Supplemental Digital Content is available in the text
Abstract
This study aimed to evaluate cancer risk and possible risk factors in patients diagnosed with empyema.
A total of 31,636 patients with newly diagnosed empyema between January 1, 1999 and December 31, 2010 were included in this study. Standardized incidence ratios (SIRs) were calculated to compare the cancer incidence in these empyema patients to that in the general population. Adjusted hazard ratios were also calculated to investigate whether characteristics increased cancer risk.
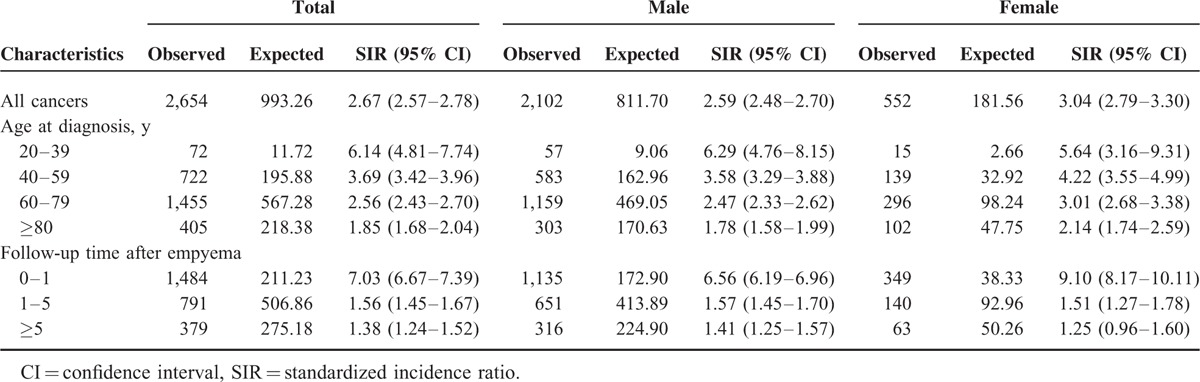
During the 12-year study period, 2,654 cancers occurred in 31,636 patients with empyema, yielding an SIR of 2.67 (95% confidence interval [CI] 2.57–2.78). We excluded cancer that occurred within 1 year to avoid surveillance bias. The cancer risk remained significantly increased (SIR 1.50, 95% CI 1.41–1.58). Specifically, patients with empyema had higher SIR of cancers of the head and neck (1.50, 95% CI 1.41–1.58), esophagus (2.56, 95% CI 1.92–3.33), stomach (1.49, 95% CI 1.16–1.89), liver and biliary tract (2.18, 95% CI 1.93–2.45), and lung and mediastinum (1.62, 95% CI 1.39–1.86). Age ≥ 60, male sex, diabetes mellitus, and liver cirrhosis were independent risk factors for cancer development.
Our study demonstrates an increased incidence of cancer development in patients with empyema, and patients’ age ≥ 60, men, and those with diabetes mellitus and liver cirrhosis showed a higher incidence of developing cancer compared to the general population. The association between such kind of infection and secondary malignancy may be elucidated by further study.
INTRODUCTION
Empyema is the accumulation of pus in the thoracic cage that often results from a progressive deposition of parapneumonic fluid. Persistent infection eventually results in the formation of scar tissue and a pleural peel encompassing the lung.1 Even with the advance of antibiotics and pneumococcal vaccines, this still accounts for approximately 5% of cases of pneumonia.2 Incidence of empyema has been increasing worldwide in recent decades.3 About 15% of empyema patients die and 30% of patients require interventional drainage of the pleural space.4,5 Most studies focus on short-term empyema complications; however, the long-term effects of this disease, including cancer, have hardly been studied.
Increased cancer risk in patients with inflammatory and infectious diseases has been reported in many studies.6,7 Furthermore, the effects of chronic pulmonary inflammatory disease on cancer have been well documented.8,9 However, no study to date has focused on the association between empyema and further cancer risk.
Using the National Health Insurance Research Dataset (NHIRD) of Taiwan, we conducted a nationwide population-based study to examine the relative risk of malignancies, including specific cancer types, in patients with empyema.
