Abstract
Previous studies suggest a link between anxiety disorders and cancer. The aim of the study was to evaluate the risk of cancer among patients with obsessive-compulsive disorder (OCD) using a nationwide population-based dataset.
We recruited newly diagnosed OCD patients without antecedent cancer from the Taiwan National Health Insurance Research Database between 2002 and 2011. The standardized incidence ratios (SIRs) were estimated for 22 specific cancer types among OCD patients and we determined the SIRs for subgroups according to age and sex group. In addition, because of a potential detection bias, a subgroup analysis stratified with the duration of the OCD diagnosis was carried out.
Among the 52,656 OCD patients, who were followed up for 259,945 person-years (median follow-up = 4.9 years), there were 718 cases of cancer. Patients with OCD did not exhibit an increased overall cancer risk relative to the general population (SIR 1.05, 95% confidence interval [CI] 0.98–1.13). An increased SIR was observed among OCD patients only within the first year of OCD diagnosis (SIR 1.21, 95% CI 1.01–1.43).
This study indicated that the overall cancer risk was not elevated among OCD patients. An increased SIR observed among OCD patients within the first year of OCD diagnosis may be caused by a surveillance bias, and because paraneoplastic manifestations presented with obsessive-compulsive behaviors. Prospective study is necessary to confirm these findings.
INTRODUCTION
Obsessive-compulsive disorder (OCD) is a type of anxiety disorder with a lifetime prevalence of 1.6% to 2.5% among the general adult population.1–3 It is characterized by the presence of either obsessions or compulsions that cause marked distress or interfere with normal functioning at work, home, or during social activities.4 Despite the availability of treatment options, OCD remains a major public health concern. It is associated with considerable impairment and disability, and patients with OCD often experience severe social and interpersonal difficulties, familial dysfunction, occupational problems, and impaired quality of life.5–8 The World Health Organization classified OCD as one of the top 10 most debilitating illnesses.9
Emotional distress, such as chronic depression and anxiety, has been associated with poorer survival among cancer patients.10 However, whether psychological factors increase the cancer incidence remains unclear.11 Although substantial studies have been performed on the associations between psychosocial factors and cancer incidence, there is no research evidence to support a direct causal relationship between the 2 events.11–22 In addition, only a few of these studies focused on anxiety or anxiety disorders.17–19,22 A study in Taiwan has suggested that patients with anxiety disorders may increase the risk of prostate cancer and marginally decrease the risk of cervical cancer,17 and a study in the United Kingdom (UK) has indicated that patients with anxiety disorders may have a higher risk for lung and brain cancers.18 In both the studies, the results showed no significant effect of anxiety disorders on overall cancer risk.
The present study was designed to determine the links between OCD and cancers. To our knowledge, this is the first large population-based retrospective cohort study to evaluate the risk of cancer among OCD patients.
METHODS
Data Sources
The National Health Insurance (NHI) program was implemented in Taiwan in 1995, and offers affordable and comprehensive health-care coverage to nearly 99% of all Taiwan residents.23 The NHI Research Database (NHIRD) contains comprehensive information about every billing order, including details on both inpatients and outpatients services. The NHIRD is managed by the National Health Research Institutes (NHRI) and confidentiality is maintained according to the directives of the Bureau of the NHI. For researchers’ convenience, the NHRI issues the Longitudinal Health Insurance Database (LHID) as a representative NHIRD subset. The LHID contains original claims data for 1,000,000 randomly selected beneficiaries in the registry of beneficiaries. Its representativeness is supported by similar distributions in age, sex, and health-care cost from the original NHIRD.
However, rather than using the LHID, in the current study we analyzed a specific database from the NHRI, which was composed of all beneficiaries with OCD in the registry of beneficiaries. Thus, nearly all OCD patients in Taiwan were included in this dataset. The registry of catastrophic illness patients in the NHIRD provides comprehensive enrollment information for all patients with severe diseases, such as cancer, who have received a copayment exemption in the NHI program.
