Abstract
The aim of the study was to evaluate the efficacy and safety of 1-h infusion of recombinant human atrial natriuretic peptide (rhANP) in combination with standard therapy in patients with acute decompensated heart failure (ADHF).
This was a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Eligible patients with ADHF were randomized to receive a 1-h infusion of either rhANP or placebo at a ratio of 3:1 in combination with standard therapy. The primary endpoint was dyspnea improvement (a decrease of at least 2 grades of dyspnea severity at 12 h from baseline). Reduction in pulmonary capillary wedge pressure (PCWP) 1 h after infusion was the co-primary endpoint for catheterized patients. Overall, 477 patients were randomized: 358 (93 catheterized) patients received rhANP and 118 (28 catheterized) received placebo. The percentage of patients with dyspnea improvement at 12 h was higher, although not statistically significant, in the rhANP group than in the placebo group (32.0% vs 25.4%, odds ratio=1.382, 95% confidence interval [CI]: 0.863–2.212, P = 0.17). Reduction in PCWP at 1 h was significantly greater in patients treated with rhANP than in patients treated with placebo (−7.74 ± 5.95 vs −1.82 ± 4.47 mm Hg, P < 0.001). The frequencies of adverse events and renal impairment within 3 days of treatment were similar between the 2 groups. Mortality at 1 month was 3.1% in the rhANP group vs 2.5% in the placebo group (hazard ratio = 1.21, 95% CI: 0.34–4.26; P > 0.99).
1-h rhANP infusion appears to result in prompt, transient hemodynamic improvement with a small, nonsignificant, effect on dyspnea in ADHF patients receiving standard therapy. The safety of 1-h infusion of rhANP seems to be acceptable. (WHO International Clinical Trials Registry Platform [ICTRP] number, ChiCTR-IPR-14005719.)
INTRODUCTION
Heart failure is one of the leading causes of hospitalization with high mortality.1 Over the past decade, little improvement has been achieved in the treatment of acute decompensated heart failure (ADHF).1
Aldosterone and hyperactivation of the adrenergic system play an important role in the pathophysiology of heart failure.2,3 Atrial natriuretic peptide (ANP) is a circulating cardiac hormone that inhibits aldosterone and the adrenergic system.4 Previous studies have reported that a relative deficiency in ANP contributes to the development of heart failure.5 Thus, we hypothesized that infusion of ANP will be effective in treating heart failure.
Small, open-labeled clinical trials reported that ANP may improve hemodynamic parameters and the long-term prognosis of patients with ADHF.6,7 Also, an observational study revealed that clinical conditions improved in 82% of patients with acute heart failure treated with ANP infusion.8 However, such benefits have not been confirmed in any randomized controlled, double-blind, large-scale clinical trial. In fact, randomized controlled trials are virtually lacking.9,10
Recombinant human ANP (rhANP) is a synthetic 28-amino-acid alpha ANP developed by a group of Chinese scientists.11 In our previous phase II study, we demonstrated that a 1-h infusion of rhANP at doses of 0.05, 0.1, or 0.2 μg/kg/min improved hemodynamic properties in patients with congestive heart failure.12 However, the sample size was small (n = 48) and rhANP efficacy on hemodynamic parameters needs to be confirmed in patients with ADHF. Moreover, rhANP efficacy on dyspnea needs to be evaluated. Therefore, the objective of this phase III, randomized, double-blind, placebo-controlled, multicenter trial was to determine the efficacy and safety of a 1-h infusion of rhANP in combination with standard therapy in patients with ADHF, with dyspnea and PCWP being the primary efficacy endpoints.
METHODS
Study Participants
Adult patients with NYHA class III or IV heart failure at the time of screening, echocardiography showing left ventricular ejection fraction (LVEF) ≤40%, systolic blood pressure (SBP) ≥90 mm Hg, and lung rales or pulmonary vascular congestion upon chest radiography were eligible for this trial. Additionally, patients in whom hemodynamic parameters were measured by a Swan-Ganz catheter and presented with a PCWP of ≥13 mm Hg were eligible for inclusion and randomization in the catheterized group. Patients with acute myocardial infarction, complex congenital heart disease, significant valvular stenosis, constrictive pericarditis, hypertrophic, obstructive or, restrictive cardiomyopathy, ventricular fibrillation or sustained ventricular tachycardia, third-degree or Mobitz type II heart block without a permanent pacemaker were excluded. Other exclusion criteria included abnormal liver function with aspartate aminotransferase (AST) or alanine aminotransferase (ALT)≥120 IU/L, abnormal renal function with serum creatinine≥160 μmol/L, abnormal serum sodium concentrations (≥160 or ≤125 mmol/L), intravenous continuous infusion of diuretics or nitroprusside that could not be withheld through the study period as judged by the investigator and pregnancy.