MATERIALS AND METHODS
Data Sources
Taiwan's National Health Insurance (NHI) program, which began in 1995, is a mandatory universal health insurance program that covers more than 99% of the Taiwanese population.10 The NHIRD is managed by the National Health Research Institutes (NHRI) of Taiwan and consists of detailed healthcare data from more than 28 million enrollees. It includes coverage for outpatient, inpatient, emergency, dental, and traditional Chinese medicine services. The Longitudinal Health Insurance Database is a subset of the NHIRD and is a representative database containing 1,000,000 patients randomly sampled from the registry of all enrollees. Cancer diagnosis was confirmed using the Registry for Catastrophic Illness Patients, which contains comprehensive enrollment information for all patients with severe diseases who have received copayment exemption under the NHI program. Patients diagnosed with cancer require histological confirmation to be enrolled in this registry.
All information that could potentially identify an individual is encrypted. Confidentiality of data is maintained in accordance with the data regulations of the Bureau of NHI and the NHRI. This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (2014-05-001BE).
Study Population
Patients newly diagnosed with empyema (International Classification of Diseases, Ninth Revision, Clinical Modification: 510.X) between January 1, 1999 and December 31, 2010 were enrolled in the empyema cohort. We excluded patients under 20 years old and those who had prior malignancies. The observation period was from January 1, 1999 to December 31, 2011.
Statistical Analysis
The follow-up period started at the date of diagnosis of empyema and ended at death or on December 31, 2011, and the occurrence of cancer was identified as the main dependent variable. We used standardized incidence ratio (SIR) as the main method of analysis. To calculate SIR, the observed number of cancer occurrences was divided by the expected number, which was computed by applying the national cancer incidence rate record in the Taiwan National Cancer Registry. The 95% confidence intervals (CI) for each SIR were calculated assuming a Poisson distribution of cancer occurrence. A 1-year lag time was used to avoid misclassification and surveillance bias, and Bonferroni correction was applied to counteract the problem of multiple comparisons.
The Cox proportional hazards model was used in univariate and multivariate analyses to identify predictors of cancer occurrence. All variables with a P value < 0.1 in the univariate analyses were entered into a multivariate analysis. The statistical analyses were conducted using SAS 9.3 software (SAS Institute, Inc., Cary, NC). The selected level of significance was P < 0.05. The Perl programming language (version 5.12.2) was used for data extraction and calculation, and Microsoft SQL Server 2012 (Microsoft Corp., Redmond, WA) for data linkage, processing, and sampling.
RESULTS
Study Population Characteristics
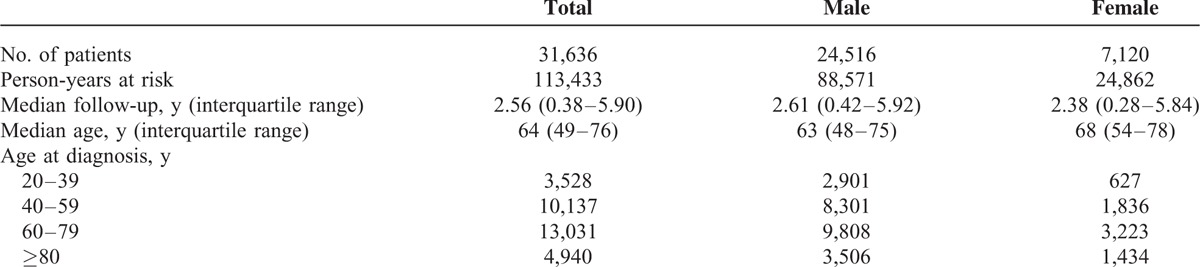
We identified 39,024 patients with newly diagnosed empyema. Of these, 2,335 were diagnosed before age 20, 72 were lost to follow-up after empyema diagnosis, and 4,981 had antecedent malignancies. The patient selection flow chart is shown in Figure 1. After excluding these patients, the final sample consisted of 31,636 patients, 77.5% of which were men. Overall, the cohort was observed for 113,433 person-years. The median age at diagnosis was 64 years (interquartile range, 49–76 years). The demographic data of the cohort are shown in Table 1.
FIGURE 1.