Standard Protocol Approvals, Registrations, and Patient Consent
The Institutional Review Board of the Taipei Veterans General Hospital approved this study (2012-12-013BC). Written consent from study patients was not obtained because the NHI dataset consists of deidentified secondary data used for research purposes, and the Institutional Review Board of Taipei Veterans General Hospital issued a formal written waiver regarding the need for consent.
Study Population
We conducted a retrospective cohort study from January 1, 2001 to December 31, 2011. Patients newly diagnosed with OCD between January 1, 2002 and December 31, 2010, who had no prior malignancies, were enrolled. OCD was defined according to the International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) code 300.3. In addition, we included only patients who were diagnosed with OCD by a clinical psychiatrist to ensure the diagnostic validity and patient homogeneity.
Statistical Analysis
The main dependent variable was the occurrence of cancer, as reported in the registry of catastrophic illness patients. The cancer diagnoses of patients in the registry were histologically confirmed. OCD patients were followed until the development of cancer, death, withdrawal from the NHI program, or the end of the year 2011.
The risk of cancer among the OCD cohort was determined based on standardized incidence ratios (SIRs), an analysis often used to determine if the occurrence of cancer in a relatively small population, such as the patients with OCD, is high or low. The SIR is obtained by dividing the observed number of cases of cancer and the expected number of cases. In our study, the observed number of cases was the absolute number of cancer diagnoses among the patients with OCD and the expected number of cancer occurrences in OCD patients was derived by multiplying the national cancer incidence rate, stratified according to calendar period, age groups in 5-year intervals and sex, with the corresponding stratum-specific person-time accrued in the patients with OCD. The national cancer incidence rates were obtained from the Taiwan Cancer Registry. The 95% confidence intervals (CIs) of the SIRs were estimated based on the assumption that the observed numbers of cancers have a Poisson probability distribution. To avoid biased conclusions, especially those caused by the potential surveillance bias, subgroups were stratified according to the follow-up duration since OCD diagnosis. For the same reason, only patients with cancers diagnosed beyond the first year following OCD diagnoses were included to calculate the SIRs for the overall cancer risk and the risk of each cancer type. In addition, the Bonferroni correction was used for the concern of the multiple comparisons in statistics. Categorical variables were compared using χ2 tests, and continuous variables were compared using independent t tests.
The Perl programming language (version 5.12.2) was used to extract, match, and compute data. The Microsoft SQL Server 2005 (Microsoft Corporation, Redmond, WA) was used for further data processing. Statistical analysis was performed using IBM SPSS 17.0 software (SPSS Inc, Chicago, IL). The comparisons resulting in a P value of less than 0.05 were considered to present a statistically significant relationship.
RESULTS
Patient Demographics
Overall, the cohort was observed for 259,945 person-years from 2002 to 2011. A total of 52,656 patients with OCD (50.9% female) were identified. The median age at diagnosis was 32 years (interquartile range [IQR] = 23–45 years), and the median follow-up period was 4.9 years (IQR = 2.5–7.4 years). The patient demographics are shown in Table 1.
TABLE 1.
Characteristics of Patients With Obsessive Compulsive Disorder

All Cancers
A total of 718 cancers occurrences were recorded within the observation period. Compared with the general population, patients with OCD did not exhibit an increased overall cancer risk, as indicated by the SIR of 1.05 (95% CI 0.98–1.13). An analysis of subgroups stratified according to sex showed that neither male nor female patients exhibited increased SIRs (1.09, 95% CI 0.98–1.21 and 1.02, 95% CI 0.92–1.13, respectively). An analysis of subgroups stratified according to age showed that patients younger than 20 years exhibited an increased SIR (2.68, 95% CI 1.34–4.80, P = 0.01), but those aged 20 years and older did not. Male patients under 20 years of age also exhibited an increased SIR (2.64, 95% CI 1.06–5.44, P = 0.04). Within the first year of OCD diagnosis, 134 cancers were reported, with an SIR of 1.21 (95% CI 1.01–1.43, P = 0.03). Excluding the first year following, did not significantly increase the SIR (1.02, 95% CI 0.94–1.11). The results of the subgroup analyses are summarized in Table 2.