Study Design
This was a phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial conducted in China. The trial protocol and relevant amendments were approved by the ethics committee at each center, the State Food and Drug Administration of China (No. 2004L00615). The trial was conducted according to standards of Good Clinical Practice and the Declaration of Helsinki of the World Medical Assembly. All patients provided written informed consent. The trial was monitored by the Monitoring Board of Giant Med-Pharma Service Group, Beijing, China. The study-data processing and statistical analyses were performed by the Medical Research & Biometrics Center, National Center for Cardiovascular Diseases (China) independently of the sponsor.
Randomization and Masking
Randomization was performed using random blocks, and each block contained a size of 4. The subjects were randomly assigned to the rhANP group or placebo group at a ratio of 3:1. In addition, one-fourth of the random numbers were assigned to the catheterized group according to the sample size calculation. The random number table was generated by the Medical Research & Biometrics Center, National Center for Cardiovascular Diseases; Beijing, China, using SAS software, version 9.13 (Cary, NC). The blind code was concealed in sealed and opaque envelopes.
Study Drug Administration
The rhANP and placebo, which were provided by National Engineering Center of Biotechnology, Shenzhen, China, were lyophilized powder in glass vials and appeared identical. They were intravenously administrated by using a calibrated infusion pump. The study drug was initiated at a rate of 0.1 μg/kg/min. After a half hour, the dose was adjusted to 0.15 μg/kg/min if SBP was >100 mm Hg and PCWP was >15 mm Hg. Dosing was stopped 1 h after initiation.
Before screening, there was no restriction on the standard therapies for the treatment of heart failure, such as intravenous bolus of diuretics and continuous infusion of nitrates, beta-blocker, angiotensin-converting enzyme inhibitor (ACEI), angiotensin-receptor blocker (ARB), spironolactone, and oral vasoactive medications. During the period from screening to baseline and afterwards, none of the above medications was withheld except the continuous intravenous infusion of diuretics or nitroprusside.
Efficacy Endpoint Assessments
The primary endpoint was dyspnea improvement at 12 h after treatment, which was determined by a decrease of at least 2 grades of severity in dyspnea at 12 h from baseline. Dyspnea, defined as sensation of severity of breathlessness of patients at rest, was assessed with the combination of the position and symptom of patients, and classified into 5 grades according to the severity, that is, 0, absence of dyspnea at rest; 1, dyspnea in the supine position; 2, paroxysmal nocturnal dyspnea; 3, dyspnea in the semi-reclining position; and 4, orthopnea. Changes in dyspnea were measured by calculating the percentage of patients with different levels of dyspnea severity changes at various time points from baseline.
Change in PCWP at 1 h after study-drug initiation in the catheterized group was a co-primary endpoint in addition to dyspnea. Secondary endpoints included other changes of dyspnea at 12 h and dyspnea improvement at 0.5, 1, 3, 6, 24, and 72 h, total urine output at 12 h for all patients, and other hemodynamic parameters including changes in PCWP at 0.25, 0.5, 0.75, 3, 6, and 12 h and cardiac index and systemic vascular resistance (SVR) at 0.5, 1, 3, 6, and 12 h in the catheterized group. PCWP was measured by a Swan-Ganz catheter using a floating inflated balloon. Hemodynamic cardiac output was derived from thermodilution measurements using ice-cold sodium chloride 0.9%. The cardiac index and SVR were calculated using standard formulas.13
Safety Endpoint Assessments
The physical examination was given at baseline, 24 and 72 h after initiation of study drug. Urine samples were collected for routine analysis, blood samples were drawn for chemical and hematological analyses, and electrocardiogram was performed at baseline and 3 days after initiation of study drug. Renal impairment was defined by a >25% decrease in the estimated glomerular filtration rate compared with baseline within 3 days of study drug, as calculated by the Cockcroft–Gault equation. Adverse events were monitored in all patients within 3 days. Death and serious adverse events that resulted in hospitalization or prolonged hospital stays lasting 1 month were monitored by hospital visits or telephone interviews after discharge.
