risk. Our data show that beta-2 agonists did not significantly affect migraine risk, consistent with Wilkinson et al,20 who reported that in schoolchildren aged 5 to 15 years, no association was observed between frequent headache and use of bronchodilators. Our data show that systemic/inhaled corticosteroids did not significantly affect migraine risk either. Because no comparative study exists, further study examining whether corticosteroids affect migraine risk is necessary for corroborating or refuting our findings.
The strengths of this study are its nationwide population-based design, relatively long follow-up (up to 12 years), and the representativeness of the cohorts. However, several limitations should be considered. First, detailed information on the lifestyle of the patients, such as cigarette smoking, alcohol consumption, dietary habits, and environmental effects, is not provided in the NHIRD, all of which might have been confounding factors. Second, the diagnoses were based on ICD-9 codes obtained from the administrative data. Each patient's clinical information, such as imaging results, serum laboratory data, lung function tests, migraine frequency, and the presence or absence of auras, was not available in the NHIRD. Therefore, it was difficult to distinguish between allergic and nonallergic asthma, and also different types of migraine. Third, because the information about pain medications was incomplete in the NHIRD, we could not determine whether these medications affected migraine development in our study. Finally, despite our meticulous study design with adequate control for confounding factors, unknown or unmeasured confounders were present and may have biased the study results. Nevertheless, in the NHI program, from which the NHIRD is derived, all the insurance claims must be reviewed and audited by medical reimbursement specialists, upholding the validity and accuracy of the asthma and migraine diagnoses. Furthermore, because of the validity of the database, and also the large sample and long follow-up period, we believe that our finding regarding the association between asthma and migraine is reliable.
In summary, our study revealed that adult patients with asthma exhibited a significantly higher risk of migraine than did those without asthma. This increased risk was significantly higher in both sexes and in adults of all ages. We suggest that clinicians be aware that asthma is a potential risk factor for migraine. Future studies are advised to confirm our findings and explore the underlying pathophysiology.
Footnotes
Abbreviations: CCI = Charlson comorbidity index, CI = confidence interval, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LABA = long-acting beta-2 agonist, LHID2000 = Longitudinal Health Insurance Database 2000, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, OPD = outpatient department, SABA = short-acting beta-2 agonist, TRPV1 = transient receptor potential vanilloid subfamily member 1.
Y-HP and K-FC contributed equally to this article.
W-CL and C-HC contributed equally.
Authors’ contributions: Y-HP, K-FC, C-HC, and W-CL contributed to the conception and design of the study; C-HK and H-JC provided administrative support; Y-HP, K-FC, and W-CL contributed to data analysis and interpretation; all of the authors were responsible for the collection and assembly of data and the final approval of the article; and Y-HP, K-FC, C-HC, and W-CL were responsible for article writing.
Funding: This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019); China Medical University Hospital; Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037); NRPB Stroke Clinical Trial Consortium (MOST 104–2325-B-039-005); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention, Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2012. http://www.cdc.gov/nchs/fastats/asthma.htm Accessed December 24, 2015. [Google Scholar]
- 2.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma. http://www.ginasthma.org Accessed November 15, 2015. [Google Scholar]
- 3.Song WJ, Kang MG, Chang YS, et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy 2014; 4:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amato G, Baena-Cagnani CE, Cecchi L, et al. Climate change, air pollution and extreme events leading to increasing prevalence of allergic respiratory diseases. Multidiscip Respir Med 2013; 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MH, Su TP, Chen YS, et al. Higher risk of developing major depression and bipolar disorder in later life among adolescents with asthma: a nationwide prospective study. J Psychiatr Res 2014; 49:25–30. [DOI] [PubMed] [Google Scholar]