TABLE 2.
Standardized Incidence Ratios (SIRs) According to Age, Sex, and Duration of Obsessive Compulsive Disorder

Specific Cancer Types (Excluding Patients Whose Cancer Was Diagnosed Within the First Year of OCD Diagnosis)
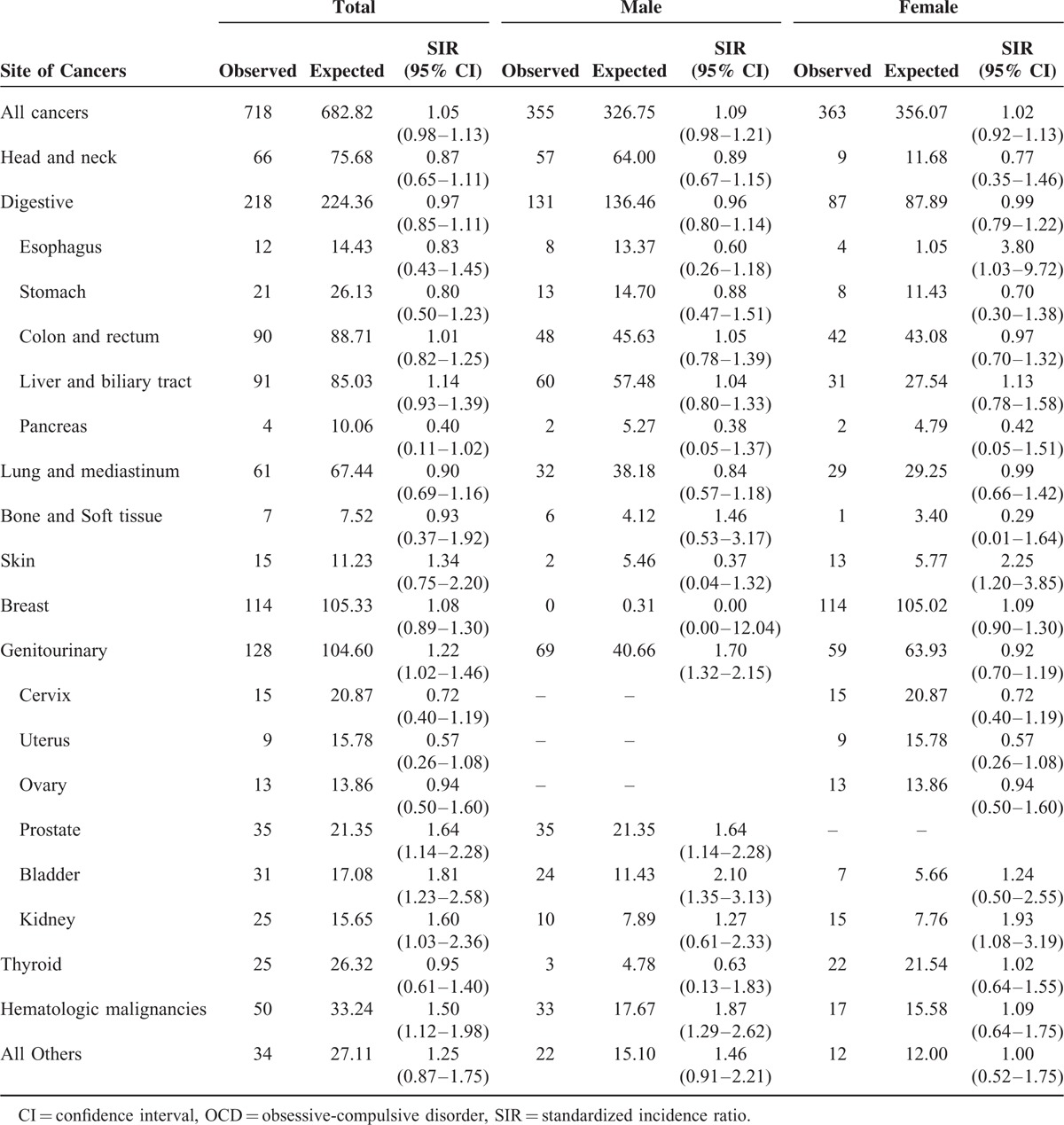
Among the OCD patients, breast cancer (n = 96) was most common, followed by liver and biliary tract cancers (n = 79), and colon and rectum cancers (n = 75). An increased SIR was observed for prostate cancer (1.64, 95% CI 1.11–2.35, P = 0.01) and bladder cancer (1.74, 95% CI 1.13–2.57, P = 0.01). However, a decreased SIR was observed for uterine cancer (0.45, 95% CI 0.16–0.98, P = 0.04). An increased SIR was observed among male OCD patients for prostate (1.64, 95% CI 1.11–2.35, P = 0.01), bladder (1.98, 95% CI 1.19–3.09, P = 0.01), and hematologic cancers (1.84, 95% CI 1.21–2.67, P = 0.01). Among female OCD patients, a decreased SIR was observed for uterine cancer (0.45, 95% CI 0.16–0.98, P = 0.04). The SIRs for specific types of cancer are presented in Table 3.
