Abstract
Uremic pruritus is common and bothersome in patients receiving either peritoneal dialysis (PD) or hemodialysis (HD). To date, the preferred dialysis modality regarding the alleviation of uremic pruritus remains controversial. We conducted this cross-sectional study to compare the prevalence, intensity, and characteristics of uremic pruritus between PD and HD patients.
Patients receiving maintenance dialysis at a referral medical center in Taiwan were recruited. Dialysis modality, patient demographic, clinical characteristics, and laboratory data were recorded. The intensity of uremic pruritus was measured using visual analogue scale (VAS) scores. Multivariate linear regression analysis was conducted to compare the severity of uremic pruritus between PD and HD patients. Generalized additive models were applied to detect nonlinear effects between pruritus intensity and continuous covariates.
A total of 380 patients completed this study, with a mean age of 60.3 years and 49.2% being female. Uremic pruritus was presented in 24 (28.6%) of the 84 PD patients and 113 (38.2%) of the 296 HD patients (P = .12). The VAS score of pruritus intensity was significantly lower among the PD patients than the HD patients (1.32 ± 2.46 vs 2.26 ± 3.30, P = .04). Multivariate linear regression analysis showed that PD was an independent predictor for lower VAS scores of pruritus intensity compared with HD (β-value −0.88, 95% confidence interval −1.62 to −0.13). The use of active vitamin D was also an independent predictor for a lower intensity of uremic pruritus, whereas hyperphosphatemia and higher serum levels of triglyceride and aspartate transaminase were significantly associated with higher pruritus intensity. There was a trend toward a less affected body surface area of uremic pruritus in the PD patients than in the HD patients, but the difference did not reach statistical significance (P = .13).
In conclusion, the severity of uremic pruritus was lower among PD patients than HD patients, and PD may provide better alleviation of pruritus symptoms. The result provides a valuable reference for clinicians and patients when choosing a dialysis modality.
INTRODUCTION
Uremic pruritus is a common and bothersome symptom in patients with end-stage renal disease (ESRD).1,2 Uremic pruritus considerably influences the quality of life, causing sleep disturbance, mood change, and uncontrollable scratching.3,4 Moreover, severe uremic pruritus is associated with an increased risk of mortality in dialysis patients.5,6 Many factors have been implicated in the pathophysiology of uremic pruritus, such as xerosis, divalent ions, calcium-phosphate product, interleukin (IL)-31, and hyperparathyroidism.7–10 Besides, we and others found that the adequacy of dialysis and the clearance of pruritogenic substances could influence the severity of pruritus.11–13
Peritoneal dialysis (PD) and hemodialysis (HD) are the 2 major renal replacement therapies for patients with ESRD. Many studies have reported a substantial prevalence of uremic pruritus among dialysis patients, ranging from 10% to 70% in PD patients, and 20% to 90% in HD patients.2,14–16 Patients with PD and HD differ in terms of demographics, clinical and biochemical conditions, all of which may influence the severity of uremic pruritus. However, few studies have directly compared the characteristics of uremic pruritus between patients with PD and HD, and their results are controversial.15–20 Some studies reported similar prevalence of uremic pruritus among patients receiving PD and HD,16–20 whereas other study found differences in favor of PD.15 Furthermore, the current standard for dialysis adequacy of solute clearance has not been considered in these studies.
To address this issue, we performed this study to evaluate differences in the prevalence, intensity, and body surface area involvement of uremic pruritus among PD and HD patients.
METHODS
Study Participants
The study population included patients with ESRD who were undergoing maintenance dialysis at Far Eastern Memorial Hospital, a tertiary medical center in Taiwan. The eligible participants were those aged 20 years or older. Patients were excluded if they had any of the following conditions: active infection; psychotic illness or other communication problems; primary skin disorders; cholestatic liver disease or acute hepatitis; active malignancy; change in dialysis modality within the last 3 months before the study; and patient refusal. In total, 98 patients were receiving maintenance PD and 420 patients were receiving maintenance HD at Far Eastern Memorial Hospital in April 2013. After excluding 138 patients, a total of 84 (86%) PD patients and 296 HD patients (70%) were included in this study.