Statistical Analysis
Calculation of the sample size of the general population was based on the primary endpoint, the change of dyspnea at 12 h after study-drug initiation. According to our rhANP phase II study, dyspnea improved in 57.0% patients in the rhANP group at 12 h compared to 40.0% in the standard therapy only group (unpublished data). At a ratio of 3:1, the enrolment of 360 patients in the rhANP group and 120 in the placebo group was estimated to provide 80% power, with the use of the chi-square test and a 2-sided alpha level of 0.05. Calculation of the sample size of the catheterized group was based on the primary endpoint, PCWP. According to our phase II, dose-finding study, the reduction of PCWP from baseline at 1 h was at least 6.83 ± 7.80 mm Hg in the rhANP group and −2 ± 6.12 mm Hg in the placebo group.31 With a 2-sided 5% significance level and a ratio of 3:1, 90 patients in the rhANP group and 30 patients in the placebo group provided 90% power.
All reported analyses were performed on an intention-to-treat (ITT) basis, which included all randomly assigned participants who received any amount of study medication. The numerical data were expressed as mean ± standard deviation. An overall comparison of continuous data between 2 groups was made with analysis of covariance. The rate of dyspnea improvement and adverse events in the groups was compared using a chi-square test or Fisher's exact test, where appropriate; odds ratios (OR) and 95% confidence interval (CI) were calculated. All reported P values were 2-sided and a P value of <0.05 was considered statistically significant. All analyses were performed using SAS software, version 9.4 (Cary, NC).
RESULTS
Study Patients
From March 2009 to July 2013, a total of 477 eligible patients were screened and underwent randomization at 12 centers in China. One patient randomized into the placebo group withdrew consent and did not receive the drug. Thus, 358 patients received rhANP and 118 patients received placebo (Figure 1). There were no significant differences in baseline characteristics between groups (Table 1). Dyspnea at baseline was also similar between groups (Table 1). There were 41.9% patients with <1 grade of dyspnea at baseline in the rhANP group compared to 43.2% in the placebo group (P = 0.80). Of all randomized 121 patients, 93 receiving rhANP and 28 receiving placebo were included in the catheterized group (Figure 1).
FIGURE 1.
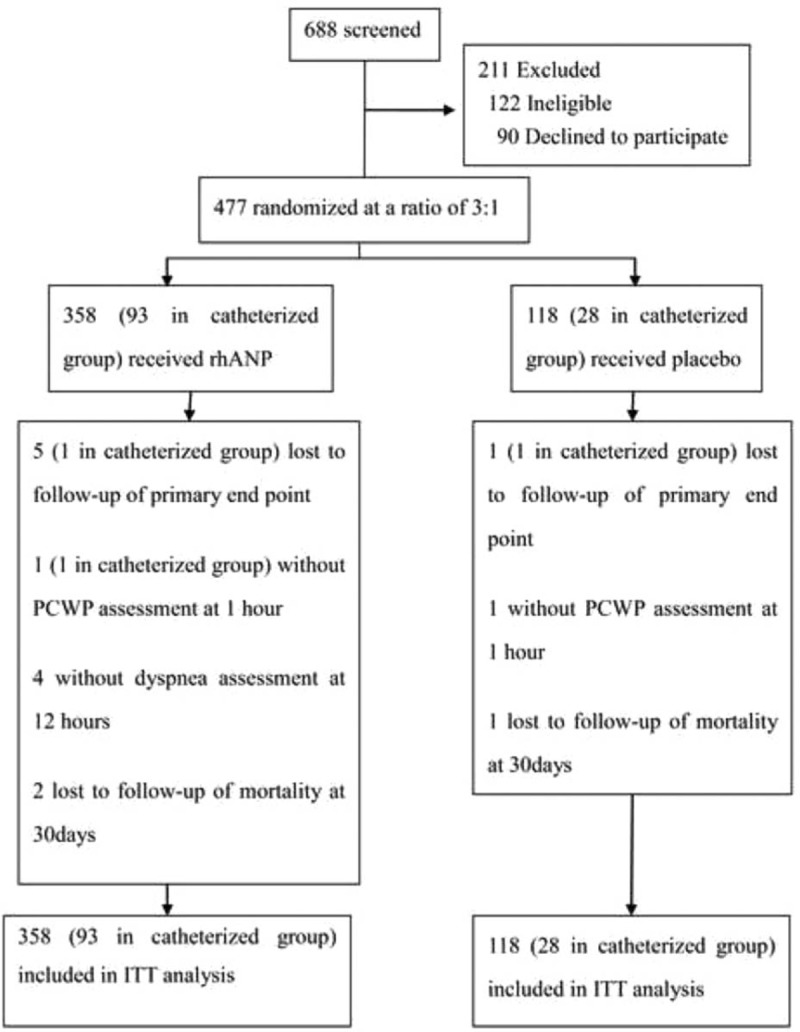
Recruitment, randomization, and follow-up of patients. The intention-to-treat (ITT) analysis includes all randomized participants who received study medication. ITT = intention-to-treat, rhANP = recombinant human atrial natriuretic peptide, PCWP = pulmonary capillary wedge pressure.