TABLE 3.
Standardized Incidence Ratios (SIRs) for Specific Cancer Types Among Patients With Obsessive Compulsive Disorder (Excluding Patients Whose Cancer Was Diagnosed Within First Year of OCD Diagnosis)

DISCUSSION
According to our thorough review of relevant research, this is the first large population-based study to evaluate the risk of cancer among patients with OCD. This study analyzed 52,656 patients followed up for 259,945 person-years. The data showed no increase in overall cancer risk among OCD patients relative to the general population. However, an increased SIR was observed among OCD patients aged less than 19 years (SIR = 2.68) and within the first year of OCD diagnosis (SIR = 1.21).
This study used unbiased patient selection and performed SIR estimation; the patients were matched according to calendar period, age, and sex. Because participation in the NHI is mandatory and offered to all residents of Taiwan, and copayments are low, referral bias was not present and the follow-up was complete. Patients with a certificate of catastrophic illness are exempted from related medical expenses, in particular, hospital costs. To apply for a catastrophic illness certificate for cancer, pathologic proof of malignancy is required, and laboratory and imaging studies must be provided. Therefore, the cancer diagnoses analyzed in this study were reliable and thorough.
Numerous studies have investigated the association between anxiety and subsequent cancer risk.17–19 A study in Taiwan reported that patients with anxiety exhibited a higher risk for prostate cancer and a marginally lower risk for cervical cancer.17 In addition, a UK study revealed that the anxiety patients appeared to be at a increased risk for lung and brain cancer.18 Moreover, a prospective large-scale study in Finland, with a 6 to 9-year follow-up, suggested that anxiety was not a risk factor for breast cancer.19 To sum up, there is no general consensus on the association between anxiety and the subsequent cancer occurrence. The inconsistency in the results from the above-mentioned studies may be attributed to the varying definitions of anxiety and the different inclusion criteria used. For example, the Taiwan study included patients with all types of anxiety disorders defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision and only anxiety patients who had been admitted for at least 3 times were included. The inclusion criteria may have caused a tendency for including only patients with severe anxiety disorders. Similarly, the UK study might have tended to include severely anxious patients, because it also included only those having been admitted for anxiety disorders according to the ICD systems, including ICD-7, ICD-8, ICD-9, and ICD-10 codes for anxiety disorders. Therefore, different ICD systems and numerous ICD codes were used to define anxiety disorders in this study. Moreover, in the Finland study, the Spielberger State-Trait Anxiety Inventory, rather than psychiatrist diagnosis, was used to evaluate anxiety.19 Thus, the diversity of results among these studies might be attributed to the different definitions of anxiety and inclusion criteria used and the fact that anxiety disorders comprise a relatively heterogeneous group of clinical conditions.
