Abstract
Acinetobacter (A.) baumannii, an opportunistic nosocomial pathogen that can cause significant morbidity and mortality, has emerged as a worldwide problem. This study aimed to analyze the clinical features and outcomes of patients with A. baumannii bacteremia and determine the factors influencing survival by using 14-day mortality as the primary endpoint.
A 6-year retrospective study of 122 cases with monomicrobial A. baumannii bacteremia was conducted in Chinese People's Liberation Army (PLA) General Hospital from January 2008 to April 2014. Predictors of 14-day mortality were identified by logistic regression analysis.
The overall 14-day mortality rate was 40.2% (49 of 122 patients). Multivariable analysis revealed that independent predictors of 14-day mortality included severity of illness defined by Pitt Bacteremia Score (PBS) (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.340–0.619; P < 0.001), neutropenia (OR, 18.02; 95% CI, 1.667–194.67; P = 0.017), and malignancy (OR, 4.63; 95% CI, 1.292–16.588; P = 0.019). The effect of malignancy was influenced by neutropenia (OR for interaction term, 1.60; 95% CI, 1.15–2.22; P = 0.005). A subgroup analysis revealed that 14-day mortality rate for patients with underlying hematological malignancies and solid tumors was 75% (12/16) and 40% (12/30), respectively. Survival analysis revealed that mortality in patients with hematological malignancies was higher than that in patients with solid tumors (P = 0.032).
The outcomes of patients with A. baumannii bacteremia were related to PBS, neutropenia, and malignancy. Compared with solid tumors, patients with hematological malignancies had a higher mortality in the setting of A. baumannii bacteremia.
INTRODUCTION
Acinetobacter (A.) baumannii is one of the most common, gram-negative pathogens causing bacteremia.1 Bacteremia is an important and prevalent cause of patient mortality and the overall mortality in patients with A. baumannii bacteremia ranges widely from 29% to 63%.1–3 Furthermore, due to increasing exposure to antibiotics, multidrug resistance (MDR) and carbapenem resistance rates have been predictably increasing these years.4,5 With limited treatment options, infections caused by multidrug resistant and carbapenem resistant A. baumannii might result in higher mortality.
Several studies have investigated predictors of mortality in patients with A. baumannii bacteremia. Risk factors independently associated with mortality include drug resistance, severity of illness, appropriate antimicrobial therapy, malignancy, and other comorbidities such as immunosuppression.6–9 However, previous studies suffered from different limitations which make it difficult to draw definitive conclusions, such as failure to distinguish between A. baumannii colonization and infection, inappropriate clinical endpoints, failure to adjust for confounders such as severity of illness and other comorbid conditions.
To further understand risk factors influencing survival in patients with A. baumannii bacteremia, this retrospective study was conducted to analyze the clinical features and outcomes of patients with A. baumannii bacteremia and determine factors influencing survival by using 14-day mortality as the primary endpoint.
METHODS
Study Design and Population
A retrospective study was conducted to investigate risk factors influencing survival in adult patients (≥18 years old) with A. baumannii bacteremia consecutively admitted to the Chinese People's Liberation Army (PLA) General Hospital, a 3000-bed, tertiary care teaching hospital in Beijing, China, from January 2008 to April 2014. Charts were reviewed for all patients with ≥1 A. baumannii bacteremic episodes who had symptoms and signs of infection. For patients with ≥2 bacteremic episodes, only the first episode was included. Patients with polymicrobial bacteremia and those with incomplete medical records were excluded. Patient records/information was anonymized and deidentified before analysis. This study was reviewed and approved by the Medical Ethics Committee of PLA General Hospital.
Organism Identification and Susceptibility Classification
Blood specimens drawn at the bedside under sterile conditions were processed in an automated blood culture machine. Identification of the isolates to the level of the A. baumannii complex and antimicrobial susceptibility tests were completed using a Vitek II system (bioMerieux, Marcy-lEtoile, France). Antimicrobial susceptibility was performed by disk diffusion method and results were interpreted according to Clinical Laboratory Standards Institute criteria. Intermediate resistance was regarded as resistance in our study. MDR was defined as resistance to ≥3 of the following classes of antimicrobials: antipseudomonal cephalosporins, antipseudomonal carbapenems, ampicillin-sulbactam, fluoroquinolones, and aminoglycosides.10 Carbapenem resistance was defined as resistance to imipenem and meropenem.