TABLE 1.
Baseline Characteristics and Standard Therapy of the Intention-to-Treat Population∗

Efficacy Endpoints
The proportion of patients with dyspnea improvement at 12 h tended to be greater in the rhANP group (32.0% vs 25.4%, OR = 1.382, 95% CI: 0.863–2.212, P = 0.17), although the difference was not statistically significant (Figure 2A). There were no differences in dyspnea improvement at other time points between the 2 groups (Figure 2B).
FIGURE 2.

Changes in dyspnea among all patients. (A) Changes in dyspnea severity at 12 h, represented by the percentage of patients with 7 levels of dyspnea severity changes at 12 h from baseline. The numbers on the right of the bars indicate the overall percentage of patients with dyspnea improvement of at least 2 grades at 12 h in the 2 groups. (B) Dyspnea improvement at various time points, represented by the percentage of patients with dyspnea improvement that was defined when there was a decrease of at least 2 grades of dyspnea at a designated time point from baseline.
In the catheterized patients, the baseline PCWP values were similar between the rhANP and placebo groups (23.71 ± 7.0 vs 25.66 ± 8.78 mm Hg, P = 0.23), both of which were decreased after study-drug initiation. At 0.5 h, the mean reduction of PCWP was greater in the rhANP group than in the placebo group (−5.45 vs −2.03 mm Hg, P = 0.002). At 1 h, the maximum reduction of PCWP was observed in the rhANP group, which was significantly greater than the placebo group (−7.74 ± 5.95 vs −1.82 ± 4.47 mm Hg, P < 0.001). At 3 h, PWCP was sustained at a lower level in the rhANP group compared to the placebo group (19.52 ± 6.55 vs 24.79 ± 8.42 mm Hg, P < 0.001). However, no significant differences in PCWP at 6 h and afterwards were found between the 2 groups (21.43 ± 6.51 vs 24.79 ± 10.67 mm Hg, P = 0.13).
Other hemodynamic parameters improved more significantly in the rhANP group. Figure 3 shows that rhANP increased cardiac index and decreased SVR, respectively, although these effects lasted <6 h. There was no difference in the urine output during 12 h post drug infusion between groups.
FIGURE 3.
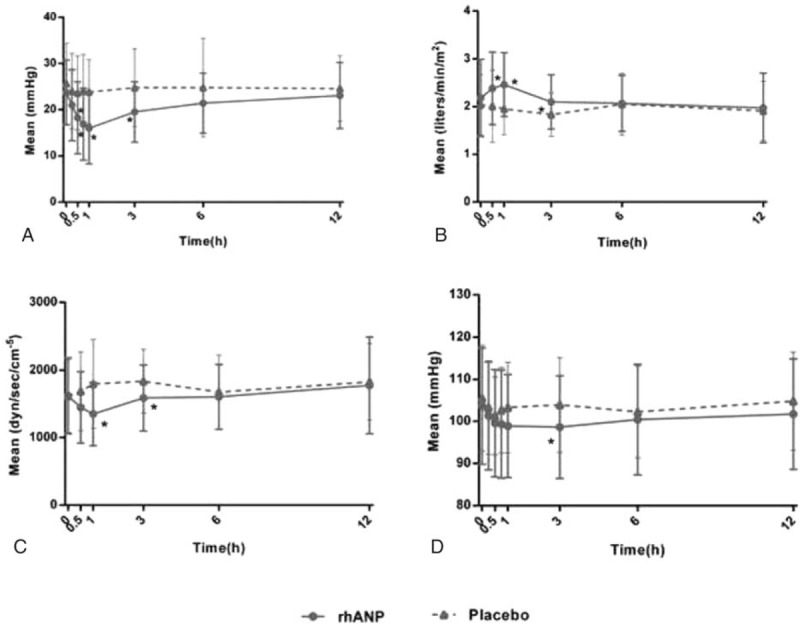
Hemodynamic changes including pulmonary capillary wedge pressure. (A) Cardiac index (B) systemic vascular resistance (C) and systolic blood pressure (D) at different time points in the catheterized group. The dots and triangles denote the mean values and the bars indicate the standard deviation. ∗, P < 0.05 compared with placebo.