Ethics
The Institutional Review Board of Far Eastern Memorial Hospital, New Taipei City, Taiwan, approved this study, and all participants provided written informed consent.
Pruritus Assessment
The patients were considered to have uremic pruritus if they had the following: at least 3 episodes of pruritus during a period of ≤2 weeks, with the symptoms occurring a few times a day, lasting at least a few minutes, and troubling the patient; or the regular occurrence of pruritus during a period of 6 months, but less frequently than listed above.16,21 A visual analogue scale (VAS) score measuring the general severity of pruritus was reported from 0 to 10 (0 = no itching, 10 = worst imaginable itching).22 The body distribution of pruritus was also assessed and categorized as a percentage of the affected body surface area as: <25%; ≥25% but <50%; ≥50% but <75%; or ≥75%.1
Patient Characteristics and Laboratory Parameters
Baseline data including sex, age, body mass index, comorbid diseases, etiology of ESRD, PD, and HD regimens, as well as the duration of dialysis therapy, were recorded for each participant. Venous blood was sampled after an overnight fast exceeding 8 hours. All laboratory tests were performed by the hospital's central laboratory, with an auto-analyzer used to determine biochemical data. Patients with a positive hepatitis B virus surface antigen test result were considered to be hepatitis B carriers, and those with a positive hepatitis C virus antibody test result were considered to have an infection caused by hepatitis C virus.
Dialysis Adequacy of Solute Clearance
The dialysis adequacy of solute clearance was assessed by the Kt/V parameter (amount of dialysis delivered: K = clearance of urea, t = time on dialysis, V = estimated total body water) based on urea kinetic modeling. The PD regimen for each patient was evaluated and prescribed during monthly follow-up. The adequacy of PD was assessed by weekly total Kt/V (the sum of peritoneal Kt/V and renal Kt/V) and should be at least 1.7 or above.23 The HD patients received 3.5 to 5 hours of HD therapy 3 times a week using bicarbonate dialysate and reverse osmosis purified water. In 91.6% of the HD participants, a high-flux polysulfone membrane was used as the dialyzer, whereas the remaining 8.4% used a low-flux synthetic membrane dialyzer. The adequacy of HD was assessed by single-pool Kt/V with a target dose of 1.4 or higher.24 For comparability between PD and HD patients, we considered the achievement of the target Kt/V as a covariate during the process of variable selection and model fitting.
Statistical Analysis
Statistical analysis was performed using R 2.14.1 software (R Foundation for Statistical Computing, Vienna, Austria). A 2-sided P value ≤0.05 was considered to be statistically significant. Data are expressed as mean ± standard deviation for normally distributed continuous variables, as median (1st quartile, 3rd quartile) for non-normally distributed continuous variables, and as number (percentage) for categorical variables. For descriptive analysis, univariate analyses were conducted using the independent 2-sample t test, Wilcoxon rank-sum test, and Pearson χ2 test, respectively.
Multivariate linear regression analysis was conducted to identify the predictive factors for the VAS score of uremic pruritus. To ensure the quality of the results, basic model-fitting techniques for variable selection, goodness-of-fit assessment, and regression diagnostics were used in our regression analyses. Generalized additive models were applied to detect nonlinear effects of continuous variables during the stepwise variable selection procedure of the regression analysis.25 Statistical tools for regression diagnostics including residual analysis, detection of influential cases, and to check for multicollinearity were used to reveal problems with the model or data.
RESULTS
Patient Characteristics
A total of 380 participants, including 84 PD patients and 296 HD patients completed this study. The basic characteristics of the participants are summarized in Tables 1 and 2. The study participants had a mean age of 60.3 years, with 49.2% being female and 46.1% having diabetes. The prevalence of uremic pruritus in the whole study population was 36.1%. Comparisons of the clinical and laboratory data between the PD and HD patients are listed in Table 2. More PD patients achieved the target dose for dialysis adequacy of solute clearance than the HD patients (88.1% vs 71.3%, P = .002). Compared with the HD patients, the PD patients had a significantly younger age, shorter duration of dialysis therapy, lower prevalence of diabetes, higher female percentage, and higher body mass index. In addition, the PD patients had significantly lower serum levels of hematocrit, uric acid, albumin, total bilirubin, ferritin, and high-sensitivity C-reactive protein (CRP), as well as significantly higher serum levels of creatinine, calcium, and total cholesterol.