Data Collection
Demographic characteristics of patients included age, gender, dates of admission and discharge, duration of hospital stay before development of bacteremia. We also collected patient medical comorbidities (diabetes, hepatic dysfunction, renal dysfunction, underlying malignancy, coronary arterial disease, congestive heart failure, and chronic obstructive pulmonary disease), recent surgery (performed within 4 weeks before the onset of bacteremia), history of immunosuppression (ie, corticosteroids or chemotherapy within the previous 6 months or HIV-positive status), absolute neutrophil count, severity of illness defined by the Pitt Bacteremia Score (PBS) within 24 hours before bacteremia onset, the presence of a ventilator, central venous catheters, a nasogastric tube, or a Foley catheter at the time of bacteremia onset, antimicrobial susceptibility, time of receipt, dose and route of therapy with individual antimicrobial drugs, the sources of bacteremia and mortality. The PBS system was an efficient index to determine the severity of sepsis in critically ill patients, to compare patient outcomes, and more importantly, to predict clinical outcomes and guide physicians in the management of patients.
Definitions
The onset of bacteremia was defined at the time when the blood specimens that eventually yielded A. baumannii was drawn. Neutropenia was defined as an absolute neutrophil count <500 cells/mm3. Renal impairment was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. Antimicrobial therapy was defined to be “appropriate” if the antibiotics, which were administered within 48 hours after the onset of bacteremia, included at least 1 antibiotic that was active in vitro and when the dosage, duration, and route of administration were in accordance with current medical standards. Antimicrobial therapy which did not meet this definition was considered “inappropriate.” Source of bacteremia was recorded as documented or indirectly inferred from the infection diagnosis on the day of bacteremia according to the definitions of the Centers for Disease Control and Prevention.11 Given 30 days is too long to interpret the cause of mortality for critically ill patients and 7 days is too short a time to witness a response to treatment,2,3,9 we chose all-cause 14-day mortality after the onset of A. baumannii bacteremia as the primary outcome measure.
Statistical Analysis
Continuous variables were presented by median values and interquartile ranges (IQRs) and calculated with the Student t test or Mann–Whitney U test as appropriate. Categorical variables presented by percentage were calculated with Pearson Chi-square test with Yate correction or Fisher exact test. Logistic regression models were used to explore independent risk factors for 14-day mortality. All biologically plausible variables with a P-value <0.10 in the univariable analysis were entered into a multivariable backward logistic regression analyses to assess their relationship with mortality. The interaction analysis between the PBS and the covariates was chosen using an interaction term. Kaplan–Meier survival analysis were performed to determine the time to mortality, which was defined as the interval between bacteremia onset and death. All the analyses were performed using Statistical Package for the Social Sciences (SPSS) software version 19.0. A P < 0.05 was considered to be statistically significant.
RESULTS
A total of 142 adult patients with ≥1 blood culture positive for the A. baumannii complex were identified during our study period. After excluding 14 patients with incomplete clinical records and 6 patients with polymicrobial bacteremia, 122 patient bacteremic episodes were included in the final analysis. Forty-nine patients (40.2%, 49/122) died within 14 days after the onset of monomicrobial A. baumannii bacteremia. Sixty-nine patients (55.6%, 69/122) were found to acquire their A. baumannii bacteremia in the ICU.