Safety Endpoints
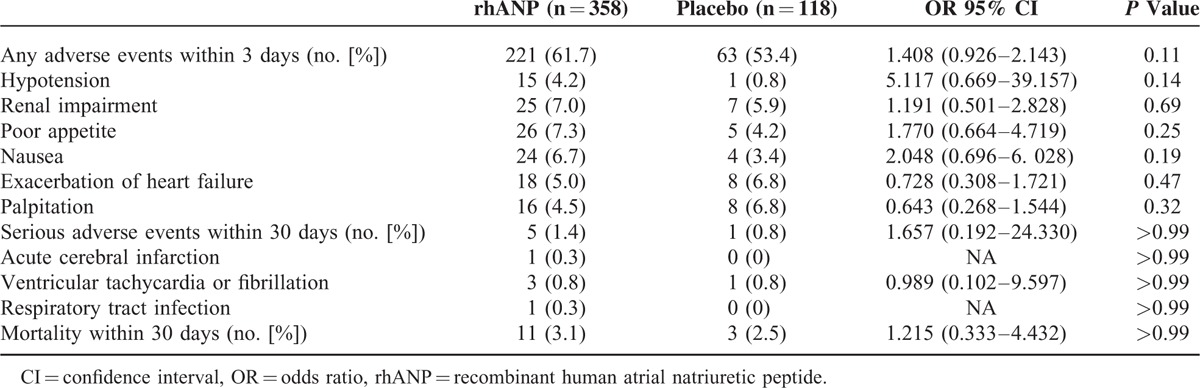
Table 2 shows the safety profiles in the 2 groups. There were no differences between the 2 groups in the frequency of adverse events and renal impairment within 3 days. The major adverse events included hypotension and renal impairment, exacerbation of heart failure, palpitation, poor appetite, and nausea. Hypotension was observed within 3 days and tended to be higher in the rhANP group. SBP reduction at 3 h was significantly greater in the rhANP group than in the placebo group (−5.04 vs −1.57 mm Hg, P = 0.04). However, the reduction was not significantly different after 3 h (Figure 3D). In addition, there was no difference in the frequency of serious adverse events and mortality between the 2 groups within 30 days. There were no significant differences in electrocardiogram and blood and urine analyses between the 2 groups.
TABLE 2.
Adverse Events of the Intention-to-Treat Population
DISCUSSION
In this trial, we found that, 1-h infusion of rhANP in combination with standard care, significantly decreased PCWP compared with placebo within 3 h, confirming that 1-h infusion of rhANP results in prompt and transient hemodynamic improvement in patients with ADHF. However, 1-h infusion of rhANP only achieved a small, nonsignificant improvement in dyspnea at 12 h. There was no difference in the urine output during the 12 h postdrug infusion between the 2 groups. Renal impairment and other adverse events at 3 days and mortality or severe adverse events at 1 month were similar between the 2 groups.
rhANP efficacy on dyspnea in this trial was in agreement with the findings of the ASCEND-HF trial, which also showed that BNP only had a small, nonsignificant, but beneficial effect on dyspnea compared with placebo treatment.14
Recently, dyspnea has been used as a common primary endpoint in clinical trials of ADHF,14,15 although the optimal method of dyspnea assessment is controversial.16 The present study adopted the combination of the patient's position and symptom to assess dyspnea. This method is more objective and sensitive than those relying only on the symptoms reported by patients, as the dyspnea of patients with ADHF is affected by their position.17,18 We note that dyspnea improvement at 12 h post drug infusion was observed only in 32.0% and 25.4% of the patients in the rhANP and placebo groups, respectively, which were much less than that (57.0% and 40.0%, respectively) estimated in our phase II study (unpublished data). There are a few reasons that may explain our observations. First, such over estimation may be attributed to the possibility that sample calculation was associated with the different methods used for the dyspnea assessment from the phase II study of rhANP, which adopted a self-reported 7-point Likert scale that was used in the ASCEND-HF trial. In the ASCEND-HF trial, 44.5% and 68.2% of patients in the nesiritide group reported marked or moderate dyspnea improvement at 6 and 24 h, respectively, whereas the rates were 42.1% and 66.1%, respectively, in the placebo group.14 In contrast, in an observational registry study of ANP in the “real world” including 3777 patients with acute heart failure, the global efficacy at 24 h was only 38.0%.8 Global efficacy was defined as the percentage of patients whose dyspnea or other symptoms and physical signs of heart failure were “improved or markedly improved” over the total treated patients in a 5-grade efficacy classification. This rate was much lower than that in the ASCEND-HF,14 but similar to that in the present study. Second, the low rate of dyspnea improvement may be altered by baseline dyspnea, which was rarely assessed in previous studies.14,15 In the present study, 42.2% patients presented with <1 grade of dyspnea at baseline, which accounted for a decreased proportion of patients that reported moderate or marked improvement (i.e., 2-grade decrease in the symptom). Third, dyspnea symptoms may have been alleviated during the period from screening to baseline in the present study, as patients were permitted to receive standard therapies, which were relatively sufficient to treat the symptom. Medications, such as intravenous nitroglycerin and a bolus of diuretics, were not restricted, except for nitroprusside and the continuous infusion of diuretics. Recently, it has been shown that the effect of diuretics on dyspnea is similar whether they are administrated by bolus or by continuous infusion in patients with ADHF.19 Furthermore, the VMAC trial reported that the effect of nitroglycerin on dyspnea was similar to that of BNP.20 Because BNP targets the same receptors as rhANP,21 standard therapies in the present study consisting of nitroglycerin may also contribute to dyspnea improvement in both the rhANP and placebo groups, and minimize the advantage of rhANP over placebo in terms of effect on dyspnea.