TABLE 1.
Demographic and Clinical Characteristics of the Study Participants

TABLE 2.
Demographic and Clinical Characteristics of the Patients Receiving Peritoneal Dialysis and Hemodialysis

Association Between Dialysis Modality and Uremic pruritus
The distributions of the severity of uremic pruritus in the PD and HD patients are shown in Figure 1 and Table 3. The prevalence of uremic pruritus was 28.6% in the PD patients and 38.2% in the HD patients (P = .12). The VAS score was significantly lower in the PD patients than in the HD patients (mean ± standard deviation [1.32 ± 2.46 vs 2.26 ± 3.30], P = .04). There might be a trend toward a less affected body surface area of uremic pruritus in the PD patients than in the HD patients; however, the difference did not reach statistical significance (P = .13) (Table 3).
FIGURE 1.
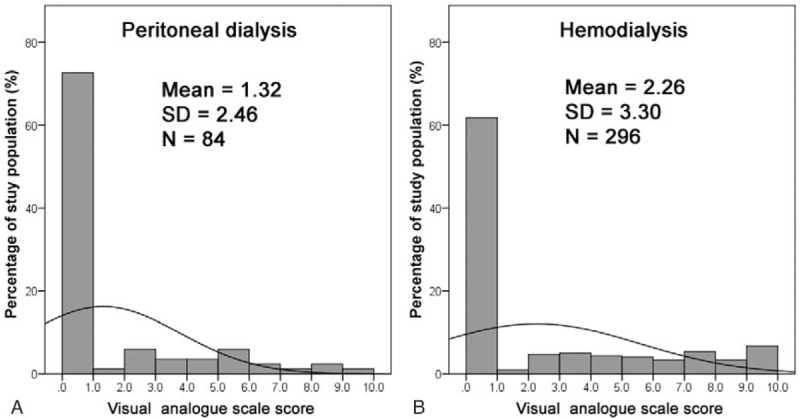
Frequency distribution of visual analogue scale score of pruritus intensity in the study population. Frequency distribution in the study participants receiving (A) peritoneal dialysis and (B) hemodialysis.
TABLE 3.
The Percentage of Body Surface Area Affected by Uremic Pruritus

Table 4 shows the independent predictors for the VAS score of uremic pruritus, after adjusting important covariates in the multivariate linear regression analysis, with the assistance of generalized additive models. Compared with HD, the result showed that PD was independently associated with a nearly one unit lower VAS score (β-value–0.88, 95% confidence interval –1.62 to –0.13, P = .02). The use of active vitamin D was also an independent predictor for a lower intensity of uremic pruritus, whereas hyperphosphatemia and higher serum levels of triglyceride and aspartate transaminase were significantly associated with higher pruritus intensity. In addition, older age showed a borderline significance for higher pruritus intensity.
TABLE 4.
Multivariate Linear Regression Analysis of the Predictors for Visual Analog Scale Scores of Pruritus Intensity

DISCUSSION
To the best of our knowledge, this is the first study comparing the characteristics of uremic pruritus between PD and HD patients taking the current standards of dialysis therapy and related parameters into consideration. We found that PD patients had a significantly lower intensity of uremic pruritus than HD patients. In addition, there may have been a trend toward a lower prevalence and less affected body surface area of uremic pruritus in the PD patients.