It has been hypothesized that psychological factors, including stress-related anxiety, may impair immune and endocrine functions,4,24,25 and that the impaired immune and endocrine function might play an essential role in specific stages of cancer development and in the pathogenesis of certain cancer types.26–29 However, the present study suggested that the overall cancer risk among OCD patients was not higher than that among general population (SIR = 1.05, 95% CI 0.98–1.13). There are 2 explanations for the possibility of false-negative finding. First, carcinogenesis is a multistage process, and second, a latency period may separate a potential carcinogenic agent and the appearance of a cancer. The phenomenon of increasing cancer incidence rates with age might further enforce these arguments.30 Although the onset of OCD typically occurs during childhood or early adulthood31which implicated that the population of OCD would have more opportunity to live long enough to see the cancer fully develop, the median follow-up period in the current study was 4.9 years, which may have been insufficient for detecting an increased risk of certain malignancies, especially cancers with long latency periods.
Among the patients with OCD, the cancer incidence significantly increased only within the first year after the diagnosis of OCD, with an SIR of 1.21. The most reasonable explanations for this result are surveillance bias32 and that patients with paraneoplastic syndrome might be misdiagnosed with OCD initially. Patients with OCD are likely to have more outpatient visits than the general population, leading to an earlier detection of undiagnosed cancers. In addition, the close temporal relationship demonstrated in this study between OCD diagnosis and newly diagnosed cancer may support the argument that obsessive-compulsive behaviors are a paraneoplastic manifestation among patients with an undiagnosed malignancy.33–36 While excluding data from OCD patients with cancer development within the first-year follow-up, the result found no statistically significant difference in cancer risk between the patient with OCD and the general population (SIR = 1.02, 95% CI 0.94–1.11).
In our study, an increased SIR was observed among OCD patients aged less than 19 years (SIR = 2.68). Previous studies have shown that testicular teratoma and anti-Ma2 antibodies were associated with OCD, and have suggested that these were causative, based on data indicating the involvement of the limbic region in OCD.36,37 Because testicular teratoma is the most common malignancy in male adolescents and young adults,38 it is reasonable to conclude that the increased SIR among OCD patients aged less than 19 years may be attributed to paraneoplastic manifestation.
After excluding patients whose cancer was diagnosed within the first year of OCD diagnosis, a higher risk of prostate (1.64, 95% CI 1.11–2.35, P = 0.01), bladder (1.98, 95% CI 1.19–3.09, P = 0.01), and hematologic cancers (1.84, 95% CI 1.21–2.67, P = 0.01) was noted among male OCD patients. By contrast, a lower risk of uterine cancer (0.45, 95% CI 0.16–0.98, P = 0.04) was noted among female OCD patients. An increased risk of prostate cancer among patients with anxiety disorder was previously reported; however, this may be attributed mainly to surveillance bias, and it was observed that a higher proportion of patients with anxiety disorder underwent a prostate-specific antigen test.17,22 However, that OCD increased the risk of bladder and hematologic cancers, and reduced the risk of uterine cancer, has not been previously reported. Our findings require confirmation by further studies, because when taking into account multiple comparisons, the P values of the results presented in this study did not indicate statistical significance (a P value equal to or less than 0.05 divided by 22, approximately 0.002, was considered significant when adjusting using the Bonferroni correction). Thus, the values determined in this study may be chance findings.
In our study, patients with a newly diagnosed cancer within the first year of OCD diagnosis were excluded from our original analysis for the concerns of detection bias and bias caused by paraneoplastic syndrome. However, based on the fact that the final numbers of cases in each cancer category are relatively small and an increased SIR was indeed observed within the first year of OCD diagnosis (Table 2), it is appropriate to perform the same analysis using all cancer cases without follow-up year stratification. As shown in Table 4, the results of the further analysis are consistent with original one in our study, showing no increase in overall cancer risk among OCD patients relative to the general population and the increased SIRs of specific types of cancer were considered to lack statistical significance after using Bonferroni correction.
TABLE 4.