The present trial demonstrated that ANP decreased PCWP and improved other hemodynamic parameters. Hemodynamic improvement, especially a decrease in PCWP, used to be regarded as an important primary endpoint of clinical efficacy of ADHF.22–25 In the present study, PCWP was used as a co-primary endpoint in a subgroup of patients and further verified the findings obtained in our phase II, dose-finding study.12 Dyspnea improvement in this subgroup appeared at 0.5 h post drug infusion and was most obvious at 12 h. Hemodynamic improvement reached a peak at 1 h and started to wane after 6 h. This is consistent with observations in previous studies that dyspnea improvement was delayed compared with the hemodynamic effects, which gradually disappeared after stopping the study drug.25,26 Similarly, the hemodynamic or dyspnea improvement may be helpful for other available treatments of acute heart failure, to advance patients past the acute phase and achieve a relatively stable chronic status.27
The present study adopted a short-term infusion, which was used in other studies of ANP carried out before 1999.28,29 Although long-term infusion has been used in most subsequent studies,4,6,7 it has been reported that some effects of ANP, such as vasodilation, inhibition of aldosterone, and potential diuresis, may be attenuated by long-term infusion.30,31
Although placebo was adopted in the control group of the present study, it was added in the standard therapies including diuretics, vasodilators, ACEI, or ARB. The background standard therapies varied among clinical trials, affecting the evaluation of a new agent for the treatment of heart failure, which may explain their inconsistency regarding the effect of dyspnea, diuresis, and prognosis.32 The VMAC trial demonstrated a significantly better effect on dyspnea at 3 h for nesiritide compared with placebo, and thus formed the basis for the Food and Drug Administration's approval of nesiritide.20 However, the use of intravenous vasodilators was restricted until there was defined assessment of dyspnea.20 Similarly, patients receiving intravenous vasodilators were excluded in the trials using relaxin.15,33 In a phase II study of ularitide for decompensated heart failure, intravenous diuretics, ACEI, and vasoactive drugs were withheld during a 5-h period (beginning 3 h before initiating study drug infusion).25 Spironolactone was prohibited in another clinical trial that showed that ANP improved the prognosis of heart failure.7 In most studies evaluating effects of ANP or BNP on diuresis or aldosterone inhibition, diuretics and/or ACEI were withheld.30,34 Therefore, it seems difficult to prove that the new drugs used for ADHF can produce an additional benefit on top of a combination of the conventional vasodilators, diuretics, ACEI or ARB, beta-blockers, and spironolactone.32
There are several major limitations in this phase III clinical trial. First, similar to other heart failure trials,14,15 patients were relatively highly selected in the present study. All study patients had reduced ejection fraction with better hepatic and renal function and were younger in age compared to most registry study patients.10,35,36 However, it has recently been indicated that heart failure trials should be designed in a selective population since the patients with heart failure are heterogeneous in pathophysiology and comorbidity.10,35,36 Second, aldosterone and adrenergic hormones are important factors involved in the pathophysiology of heart failure, and previous studies have reported that ANP causes inhibition of aldosterone and the adrenergic system in patients with congestive heart failure.2,3 In the present study, these hormones were not measured and thus the potential effects of ANP on these hormones in patients with ADHF remains to be elucidated. Third, more biomarkers such as N-terminal pro B-type natriuretic peptide and N-terminal propeptide of procollagen type III that are associated with the development of heart failure37,38 were not included as inclusion criteria for the diagnosis of heart failure. Fourth, as the patients all had systolic dysfunction with class III or IV NYHA, the effects of ANP on impairment of excitation–contraction coupling and nutritional risk index should be further studied.39,40 Last, this clinical trial of rhANP only included Chinese patients and the number of patients was low despite sample-size estimation. Thus the efficacy and safety of rhANP for patients should be further evaluated in large-scale international, multicenter trials in other countries.