One of the possible explanations for the lower severity of uremic pruritus among the PD patients is the better preservation of residual renal function through PD therapy.26–28 The residual renal function has been reported to be better preserved in PD patients, with a 65% lower risk of losing residual renal function compared PD with HD among incident dialysis patients.26,27 The previous studies by us and other groups have indicated an inverse relationship between the severity of uremic pruritus and the clearance of small and middle sized molecules in HD patients, and a variety of uremic toxins have been proposed to be pruritogenic substances.5,11 Abnormalities of calcium, phosphorus, parathyroid hormone, and vitamin D metabolism have all been reported to be associated with the severity of uremic pruritus.5,10 A greater residual renal function is associated with better clearance of middle-sized uremic toxins, better preservation of erythropoietin productivity, and better homeostasis of calcium, phosphorus, and vitamin D, all of which may reduce the severity of uremic pruritus.29–31
Another proposed mechanism for the milder uremic pruritus in PD patients is less inflammation compared with HD patients. Our previous studies have shown that inflammation plays an important role in uremic pruritus,6,32 and various inflammatory factors including IL-31, IL-2, IL-6, and histamine have been reported to be associated with uremic pruritus.10,19,33,34 In patients treated with PD, the induction of inflammation, such as the production of IL-6 and CRP, has been found to be lower than in patients undergoing HD.35,36 In this study, high-sensitivity CRP, which is a good surrogate of inflammation in dialysis patients,6,37 was significantly lower in PD patients than in HD patients. However, the multivariate linear regression analysis did not demonstrate a significant role of high-sensitivity CRP. The dysregulation of the immune system in PD and HD patients continues to be an interesting topic and deserves further investigation.
We also found that age, use of active vitamin D, serum level of phosphate, aspartate transaminase, and triglyceride are associated with the severity of uremic pruritus. Aging results in the alternation of skin structure and decline of skin function that could contribute to higher pruritus intensity.38 The importance of hemostasis among vitamin D metabolism, calcium, phosphorus, and parathyroid hormone in uremic pruritus has also been reported.5,10,39 Fatty liver, chronic active or persistent hepatitis, alcohol consumption, obesity, hypercholesterolemia, and hyperglycemia have all been reported to cause hepatic dysfunction and increased serum transaminase concentration.40,41 We postulated that this hepatic dysfunction may lead to the association between elevated serum aspartate transaminase and uremic pruritus. As triglyceride is a risk factor for neuropathy, which is a trigger factor for uremic pruritus, the association between hypertriglyceridemia and uremic pruritus might be resulted from uremic neuropathy.42,43
As diabetes has been shown to be associated with pruritus, we have considered diabetes in the process of multivariate linear regression analysis.44 During the process of variable selection and model fitting, no significant role was found for diabetes in the comparison of uremic pruritus between PD and HD patients. Several studies have reported that dialysis adequacy is an independent predictor of pruritus intensity in uremic patients.11–13 However, the achievement of the target Kt/V did not show statistically significance during variable selection of multivariate linear regression analyses. A possible reason is that most of the study participants have achieved the target dose of Kt/V. Moreover, as PD and HD patients used different standards and calculation process, Kt/V could not be analyzed as a continuous variable, which results in loss of statistical power for dialysis adequacy in the linear regression.
In this study, fewer patients received PD (22.1%) than HD (77.9%). This trend is consistent with other dialysis facilities in Taiwan and in many other countries.45,46 The selection of an appropriate dialysis modality is influenced by medical and social factors, patient preference, clinical needs, and resource availability, as well as a possible influence of bias from health care professionals.47,48 As shown in Table 2, these factors resulted in different individual characteristics between the PD and HD patients. Considering potential selection bias in this study, we analyzed our data using multivariate linear regression to balance the important covariates among the 2 dialysis groups.
There are several limitations to this study. First, we did not collect data regarding uremic pruritus before the participants started dialysis therapy. As this is a cross-sectional study, we could not establish causality and temporality between dialysis modality and uremic pruritus. Second, this study is observational in nature. Although we have controlled for most of the important variables, the influence of residual confounding could still remain. Third, information regarding residual renal function was not routinely collected in the HD patients because most HD patients lose their residual renal function within 1 year after commencing HD,27 and assessments of HD solute clearance do not require residual renal function.24 We therefore could not further explore the influence of residual renal function on uremic pruritus. Instead, we assessed whether the participants achieved the target Kt/V suggested by current guidelines as a representative variable when fitting the model. Finally, as the participants were enrolled from a single medical center in Taiwan, the generalizability of the study results may be limited. Nevertheless, the characteristics of the study participants were typical for PD and HD patients in Taiwan, and should reflect the actual condition of dialysis patients. The generalizability of our findings to dialysis patients in other countries or ethnicities may need to be clarified with additional studies.
In conclusion, our results show that the severity of uremic pruritus is lower in PD patients than in HD patients, with a trend of a lower prevalence and less affected body surface area of uremic pruritus in patients receiving PD. Therefore, PD may provide better alleviation of pruritus symptoms. The results of this study provide a valuable reference for clinicians and patients when choosing a dialysis modality.