Standardized Incidence Ratios (SIRs) for Specific Cancer Types Among Patients With Obsessive Compulsive Disorder
There are limitations to our findings. First, information regarding the family history of cancer, environmental exposures, diet, cigarette smoking, and alcohol use is not included in the NHIRD. Second, the NHIRD does not specify how diagnostic classifications were performed. Therefore, the diagnostic accuracy of OCD in our study could not be ascertained. Additional studies of OCD patients who were diagnosed through structured interviews should be conducted. Third, OCD severity was not considered in this study, and whether severity influences the risk of developing cancer warrants further study. Finally, the duration of the follow-up period in this study may have been insufficient for detecting the carcinogenesis of certain types of cancer. Thus, future studies with longer follow-up periods are required to determine the long-term risk of cancer among OCD patients.
CONCLUSIONS
In conclusion, this population-based retrospective cohort study observed no increase of overall cancer risk among patients with OCD compared with the general population. An increased cancer risk in the first year after the diagnosis of OCD among OCD patients may be attributed to surveillance bias and the paraneoplastic manifestation of certain cancers. Risks of prostate, bladder, and hematologic cancers were specifically determined to have increased among male OCD patients, whereas the risk of uterine cancer among female OCD patients was observed to be reduced. Additional large, unbiased population-based prospective studies with longer follow-up periods are required to investigate the association between OCD and cancer risk.
Acknowledgments
We thank the Research Center of Medical informatics, Kaohsiung Veterans General Hospital for their technical support, and the Economy Company Ltd for their academic English editing service.
Footnotes
Abbreviations: CI = confidence interval, GAD = generalized anxiety disorder, ICD-9-CM = the International Classification of Diseases, ninth revision, Clinical Modification, IQR = interquartile range, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = the National Health Research Institutes, OCD = obsessive-compulsive disorder, SIRs = standardized incidence ratios, UK = the United Kingdom.
The study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
L-YH and C-CS contributed equally to this manuscript.
The study is supported by a grant from Taipei Veterans General Hospital (V101D-001-2), a grant awarded for 2015 (104B-001) from Yuanshan and Suao Branch of Taipei Veterans General Hospital and grants from Kaohsiung Veterans General Hospital (VGHKS15-EM4-01).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602. [DOI] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres AR, Lima MC. Epidemiology of obsessive-compulsive disorder: a review. Rev Bras Psiquiatr 2005; 27:237–242. [DOI] [PubMed] [Google Scholar]
- 4.Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep 2012; 14:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gururaj GP, Math SB, Reddy JY, et al. Family burden, quality of life and disability in obsessive compulsive disorder: an Indian perspective. J Postgrad Med 2008; 54:91–97. [DOI] [PubMed] [Google Scholar]
- 6.Subramaniam M, Soh P, Vaingankar JA, et al. Quality of life in obsessive-compulsive disorder: impact of the disorder and of treatment. CNS Drugs 2013; 27:367–383. [DOI] [PubMed] [Google Scholar]
- 7.Fontenelle IS, Fontenelle LF, Borges MC, et al. Quality of life and symptom dimensions of patients with obsessive-compulsive disorder. Psychiatry Res 2010; 179:198–203. [DOI] [PubMed] [Google Scholar]
- 8.Bobes J, Gonzalez MP, Bascaran MT, et al. Quality of life and disability in patients with obsessive-compulsive disorder. Eur Psychiatry 2001; 16:239–245. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga H. Clinical features, treatments and outcome of obsessive-compulsive disorder (OCD) focusing on the assessment and characteristics of patients with treatment-refractory OCD. Seishin Shinkeigaku Zasshi 2013; 115:967–974. [PubMed] [Google Scholar]
- 10.Hamer M, Chida Y, Molloy GJ. Psychological distress and cancer mortality. J Psychosom Res 2009; 66:255–258. [DOI] [PubMed] [Google Scholar]