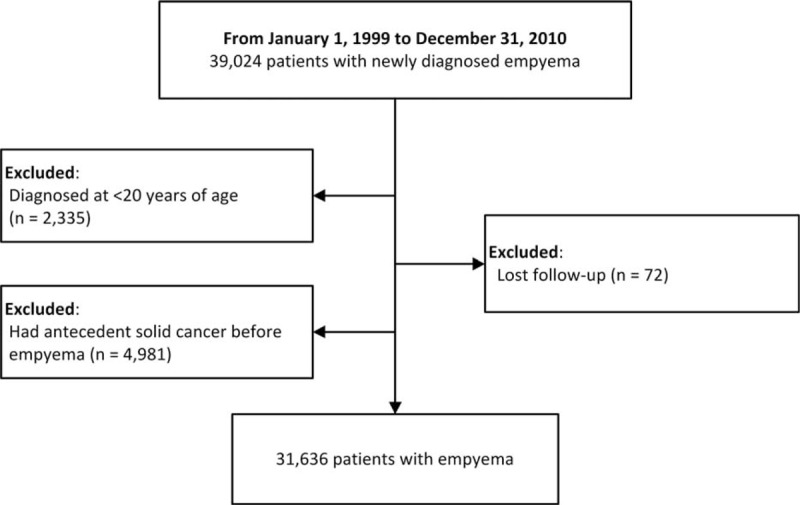
Patient selection flow chart.
TABLE 1.
Characteristics of Patients With Empyema
All Cancers
During the study period, 2,654 cancers occurred. Compared with the general population, patients with empyema had a significantly greater cancer risk, with an SIR of 2.67 (95% CI 2.57–2.78; P < 0.001). The SIR was 2.59 (95% CI 2.48–2.70; P < 0.001) for men and 3.04 (95% CI 2.79–3.304; P < 0.001) for women. A subgroup analysis performed according to patient age at diagnosis of empyema revealed that younger patients tended to have a greater cancer SIR. In the subgroup analysis performed according to follow-up duration after diagnosis of empyema, 1,484 cases of cancer were diagnosed during the first year of follow-up, only 211.23 of which were expected. This yielded an SIR of 7.03 (95% CI 6.67–7.39; P < 0.001). After excluding the first year of follow-up, a total of 1,170 cancer cases were observed during the remainder of the observation period, yielding an SIR of 1.50 (95% CI 1.41–1.48; P < 0.001). Patients still had a greater cancer risk than the general population after excluding the first year of follow-up. The cumulative incidence of cancer after empyema is included in Figure 2. Furthermore, increased cancer risks were still present after 5 years of follow-up (SIR 1.38; 95% CI 1.24–1.52; P < 0.001). The results of subgroup analyses are summarized in Table 2.
FIGURE 2.
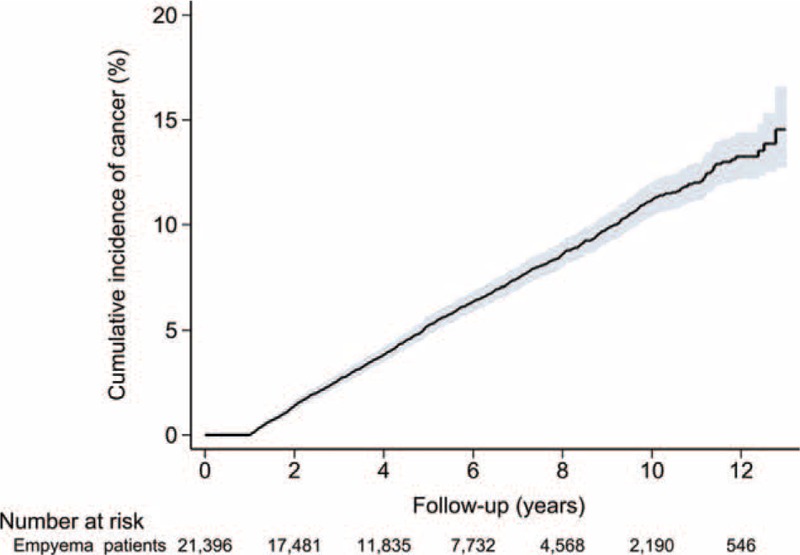
Cumulative incidence of cancer in patients with empyema.
TABLE 2.