In conclusion, a 1-h infusion of rhANP in combination with standard therapy appears to result in prompt, transient hemodynamic improvement, with a small, nonsignificant effect on dyspnea in ADHF patients. The safety of a 1-h infusion of rhANP seems to be acceptable. However, an international multicenter trial with a large number of patients is needed to further verify our findings.
Acknowledgments
The authors thank all study coordinators at the participating centers and all patients who participated in the study. The authors also thank Dr. Harry Hua-Xiang Xia, Medjaden Bioscience Inc, USA, for assistance in manuscript preparation.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor, ADHF = acute decompensated heart failure, ALT = alanine aminotransferase, ANP = atrial natriuretic peptide, ARB = angiotensin-receptor blocker, AST = aspartate aminotransferase, CI = confidence interval, ICTRP = WHO International Clinical Trials Registry Platform, ITT = intention-to-treat, LVEF = left ventricular ejection fraction, OR = odds ratios, PCWP = pulmonary capillary wedge pressure, rhANP = recombinant human atrial natriuretic peptide, SBP = systolic blood pressure, SVR = systemic vascular resistance.
Funding: the present study was financially sponsored by the National Engineering Center of Biotechnology; Shenzhen, China (No#2004L00615).
G. Wang and P. wang contributed equally to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015; 385:812–824. [DOI] [PubMed] [Google Scholar]
- 2.Odedra K, Ferro A. Neurohormones and heart failure: the importance of aldosterone. Int J Clin Pract 2006; 60:835–846. [DOI] [PubMed] [Google Scholar]
- 3.Santulli G. Adrenal signaling in heart failure: something more than a distant ship's smoke on the horizon. Hypertension 2014; 63:215–216. [DOI] [PubMed] [Google Scholar]
- 4.Kasama S, Toyama T, Kumakura H, et al. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity in patients with decompensated congestive heart failure. J Nucl Med 2004; 45:1108–1113. [PubMed] [Google Scholar]
- 5.Volpe M, Tritto C, De Luca N, et al. Failure of atrial natriuretic factor to increase with saline load in patients with dilated cardiomyopathy and mild heart failure. J Clin Invest 1991; 88:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitashiro S, Sugiura T, Takayama Y, et al. Long-term administration of atrial natriuretic peptide in patients with acute heart failure. J Cardiovasc Pharmacol 1999; 33:948–952. [DOI] [PubMed] [Google Scholar]
- 7.Hata N, Seino Y, Tsutamoto T, et al. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J 2008; 72:1787–1793. [DOI] [PubMed] [Google Scholar]
- 8.Suwa M, Seino Y, Nomachi Y, et al. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ’real world’ of therapy. Circ J 2005; 69:283–290. [DOI] [PubMed] [Google Scholar]
- 9.Morita Y, Kohsaka S, Oshima K, et al. Use of carperitide infusion for acutely decompensated heart failure. Crit Care 2012; 16:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (From the ATTEND Registry). Circ J 2013; 77:944–951. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Chen W, Lu J, et al. Over expression and purification of recombinant atrial natriuretic peptide using hybrid fusion protein REF-ANP in Escherichia coli. Protein Expres Purif 2003; 28:49–56. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Luan X, Wang G, et al. Efficacy and safety of short-term administration of recombinant human atrial natriuretic peptide (rhANP) for congestive heart failure: a phase II, multicentre randomized controlled dose-finding study. J Clin Pharm Ther 2013; 38:388–393. [DOI] [PubMed] [Google Scholar]
- 13.Gidwani UK, Mohanty B, Chatterjee K. The pulmonary artery catheter: a critical reappraisal. Cardiol Clin 2013; 31:545–565. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. New Engl J Med 2011; 365:32–43. [DOI] [PubMed] [Google Scholar]
- 15.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013; 381:29–39. [DOI] [PubMed] [Google Scholar]
- 16.Mentz RJ, Felker GM, Ahmad T, et al. Learning from recent trials and shaping the future of acute heart failure trials. Am Heart J 2013; 166:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold JMO, Porepa L. Acute decompensated heart failure: the quest to live longer and feel better. J Am Coll Cardiol 2012; 59:1449–1451. [DOI] [PubMed] [Google Scholar]