Acknowledgements
The authors gratefully thank Dr Fu-Chang Hu for his valuable advice on statistical analysis.
Footnotes
Abbreviations: CRP = C-reactive protein, ESRD = end-stage renal disease, HD = hemodialysis, IL = interleukin, PD = peritoneal dialysis, VAS = visual analogue scale.
This study was supported by research grants to Dr. Mei-Ju Ko from Department of Health, Taipei City Government, Taipei, Taiwan (10301–62–030) and Taipei City Hospital, Ren-Ai Branch, Taipei, Taiwan (No.15, 2015); and research grants to Dr. Hon-Yen Wu from the Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH 103–2314-B-418–003-MY2, FEMH-2015-C-030, FEMH 2016-C-027). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ko MJ, Yang JY, Wu HY, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. Br J Dermatol 2011; 165:633–639. [DOI] [PubMed] [Google Scholar]
- 2.Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5:1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yosipovitch G, Zucker I, Boner G, et al. A questionnaire for the assessment of pruritus: validation in uremic patients. Acta Derm Venereol 2001; 81:108–111. [DOI] [PubMed] [Google Scholar]
- 4.Pisoni RL, Wikstrom B, Elder SJ, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21:3495–3505. [DOI] [PubMed] [Google Scholar]
- 5.Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int 2006; 69:1626–1632. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Chiu YL, Hsu SP, et al. Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM 2010; 103:837–846. [DOI] [PubMed] [Google Scholar]
- 7.Morton CA, Lafferty M, Hau C, et al. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant 1996; 11:2031–2036. [DOI] [PubMed] [Google Scholar]
- 8.Massry SG, Popovtzer MM, Coburn JW, et al. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N Engl J Med 1968; 279:697–700. [DOI] [PubMed] [Google Scholar]
- 9.Graf H, Kovarik J, Stummvoll HK, et al. Disappearance of uraemic pruritus after lowering dialysate magnesium concentration. Br Med J 1979; 2:1478–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko MJ, Peng YS, Chen HY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol 2014; 71:1151–1159.e1151. [DOI] [PubMed] [Google Scholar]
- 11.Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One 2013; 8:e71404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duque MI, Thevarajah S, Chan YH, et al. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol 2006; 66:184–191. [DOI] [PubMed] [Google Scholar]
- 13.Malekmakan L, Malekmakan A, Sayadi M, et al. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J Kidney Dis Transpl 2015; 26:890–895. [DOI] [PubMed] [Google Scholar]
- 14.Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol 2000; 11:514–519. [DOI] [PubMed] [Google Scholar]
- 15.Bencini PL, Montagnino G, Citterio A, et al. Cutaneous abnormalities in uremic patients. Nephron 1985; 40:316–321. [DOI] [PubMed] [Google Scholar]
- 16.Mistik S, Utas S, Ferahbas A, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol 2006; 20:672–678. [DOI] [PubMed] [Google Scholar]
- 17.Mettang T, Fritz P, Weber J, et al. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clin Nephrol 1990; 34:136–141. [PubMed] [Google Scholar]
- 18.Balaskas EV, Chu M, Uldall RP, et al. Pruritus in continuous ambulatory peritoneal dialysis and hemodialysis patients. Perit Dial Int 1993; 13 Suppl 2:S527–532. [PubMed] [Google Scholar]
- 19.Balaskas EV, Bamihas GI, Karamouzis M, et al. Histamine and serotonin in uremic pruritus: effect of ondansetron in CAPD-pruritic patients. Nephron 1998; 78:395–402. [DOI] [PubMed] [Google Scholar]
- 20.Snit M, Gawlik R, Lacka-Gazdzik B, et al. Substance P and intensity of pruritus in hemodialysis and peritoneal dialysis patients. Med Sci Monit 2013; 19:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker I, Yosipovitch G, David M, et al. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol 2003; 49:842–846. [DOI] [PubMed] [Google Scholar]
- 22.Furue M, Ebata T, Ikoma A, et al. Verbalizing Extremes of the Visual Analogue Scale for Pruritus: A Consensus Statement. Acta Derm Venereol 2013; 93:214–215. [DOI] [PubMed] [Google Scholar]