Standardized Incidence Ratios According to Sex, Age at Diagnosis, and Follow-Up Time after Empyema
Specific Cancer Types
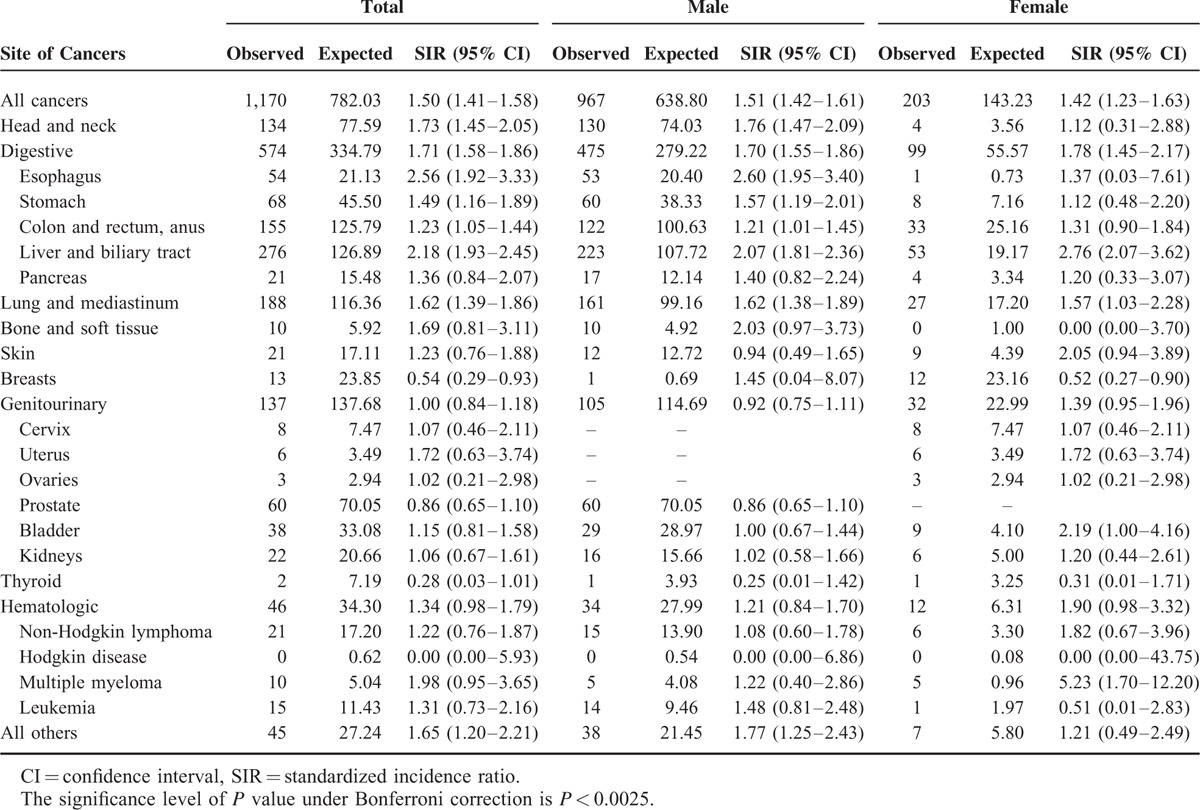
The cancer incidence for specific cancer types among patients with empyema during the whole follow-up period is shown in Supplemental Table 1. After excluding the first year of follow-up, patients with empyema still had a greater risk of cancer of the head and neck (SIR 1.73; 95% CI 1.45–2.05), esophagus (SIR 2.56, 95% CI 1.92–3.33), stomach (SIR 1.49, 95% CI 1.16–1.89), liver and biliary tract (SIR 2.18, 95% CI 1.93–2.45), and lung and mediastinum (SIR 1.62, 95% CI 1.39–1.86). The SIRs for specific types of cancers beyond the first year of follow-up are in Table 3.
TABLE 3.