The demographic and clinical characteristics of the 122 patients with A. baumannii bacteremia stratified by 14-day mortality are summarized in Table 1. there weren’t other differences in demographic characteristics and comorbid conditions besides those shown in Table 1. The group of patients who died within 14 days after the onset of bacteremia were significantly more likely to have malignancy, neutropenia, receive immunosuppressive therapy, have higher PBS, and higher rates of nasogastric tubes and ventilator use. The percentage of MDR of all patients was over 84.4%(103/122), and the percentage among the group who died reached up to 98%, which is significantly higher that of the alive group (75.3%). The blood isolates from the group who died also had a significantly greater rate of resistance to carbapenems than the control group (98.0% vs 76.7%, P < 0.001). No significant differences were found in resistance to antipseudomonal cephalosporins, ampicillin-sulbactam, fluoroquinolones, aminoglycosides, and piperacillin-tazobactam between isolates from these 2 groups (data not shown). The group of patients who died within 14 days tended to be less likely to received appropriate antimicrobial therapy than the alive group (4.1% vs 20.5%, P = 0.01). There were also no significant difference in the percentage of using sulbactam containing regimens and the source of bacteremia between these 2 groups.
TABLE 1.
Demographic and Clinical Characteristics of Patients With A. baumannii Bacteremia Stratified by 14-Day Mortality

Logistic regression analysis results are shown in Table 2. Independent predictors of 14-day mortality were revealed to be PBS (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.340–.619; P < 0.001), neutropenia (OR, 18.02; 95% CI, 1.667–194.67; P = 0.017), and malignancy (OR, 4.63; 95% CI, 1.292–16.588; P = 0.019) in multivariable analysis. Whereas the use of appropriate antimicrobial therapy or drug resistance was not found to be associated with the mortality in multivariable analysis. Similar results were also obtained using the multivariate Cox regression model (data not shown).
TABLE 2.
Logistic Regression Analysis of Predictors for 14-Day Mortality Among Patients With A. baumannii Bacteremia

To further investigate the relationship between malignancy and neutropenia, interactions between these 2 variables were added to the logistic regression model. The interaction term was statistically significant (OR for interaction term, 1.60; 95% CI, 1.15–2.22; P = 0.005). Through further investigation we found that if the patients with malignancy were divided into patients with hematological malignancies and patients with solid tumors, almost all the patients with neutropenia (11/13) suffered from hematological malignancies (13 patients with neutropenia: 11 patients with hematological malignancies, 1 patient with solid tumors, and 1 from nontumor patient). To clarify the impact of different underlying tumors on mortality, Kaplan–Meier survival curves were developed for patients with hematological malignancies and patients with solid tumors (P = 0.032, Figure 1). A comparison of the demographic and clinical characteristics between patients with hematological malignancies (16 patients) and patients with solid tumors (30 patients) revealed that patients with hematological malignancies were younger and more likely to have had neutropenia and immunosuppressive therapy, but less likely to have had recent surgical procedures (Table 3). There was no significant difference in sex, PBS, drug resistance, and appropriate antimicrobial therapy (Table 3). Further classification of the 16 patients with hematologic malignancies revealed that this group was composed of 7 patients with lymphoma, 5 patients with acute myeloid leukemia, 3 patients with acute lymphoblastic leukemia, and 1 patient with chronic myeloid leukemia.
FIGURE 1.