- 18.Pang PS, Cleland JGF, Teerlink JR, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J 2008; 29:816–824. [DOI] [PubMed] [Google Scholar]
- 19.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. New Engl J Med 2011; 364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002; 287:1531–1540. [DOI] [PubMed] [Google Scholar]
- 21.Gassanov N, Biesenbach E, Caglayan E, et al. Natriuretic peptides in therapy for decompensated heart failure. Eur J Clin Pharmacol 2012; 68:223–230. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P, Mitrovic V, Ruda M, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 2014; 35:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao R, Zhang J, Cheng L, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 2010; 55:1907–1914. [DOI] [PubMed] [Google Scholar]
- 24.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002; 360:196–202. [DOI] [PubMed] [Google Scholar]
- 25.Mitrovic V, Seferovic PM, Simeunovic D, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J 2006; 27:2823–2832. [DOI] [PubMed] [Google Scholar]
- 26.Mills RM, LeJemtel TH, Horton DP, et al. Sustained hemodynamic effects of an infusion of nesiritide (human b-type natriuretic peptide) in heart failure: a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol 1999; 34:155–162. [DOI] [PubMed] [Google Scholar]
- 27.George M, Rajaram M, Shanmugam E, et al. Novel drug targets in clinical development for heart failure. Eur J Clin Pharmacol 2014; 70:765–774. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Nakao K, Nishimura K, et al. Clinical application of atrial natriuretic polypeptide in patients with congestive heart failure: beneficial effects on left ventricular function. Circulation 1987; 76:115–124. [DOI] [PubMed] [Google Scholar]
- 29.Molina CR, Fowler MB, McCrory S, et al. Hemodynamic, renal and endocrine effects of atrial natriuretic peptide infusion in severe heart failure. J Am Coll Cardiol 1988; 12:175–186. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa C, Tsutamoto T, Wada A, et al. Inhibition of aldosterone and endothelin-1 by carperitide was attenuated with more than 1 week of infusion in patients with congestive heart failure. J Cardiovasc Pharmacol 2005; 46:513–518. [DOI] [PubMed] [Google Scholar]
- 31.Munzel T, Drexler H, Holtz J, et al. Mechanisms involved in the response to prolonged infusion of atrial natriuretic factor in patients with chronic heart failure. Circulation 1991; 83:191–201. [DOI] [PubMed] [Google Scholar]
- 32.Tamargo J, Pez-Send LO. ONJ. Novel therapeutic targets for the treatment of heart failure. Nat Rev Drug Discov 2011; 10:536–555. [DOI] [PubMed] [Google Scholar]
- 33.Teerlink JR, Metra M, Felker GM, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 2009; 373:1429–1439. [DOI] [PubMed] [Google Scholar]
- 34.Abraham WT, Lowes BD, Ferguson DA, et al. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail 1998; 4:37–44. [DOI] [PubMed] [Google Scholar]
- 35.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296:2217. [DOI] [PubMed] [Google Scholar]
- 36.Sulaiman K, Panduranga P, Al-Zakwani I, et al. Clinical characteristics, management, and outcomes of acute heart failure patients: observations from the Gulf acute heart failure registry (Gulf CARE). Eur J Heart Fail 2015; 17:374–384. [DOI] [PubMed] [Google Scholar]
- 37.Bielecka-Dabrowa A, Michalska-Kasiczak M, Gluba A, et al. Biomarkers and echocardiographic predictors of myocardial dysfunction in patients with hypertension. Sci Rep-UK 2015; 5:8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fazal IA, Bhagra SK, Bailey KM, et al. Impact of using different guideline recommended serum natriuretic peptide thresholds on the diagnosis and referral rates of a diagnostic heart failure clinic. Int J Clin Pract 2015. [DOI] [PubMed] [Google Scholar]
- 39.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol 2015; 8:206–222. [DOI] [PubMed] [Google Scholar]
- 40.Gouya G, Voithofer P, Neuhold S, et al. Association of nutritional risk index with metabolic biomarkers, appetite-regulatory hormones and inflammatory biomarkers and outcome in patients with chronic heart failure. Int J Clin Pract 2014; 68:1293–1300. [DOI] [PubMed] [Google Scholar]