Standardized Incidence Ratios for Specific Cancer Types Among Patients With Empyema (Follow-Up More Than 1 y)
Cancer Risk Predictors
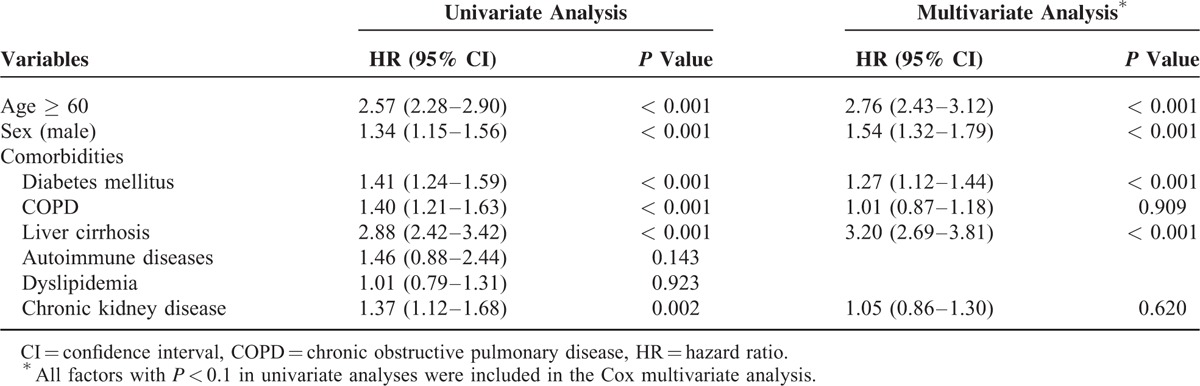
Univariate Cox proportional hazards analysis showed age ≥ 60 years (hazard ratio [HR] 2.57, 95% CI 2.28–2.90, P < 0.001), male sex (HR 1.34, 95% CI 1.15–1.56, P < 0.001), diabetes mellitus (HR 1.41, 95% CI 1.24–1.59, P < 0.001), chronic obstructive pulmonary disease (HR 1.40, 95% CI 1.21–1.63, P < 0.001), liver cirrhosis (HR 2.88, 95% CI 2.42–3.42, P < 0.001), and chronic kidney disease (HR 1.37, 95% CI 1.12–1.68, P = 0.002) to be associated with a higher risk of developing cancer. In the multivariate analysis, age ≥ 60 (HR 2.76, 95% CI 2.43–3.12, P < 0.001), male sex (HR 1.54, 95% CI 1.32–1.79, P < 0.001), diabetes mellitus (HR 1.27, 95% CI 1.12–1.44, P < 0.001), and liver cirrhosis (HR 3.20, 95% CI 2.69–3.81, P < 0.001) were still significant predictors of cancer development. The results of univariate and multivariate analyses are described in Table 4.
TABLE 4.
Risk Factors for Cancer Development in Patients With Empyema (Follow-Up More Than 1 y)
DISCUSSION
This nationwide population-based study shows that patients with empyema have increased cancer risk. Most cancer cases in our cohort were detected within 1 year after diagnosis according to the subgroup analysis of follow-up time. Comprehensive surveillance for cancer during that period may have led to a surveillance bias.11 However, after excluding the first-year follow-up, increased cancer risk was still observed for patients with empyema. The risk remained increased with more than 5 years of follow-up. This finding indicates a positive association between these diseases. Additionally, the SIRs of subgroups according to sex were similar, suggesting that sex is not a significant influence.
The cancer diagnoses in our study are considered reliable. The bureau of NHI has implemented a strict verification process for enrollment in the Registry of Catastrophic Illness Patients, which requires pathological proof of malignancies. On the other hand, certification of an NHI-defined catastrophic illness, such as various types of cancer, can exempt patients from paying additional medical expenses; thus, the diagnosis is reliable and exhaustive.
Carcinogenesis is a time-dependent process, and cancer is most likely to develop in people with chronic infection or inflammation.12–14 Several inflammatory cytokines, such as interleukin 6, interleukin 21, and tumor necrosis factor alpha, have been proven to play important roles in carcinogenesis.15–17 Inflammatory angiogenesis and the microenvironment can also promote tumor growth.18
Malignant pleural effusion might be the first presentation of certain cancers, especially for lung, breast, and ovarian cancers.19–21 However, malignant pleural effusion might be misclassified as empyema. Furthermore, patients with empyema might undergo more investigations such as imaging studies, which might lead to cancer being detected incidentally. Therefore, we excluded the first-year follow-up to avoid misclassification and surveillance bias. The cancer risk of patients with empyema was significantly increased whether first-year follow-up was included or excluded.