- 23.Peritoneal Dialysis Adequacy Work Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 2006; 48 Suppl 1:S98–S129. [DOI] [PubMed] [Google Scholar]
- 24.Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update, 2006. Am J Kidney Dis 2006; 48 Suppl 1:S2–S90. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti A, Abrahamowicz M, Leffondré K, et al. Using generalized additive models to detect and estimate threshold associations. Int J Biostat 2009; 5: Article 26. [Google Scholar]
- 26.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62:1046–1053. [DOI] [PubMed] [Google Scholar]
- 27.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556–564. [DOI] [PubMed] [Google Scholar]
- 28.Wu HY, Hung KY, Hu FC, et al. Risk factors for high dialysate glucose use in PD patients–a retrospective 5-year cohort study. Perit Dial Int 2010; 30:448–455. [DOI] [PubMed] [Google Scholar]
- 29.Mamoun AH, Sodersten P, Anderstam B, et al. Evidence of splanchnic-brain signaling in inhibition of ingestive behavior by middle molecules. J Am Soc Nephrol 1999; 10:309–314. [DOI] [PubMed] [Google Scholar]
- 30.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–2162. [DOI] [PubMed] [Google Scholar]
- 31.Wu HY, Hung KY, Huang TM, et al. Safety issues of long-term glucose load in patients on peritoneal dialysis–a 7-year cohort study. PLoS One 2012; 7:e30337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu YL, Chen HY, Chuang YF, et al. Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrol Dial Transplant 2008; 23:3685–3689. [DOI] [PubMed] [Google Scholar]
- 33.Fallahzadeh MK, Roozbeh J, Geramizadeh B, et al. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant 2011; 26:3338–3344. [DOI] [PubMed] [Google Scholar]
- 34.Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 2006; 21:749–755. [DOI] [PubMed] [Google Scholar]
- 35.Haubitz M, Brunkhorst R, Wrenger E, et al. Chronic induction of C-reactive protein by hemodialysis, but not by peritoneal dialysis therapy. Perit Dial Int 1996; 16:158–162. [PubMed] [Google Scholar]
- 36.Takahashi T, Kubota M, Nakamura T, et al. Interleukin-6 gene expression in peripheral blood mononuclear cells from patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Ren Fail 2000; 22:345–354. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–843. [DOI] [PubMed] [Google Scholar]
- 38.Rittie L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med 2015; 5:a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noordzij M, Boeschoten EW, Bos WJ, et al. Disturbed mineral metabolism is associated with muscle and skin complaints in a prospective cohort of dialysis patients. Nephrol Dial Transplant 2007; 22:2944–2949. [DOI] [PubMed] [Google Scholar]
- 40.Hultcrantz R, Glaumann H, Lindberg G, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol 1986; 21:109–113. [DOI] [PubMed] [Google Scholar]
- 41.Kim WR, Flamm SL, Di Bisceglie AM, et al. Public Policy Committee of the American Association for the Study of Liver D. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008; 47:1363–1370. [DOI] [PubMed] [Google Scholar]
- 42.Yang CP, Lin CC, Li CI, et al. Cardiovascular Risk Factors Increase the Risks of Diabetic Peripheral Neuropathy in Patients With Type 2 Diabetes Mellitus: The Taiwan Diabetes Study. Medicine (Baltimore) 2015; 94:e1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin 2001; 31:181–193. [DOI] [PubMed] [Google Scholar]
- 44.Ko MJ, Chiu HC, Jee SH, et al. Postprandial blood glucose is associated with generalized pruritus in patients with type 2 diabetes. Eur J Dermatol 2013; 23:688–693. [DOI] [PubMed] [Google Scholar]
- 45.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2015; 66:S1–S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lameire N, Van Biesen W. Epidemiology of peritoneal dialysis: a story of believers and nonbelievers. Nat Rev Nephrol 2010; 6:75–82. [DOI] [PubMed] [Google Scholar]
- 48.Griva K, Li ZH, Lai AY, et al. Perspectives of patients, families, and health care professionals on decision-making about dialysis modality–the good, the bad, and the misunderstandings!. Perit Dial Int 2013; 33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]