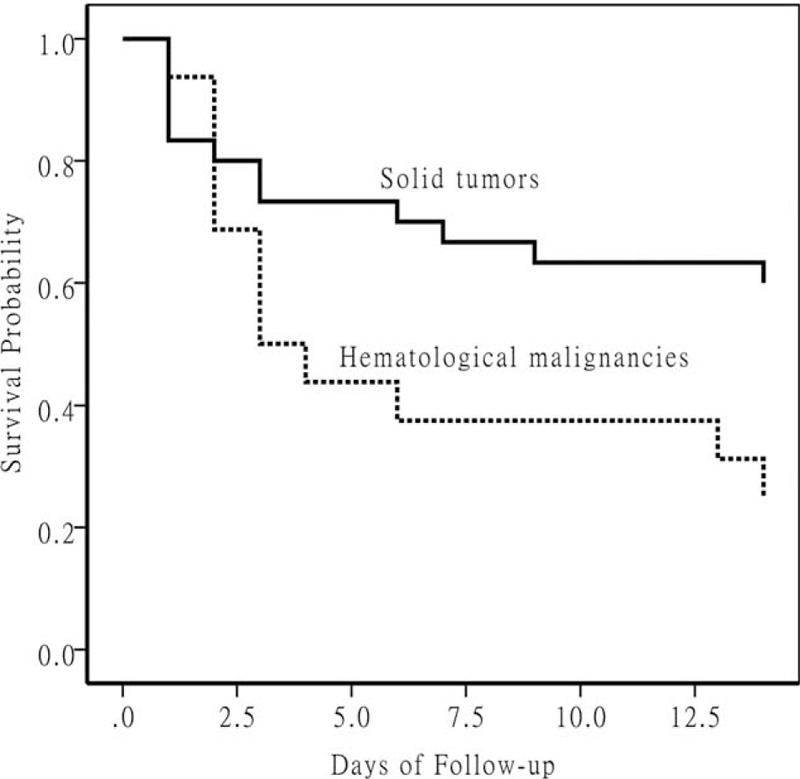
Kaplan–Meier survival curves at 14 days after Acinetobacter baumannii bacteremia onset for patients with hematological malignancies versus patients with solid tumors (P = 0.032).
TABLE 3.
Demographic and Clinical Characteristics of A. baumannii Bacteremia Patients With Different Underlying Tumors: Solid Tumors Versus Hematological Malignancies

DISCUSSION
We attempted to identify risk factors influencing early death in patients with monomicrobial A. baumannii bacteremia through this retrospective study, which consisted of 122 patients admitted to a large teaching hospital in China. Severity of illness defined by PBS, neutropenia, and malignancy were found to be independent factors for 14-day mortality. Whereas the use of appropriate antimicrobial therapy or drug resistance was not found to be associated with the mortality in multivariable analysis. Further analysis also revealed that for patients with A. baumannii bacteremia, patients who suffered hematological malignancies had a higher mortality than that of those who suffered solid tumors.
In our study, the mortality rate is 40.2%, which is comparable to the range of 29% to 63% reported by others.1–3 Thus, crude mortality rate differed significantly between patients in general wards and ICUs, and patients infected by A. baumannii with various drug resistance profiles.12 In this study, a total of 69 patients (55.6%, 69/122) acquired A. baumannii bacteremia in the ICU. And the percentage of MDR and carbapenems resistance was 84.4%(103/122) and 85.2%(105/122), respectively.
Like others, both drug resistance and the use of appropriate antimicrobial therapy were significantly different in bivariable analysis.2,13,14 But neither of these 2 variables reached difference as an independent risk factor for mortality in subsequent multivariable analysis. It is contrary to previous argument about the association of high mortality with antibiotic resistance in patients with A. baumannii bacteremia.3,9 Patients with short life expectancies have been proved to be unable to benefit significantly from appropriate antimicrobial therapy.15 In our study, 55.6% of the patients acquired A. baumannii bacteremia in the ICUs. So these results can at least be partly explained by the fact that our study population might contain many severely ill patients. Nevertheless, the rates of MDR and carbapenems resistance both excessed 84% in our study. And together with the fact that most patients have no access to tigecycline and colistin during our study period, only 13.9% (17/122) of patients received appropriate antimicrobial therapy. These results may be also confounded by the small sample size of this study.
In this study, we also analyzed the effectiveness of sulbactam containing regimens for A. baumannii bacteremia. Unfortunately, it was found to be not significantly associated with decreased mortality both in bivariable analysis and multivariable analysis. This was consistent with the results of a recent systematic review.16 With the increasing rate of drug resistance of A. baumannii, new antimicrobial agents are urgently needed. Tigecycline has been proved to be promising in the treatment of A. baumannii infections.17–19 However, tigecyclines were not commercially available until 2013 in China. In our study, 7 patients were treated with tigecycline and only 2 of them died. This supports the need for further clinical studies investigating the role of tigecycline in patients with A. baumannii bacteremia.