By analyzing and comparing the results of including versus excluding events in the first year of follow-up, we could find a sharp decline of SIR in cancers of the esophagus (including within 1-year event, SIR 7.43, 95% CI 6.43–8.54 vs excluding within 1-year event, SIR 2.56, 95% CI 1.92–3.33) and lung/mediastinum (including within 1-year event, SIR 6.63, 95% CI 6.22–7.06 vs excluding within 1-year event, SIR 1.62, 95% CI 1.39–1.86). The empyema may have been the presenting complication of the cancer. This phenomenon was supported in several studies.22–24
In the empyema cohort, age ≥ 60, male sex, having diabetes mellitus, and having liver cirrhosis were significant predictors of cancer development. Aging-related alteration in DNA methylation, histone modifications, chromatin structure, and epigenetic regulation contribute to tumor susceptibility and tumorigenesis.25 Men might be more prone than women to unhealthy behaviors such as high-fat diets, physical inactivity, tobacco use, and alcohol consumption.26,27 Hormonal stimuli might also play a role in the sex difference.28 The potential mechanisms linking diabetes mellitus to tumor growth consist of oxidative stress, hyperinsulinemia, insulin resistance, chronic inflammation, and hyperglycemia.29 Cirrhosis-associated immune dysfunction leads to an increased susceptibility to bacterial infection, immunodeficiency, and systemic inflammation.30
This study has several limitations. First, we excluded patients who had antecedent cancer before empyema diagnosis. Although the exclusion of these patients enabled us to clarify the relationship between cancer development and newly diagnosed empyema, it was difficult to clarify whether empyema is a kind of initial manifestation of cancer. However, the increased cancer risk remains significant after excluding the first-year follow-up. Second, this study has inherent limitations in the use of administrative data that did not include smoking status, body mass index, alcohol consumption, socioeconomic status, performance status, severity of empyema, or laboratory data, including biochemistry and culture results and viral hepatitis markers. We thus performed analysis focusing on two different outcomes (i.e., hepatocellular carcinoma and nonliver cancers) in the analysis of risk factors for secondary malignancy. The multivariate analysis showed that liver cirrhosis remained as a significant predictor of both hepatocellular carcinoma and nonliver cancers (Supplemental Table 2A and B). Third, this study defined cancer occurrence as enrollment in the Registry of Catastrophic Illness Patients. Some critically ill patients with empyema might not have a chance to get pathological proof. Therefore, this may have caused an underestimation of cancer risk in empyema patients, resulting in statistical movement toward the null. Finally, because of limited information on surgical intervention, drug effects, and drug sensitivity, we were unable to assess the association between empyema management and further cancer development.
CONCLUSIONS
Patients with empyema may have greater risk of cancers, especially neoplasms of the head and neck, esophagus, stomach, liver and biliary tract, and lung and mediastinum. The association between such infection and secondary malignancy may be elucidated by further study involving patient characteristics, clinical course and microbiology information, and controlling for confounders such as smoking, alcoholism, and obesity.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Dataset, NHRI = National Health Research Institutes, SIR = standardized incidence ratio.
Study concept and design: C-JT, C-JL, and T-JL; acquisition of data: C-MY and C-JL; statistical analysis and interpretation of data: Y-WH, C-MY, and C-JT; drafting of manuscript: C-JT and Y-CL; critical revision: Y-WH, C-MY, and T-JC; and study supervision: T-JC and Y-CL.
This study is supported by research grants of Far Eastern Memorial Hospital (FEMH-2015-C-0312), Taipei Veterans General Hospital (V105B-016 and V105E10-002-MY2-1), the Ministry of Science and Technology (MOST 104-2314-B-075-085-MY2), Taiwan Clinical Oncology Research Foundation, Szu-Yuan Research Foundation of Internal Medicine, and Chong Hin Loon Memorial Cancer and Biotherapy Research Center, at National Yang-Ming University.