- 6.Peng YH, Wu BR, Su CH, et al. Adult asthma increases dementia risk: a nationwide cohort study. J Epidemiol Community Health 2015; 69:123–128. [DOI] [PubMed] [Google Scholar]
- 7.Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24 Suppl 1:9–160. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008; 28:1170–1178. [DOI] [PubMed] [Google Scholar]
- 9.Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep 2014; 18:454. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ, Charbit AR, Andreou AP, et al. Neurobiology of migraine. Neuroscience 2009; 161:327–341. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T. Asthma and migraine: is asthma a part of acephalgic migraine? A hypothesis. Ann Allergy 1988; 60:374. [PubMed] [Google Scholar]
- 12.Tucker GF., Jr Pulmonary migraine. Ann Otol Rhinol Laryngol 1977; 86:671–676. [DOI] [PubMed] [Google Scholar]
- 13.Davey G, Sedgwick P, Maier W, et al. Association between migraine and asthma: matched case-control study. Br J Gen Pract 2002; 52:723–727. [PMC free article] [PubMed] [Google Scholar]
- 14.Aamodt AH, Stovner LJ, Langhammer A, et al. Is headache related to asthma, hay fever, and chronic bronchitis? The Head-HUNT Study. Headache 2007; 47:204–212. [DOI] [PubMed] [Google Scholar]
- 15.Ozge A, Ozge C, Ozturk C, et al. The relationship between migraine and atopic disorders-the contribution of pulmonary function tests and immunological screening. Cephalalgia 2006; 26:172–179. [DOI] [PubMed] [Google Scholar]
- 16.Kaleagasi H, Ozgur E, Ozge C, et al. Bronchial hyper-reactivity in migraine without aura: is it a new clue for inflammation? Headache 2011; 51:426–431. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Chen TC, Leviton A. Asthma and eczema in children born to women with migraine. Arch Neurol 1990; 47:1227–1230. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson IA, Halliday JA, Henry RL, et al. Headache and asthma. J Paediatr Child Health 1994; 30:253–256. [DOI] [PubMed] [Google Scholar]
- 21.Anderson HR, Bailey PA, Cooper JS, et al. Morbidity and school absence caused by asthma and wheezing illness. Arch Dis Child 1983; 58:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 2007; 1772:915–927. [DOI] [PubMed] [Google Scholar]
- 23.Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med 2010; 16:153–159. [DOI] [PubMed] [Google Scholar]
- 24.Takemura M, Quarcoo D, Niimi A, et al. Is TRPV1 a useful target in respiratory diseases? Pulm Pharmacol Ther 2008; 21:833–839. [DOI] [PubMed] [Google Scholar]
- 25.Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther 2009; 22:65–70. [DOI] [PubMed] [Google Scholar]
- 26.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology 2005; 64:S9–15. [DOI] [PubMed] [Google Scholar]
- 27.Lassen LH, Jacobsen VB, Haderslev PA, et al. Involvement of calcitonin gene-related peptide in migraine: regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain 2008; 9:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 2011; 38:555–563. [DOI] [PubMed] [Google Scholar]
- 29.Brightling CE, Ammit AJ, Kaur D, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 2005; 171:1103–1108. [DOI] [PubMed] [Google Scholar]
- 30.Brightling CE, Bradding P, Symon FA, et al. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002; 346:1699–1705. [DOI] [PubMed] [Google Scholar]
- 31.Levy D, Burstein R, Kainz V, et al. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 2007; 130:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theoharides TC, Donelan J, Kandere-Grzybowska K, et al. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev 2005; 49:65–76. [DOI] [PubMed] [Google Scholar]
- 33.Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep 2009; 13:237–240. [DOI] [PubMed] [Google Scholar]
- 34.Cuss FM, Dixon CM, Barnes PJ. Effects of inhaled platelet activating factor on pulmonary function and bronchial responsiveness in man. Lancet 1986; 2:189–192. [DOI] [PubMed] [Google Scholar]
- 35.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation 2008; 31:112–120. [DOI] [PubMed] [Google Scholar]
- 36.Sarchielli P, Alberti A, Coppola F, et al. Platelet-activating factor (PAF) in internal jugular venous blood of migraine without aura patients assessed during migraine attacks. Cephalalgia 2004; 24:623–630. [DOI] [PubMed] [Google Scholar]
- 37.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005; 45 Suppl 1:S3–S13. [DOI] [PubMed] [Google Scholar]
- 38.Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992; 267:64–69. [PubMed] [Google Scholar]
- 39.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology 1994; 44:S17–23. [PubMed] [Google Scholar]
- 40.Woldeamanuel YW, Rapoport AM, Cowan RP. What is the evidence for the use of corticosteroids in migraine? Curr Pain Headache Rep 2014; 18:464. [DOI] [PubMed] [Google Scholar]