- 11.Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008; 5:466–475. [DOI] [PubMed] [Google Scholar]
- 12.Schraub S, Sancho-Garnier H, Velten M. Should psychological events be considered cancer risk factors? Rev Epidemiol Sante Publique 2009; 57:113–123. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz S, Messerschmidt H, Doren M. Psychosocial risk factors for cancer development. Med Klin (Munich) 2007; 102:967–979. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen NR, Gronbaek M. Stress and breast cancer: a systematic update on the current knowledge. Nat Clin Pract Oncol 2006; 3:612–620. [DOI] [PubMed] [Google Scholar]
- 15.Kruk J, Aboul-Enein HY. Psychological stress and the risk of breast cancer: a case-control study. Cancer Detect Prev 2004; 28:399–408. [DOI] [PubMed] [Google Scholar]
- 16.Lillberg K, Verkasalo PK, Kaprio J, et al. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol 2003; 157:415–423. [DOI] [PubMed] [Google Scholar]
- 17.Liang JA, Sun LM, Su KP, et al. A nationwide population-based cohort study: will anxiety disorders increase subsequent cancer risk? PLoS One 2012; 7:e36370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldacre MJ, Wotton CJ, Yeates D, et al. Cancer in people with depression or anxiety: record-linkage study. Soc Psychiatry Psychiatr Epidemiol 2007; 42:683–689. [DOI] [PubMed] [Google Scholar]
- 19.Aro AR, De Koning HJ, Schreck M, et al. Psychological risk factors of incidence of breast cancer: a prospective cohort study in Finland. Psychol Med 2005; 35:1515–1521. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lin HC. Increased risk of cancer subsequent to severe depression: a nationwide population-based study. J Affect Disord 2011; 131:200–206. [DOI] [PubMed] [Google Scholar]
- 21.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control 2010; 21:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen CC, Hu YW, Hu LY, et al. The risk of cancer in patients with generalized anxiety disorder: a nationwide population-based study. PLoS One 2013; 8:e57399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012; 308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 24.Reiche EM, Morimoto HK, Nunes SM. Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry 2005; 17:515–527. [DOI] [PubMed] [Google Scholar]
- 25.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004; 5:617–625. [DOI] [PubMed] [Google Scholar]
- 26.Yang EV, Glaser R. Stress-induced immunomodulation: implications for tumorigenesis. Brain Behav Immun 2003; 17 Suppl 1:S37–S40. [DOI] [PubMed] [Google Scholar]
- 27.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun 2003; 17:321–328. [DOI] [PubMed] [Google Scholar]
- 28.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 2006; 12:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006; 6:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer 2004; 91:1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire JF, Lewin AB, Horng B, et al. The nature, assessment, and treatment of obsessive-compulsive disorder. Postgrad Med 2012; 124:152–165. [DOI] [PubMed] [Google Scholar]
- 32.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA 2011; 305:2462–2463. [DOI] [PubMed] [Google Scholar]
- 33.Jaeckle KA. Paraneoplastic neurologic diseases. Cancer of the Nervous System. 1996; New York: Churchill Livingstone, 361–372. [Google Scholar]
- 34.Kayser MS, Kohler CG, Dalmau J. Psychiatric manifestations of paraneoplastic disorders. Am J Psychiatry 2010; 167:1039–1050. [DOI] [PubMed] [Google Scholar]
- 35.Muehlschlegel S, Okun MS, Foote KD, et al. Paraneoplastic chorea with leukoencephalopathy presenting with obsessive-compulsive and behavioral disorder. Mov Disord 2005; 20:1523–1527. [DOI] [PubMed] [Google Scholar]
- 36.Scheid R, Voltz R, Guthke T, et al. Neuropsychiatric findings in anti-Ma2-positive paraneoplastic limbic encephalitis. Neurology 2003; 61:1159–1161. [DOI] [PubMed] [Google Scholar]
- 37.Breiter HC, Rauch SL, Kwong KK, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53:595–606. [DOI] [PubMed] [Google Scholar]
- 38.Chieffi P, Chieffi S. Molecular biomarkers as potential targets for therapeutic strategies in human testicular germ cell tumors: an overview. J Cell Physiol 2013; 228:1641–1646. [DOI] [PubMed] [Google Scholar]