The study is based in part on data from the National Health Insurance Research Database, provided by the Bureau of National Health Insurance, Department of Health, and managed by NHRI. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996; 89:285–289. [DOI] [PubMed] [Google Scholar]
- 2.Burgos J, Falco V, Pahissa A. The increasing incidence of empyema. Curr Opin Pulm Med 2013; 19:350–356. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YS. Pleural infection and empyema. Tuberc Respir Dis (Seoul) 2014; 76:160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006; 174:817–823. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed RA, Marrie TJ, Huang JQ. Thoracic empyema in patients with community-acquired pneumonia. Am J Med 2006; 119:877–883. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PC, Hu YW, Hung MH, et al. The risk of cancer in patients with benign anal lesions: a nationwide population-based study. Am J Med 2013; 126:1143.e1149–e1118. [DOI] [PubMed] [Google Scholar]
- 8.Hung YP, Teng CJ, Liu CJ, et al. Cancer risk among patients with coal workers’ pneumoconiosis in Taiwan: a nationwide population-based study. Int J Cancer 2014; 134:2910–2916. [DOI] [PubMed] [Google Scholar]
- 9.Kuo SC, Hu YW, Liu CJ, et al. Association between tuberculosis infections and non-pulmonary malignancies: a nationwide population-based study. Br J Cancer 2013; 109:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012; 308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 11.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA 2011; 305:2462–2463. [DOI] [PubMed] [Google Scholar]
- 12.Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol 2012; 3:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAlpine JN, Lisonkova S, Joseph KS, et al. Pelvic inflammation and the pathogenesis of ovarian cancer: a cohort study. Int J Gynecol Cancer 2014; 24:1406–1413. [DOI] [PubMed] [Google Scholar]
- 14.Norgaard M, Thomsen RW, Farkas DK, et al. Candida infection and cancer risk: a Danish nationwide cohort study. Eur J Intern Med 2013; 24:451–455. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo A, Pallone F, Monteleone G, et al. Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol 2011; 17:3092–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Pfeiffer RM, Hildesheim A, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013; 105:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolfi C, Rizzo A, Franze E, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med 2011; 208:2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno A, Pagani A, Magnani E, et al. Inflammatory angiogenesis and the tumor microenvironment as targets for cancer therapy and prevention. Cancer Treat Res 2014; 159:401–426. [DOI] [PubMed] [Google Scholar]
- 19.Santos GT, Prolla JC, Camillo ND, et al. Clinical and pathological factors influencing the survival of breast cancer patients with malignant pleural effusion. J Bras Pneumol 2012; 38:487–493. [DOI] [PubMed] [Google Scholar]
- 20.Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 2012; 7:1485–1489. [DOI] [PubMed] [Google Scholar]
- 21.Kim KW, Choi HJ, Kang S, et al. The utility of multi-detector computed tomography in the diagnosis of malignant pleural effusion in the patients with ovarian cancer. Eur J Radiol 2010; 75:230–235. [DOI] [PubMed] [Google Scholar]
- 22.Froeschle P, Wanke W, Granetzny A. Video-thoracoscopy and staged management of preoperative empyema in lung cancer. Thorac Cardiovasc Surg 2005; 53:188–190. [DOI] [PubMed] [Google Scholar]
- 23.Subotic D, Petrov D, Gajic M. Lung resection for lung cancer after pleural empyema. Thorac Cardiovasc Surg 2013; 61:612–618. [DOI] [PubMed] [Google Scholar]
- 24.Tsai YM, Lin YC, Huang TW, et al. Metastatic lung cancer presenting as thoracic empyema in an old patient. West Indian Med J 2014; 63:514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zane L, Sharma V, Misteli T. Common features of chromatin in aging and cancer: cause or coincidence? Trends Cell Biol 2014; 24:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buffart LM, Singh AS, van Loon EC, et al. Physical activity and the risk of developing lung cancer among smokers: a meta-analysis. J Sci Med Sport 2014; 17:67–71. [DOI] [PubMed] [Google Scholar]
- 27.Boniol M, Autier P. Prevalence of main cancer lifestyle risk factors in Europe in 2000. Eur J Cancer 2010; 46:2534–2544. [DOI] [PubMed] [Google Scholar]
- 28.Kligerman S, White C. Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am J Roentgenol 2011; 196:287–295. [DOI] [PubMed] [Google Scholar]
- 29.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J 2014; 38:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014; 61:1385–1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