In our study, severity of illness defined by PBS, neutropenia, and malignancy were all found to be independent factors for 14-day mortality. All these variables have been reported as independent predictors of death in patients with A. baumannii bacteremia.9,14,20–23 Nevertheless, crude mortality can be caused by patients’ underlying diseases as well as infections, which tend to be both frequent and severe in such patients.24,25 The point that outcomes of patients with infection correlated more closely with their underlying illness than with other factors was reported in an early study of A. baumannii bacteremia.26 Case–control studies also demonstrate that the presence of A. baumannii bacteremia did not correlate with a significantly increased mortality rate in critically ill patients.25,27 Both of them concluded that underlying illnesses seemed to play a more important role than the infection itself as the cause of death. PBS, neutropenia, and malignancy can reflect different aspects of underlying diseases. So our findings were not unexpected.
Our study demonstrated that underlying malignancy was an independent factor for mortality in patients with A. baumannii bacteremia, which concurred with previous results.9,22 Thus our investigation further pointed out that for patients with A. baumannii bacteremia, patients who suffered hematological malignancies tended to have a higher mortality than that of patients who suffered solid tumors. And patients with hematological malignancies also tended to be more likely to experience neutropenia and receive immunosuppressive therapy. New chemotherapeutic approaches with solid tumors have substantially decreased neutropenia-associated toxicity.28 However, patients with hematological malignancies still receive conventional chemotherapy and intensive immunosuppressive therapy. So neutropenia and immunosuppression was prevalent in these kind of patients.29,30
There were some inherent limitations in our study. First, this was a retrospective study with a relatively small number of patients. Second, patients with all A. baumannii complex bacteremia were all included in this study based on a single tertiary care medical center. Nevertheless, this study was strengthened by the exclusion of subjects deemed to be colonized and patients with polymicrobial bacteremia, adjustment for confounders and a well-defined end point of 14-day mortality.
Our study revealed that the independent risk factors influencing 14-day mortality for patients with A. baumannii bacteremia were severity of illness defined by PBS, neutropenia, and underlying malignancy, which implied that underlying illnesses seemed to play a more important role than the infection itself as the cause of death. Compared with solid tumors, underlying hematological malignancies were associated with higher mortality for patients with A. baumannii bacteremia.
Acknowledgment
We thank Jin Zhao, Mengjia Liu, and Wentao Ni for the statistical guidance and helpful discussions.
Footnotes
Abbreviations: A. = Acinetobacter, CI = confidence interval, IQRs = interquartile ranges, MDR = multidrug resistance, OR = odds ratio, PBS = Pitt Bacteremia Score, PLA = People's Liberation Army.
ZG, YH, and TM contributed equally to this work.
This work was supported by the fund from National Natural Science Foundation of China (NSFC) (no. 81070451) and Capital Medical Development Scientific Research Fund (SF2001-5001-07).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–317. [DOI] [PubMed] [Google Scholar]
- 2.Metan G, Sariguzel F, Sumerkan B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med 2009; 20:540–544. [DOI] [PubMed] [Google Scholar]
- 3.Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect 2014; 20:O1028–O1034. [DOI] [PubMed] [Google Scholar]
- 4.Gales AC, Castanheira M, Jones RN, et al. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn Microbiol Infect Dis 2012; 73:354–360. [DOI] [PubMed] [Google Scholar]
- 5.Kiratisin P, Chongthaleong A, Tan TY, et al. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: results of Asia-Pacific surveillance from the COMPACT II study. Int J Antimicrob Agents 2012; 39:311–316. [DOI] [PubMed] [Google Scholar]
- 6.Abbo A, Carmeli Y, Navon-Venezia S, et al. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis 2007; 26:793–800. [DOI] [PubMed] [Google Scholar]
- 7.Kim YA, Choi JY, Kim CK, et al. Risk factors and outcomes of bloodstream infections with metallo-beta-lactamase-producing Acinetobacter. Scand J Infect Dis 2008; 40:234–240. [DOI] [PubMed] [Google Scholar]
- 8.Munoz-Price LS, Zembower T, Penugonda S, et al. Clinical outcomes of carbapenem-resistant Acinetobacter baumannii bloodstream infections: study of a 2-state monoclonal outbreak. Infect Control Hosp Epidemiol 2010; 31:1057–1062. [DOI] [PubMed] [Google Scholar]
- 9.Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis 2012; 55:209–215. [DOI] [PubMed] [Google Scholar]
- 10.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008; 21:538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16:128–140. [DOI] [PubMed] [Google Scholar]
- 12.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care 2007; 11:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas ME, Kasiakou SK, Rafailidis PI, et al. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother 2006; 57:1251–1254. [DOI] [PubMed] [Google Scholar]
- 14.Kwon KT, Oh WS, Song JH, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 2007; 59:525–530. [DOI] [PubMed] [Google Scholar]
- 15.Harbarth S, Nobre V, Pittet D. Does antibiotic selection impact patient outcome? Clin Infect Dis 2007; 44:87–93. [DOI] [PubMed] [Google Scholar]
- 16.Chu H, Zhao L, Wang M, et al. Sulbactam-based therapy for Acinetobacter baumannii infection: a systematic review and meta-analysis. Braz J Infect Dis 2013; 17:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasilev K, Reshedko G, Orasan R, et al. A Phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J Antimicrob Chemother 2008; 62 (Suppl. 1):i29–i40. [DOI] [PubMed] [Google Scholar]
- 18.Morfin-Otero R, Dowzicky MJ. Changes in MIC within a global collection of Acinetobacter baumannii collected as part of the Tigecycline Evaluation and Surveillance Trial, 2004 to 2009. Clin Ther 2012; 34:101–112. [DOI] [PubMed] [Google Scholar]
- 19.Ni W, Cui J, Liang B, et al. In vitro effects of tigecycline in combination with colistin (polymyxin E) and sulbactam against multidrug-resistant Acinetobacter baumannii. J Antibiot 2013; 66:705–708. [DOI] [PubMed] [Google Scholar]
- 20.Song JY, Cheong HJ, Choi WS, et al. Clinical and microbiological characterization of carbapenem-resistant Acinetobacter baumannii bloodstream infections. J Med Microbiol 2011; 60 (Pt 5):605–611. [DOI] [PubMed] [Google Scholar]
- 21.Huang ST, Chiang MC, Kuo SC, et al. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect 2012; 45:356–362. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Choo JW, Kwon SH, et al. Risk factors for mortality in patients with Acinetobacter baumannii Bacteremia. Infect Chemother 2013; 45:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JY, Park YS, Kim CO, et al. Mortality risk factors of Acinetobacter baumannii bacteraemia. Intern Med J 2005; 35:599–603. [DOI] [PubMed] [Google Scholar]
- 24.Cisneros JM, Rodriguez-Bano J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect 2002; 8:687–693. [DOI] [PubMed] [Google Scholar]
- 25.Jang TN, Lee SH, Huang CH, et al. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect 2009; 73:143–150. [DOI] [PubMed] [Google Scholar]
- 26.Tilley PA, Roberts FJ. Bacteremia with Acinetobacter species: risk factors and prognosis in different clinical settings. Clin Infect Dis 1994; 18:896–900. [DOI] [PubMed] [Google Scholar]
- 27.Blot S, Vandewoude K, Colardyn F. Nosocomial bacteremia involving Acinetobacter baumannii in critically ill patients: a matched cohort study. Intensive Care Med 2003; 29:471–475. [DOI] [PubMed] [Google Scholar]
- 28.Chen CY, Tsay W, Tang JL, et al. Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiol Infect 2010; 138:1044–1051. [DOI] [PubMed] [Google Scholar]
- 29.Gedik H, Simsek F, Kanturk A, et al. Bloodstream infections in patients with hematological malignancies: which is more fatal—cancer or resistant pathogens? Ther Clin Risk Manag 2014; 10:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Zhao J, Xing Y, et al. Nosocomial infection in adult admissions with hematological malignancies originating from different lineages: a prospective observational study. PLoS ONE 2014; 9:e113506. [DOI] [PMC free article] [PubMed] [Google Scholar]


