Abstract
To retrospectively investigate whether background parenchymal enhancement (BPE) of the contralateral breast on preoperative dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is associated with therapeutic outcomes following neoadjuvant chemotherapy (NAC) in unilateral invasive breast cancer.
The institutional review board approved this study, and informed consent was waived. Between 2009 and 2011, 93 women with unilateral invasive breast cancer (43 premenopausal women who performed pre-NAC MRI between days 7 and 20 of the menstrual cycle and 50 postmenopausal women) underwent NAC with pre- and post-NAC DCE-MRI before surgery. MRI features (BPE [minimal, mild, moderate, marked] of the contralateral breast, lesion size and number, lesion kinetics, and changes in lesion size) and clinicopathologic features were analyzed. Patients were grouped according to BPE category (high [moderate or marked] or low [minimal or mild]). Cox regression modeling was used to determine associations between MRI features and recurrence-free survival (RFS) after controlling for clinicopathologic variables.
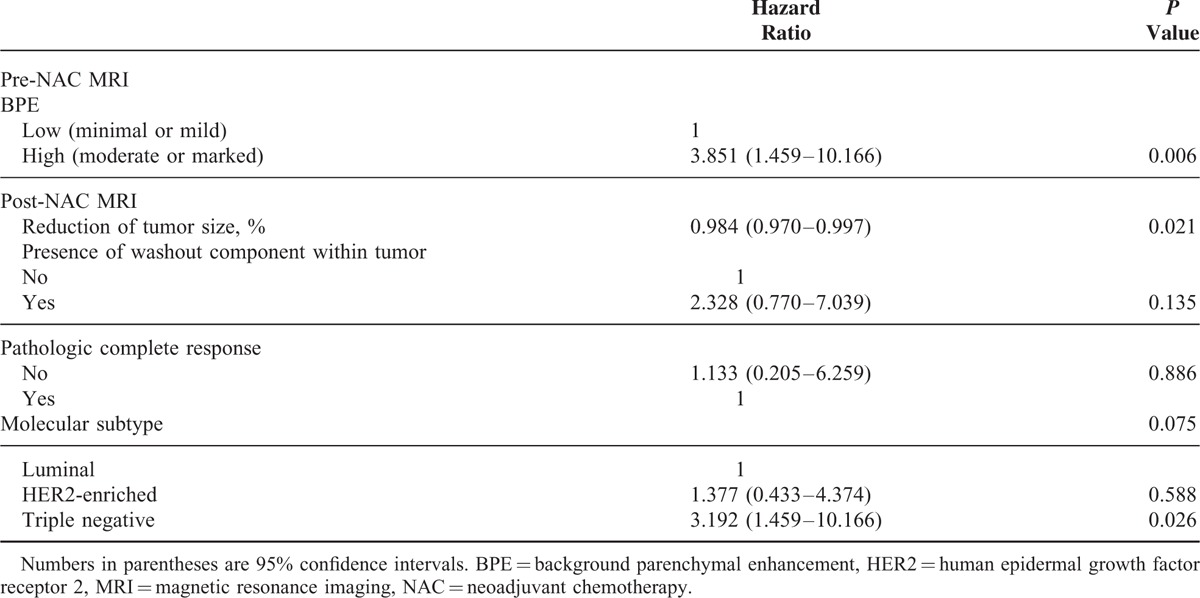
The mean follow-up period was 48.2 months. Twenty-three recurrences occurred (2 ipsilateral breasts, 6 regional, and 15 distant). On multivariate analysis, high BPE on pre-NAC MRI (hazard ratio [HR] = 3.851, P = 0.006) and triple-negative cancer (HR = 3.192, P = 0.002) were independent factors associated with worse RFS. A greater reduction of lesion size on post-NAC MRI (HR = 0.984, P = 0.021) was associated with better RFS.
High BPE on pre-NAC MRI is significantly associated with worse RFS in an NAC setting. This study suggests that BPE on pre-NAC DCE-MRI may have potential as a predictor of long-term outcomes in breast cancer patients who undergo NAC.
INTRODUCTION
Neoadjuvant chemotherapy (NAC) has been widely used for patients with locally advanced breast cancers because it renders primarily inoperable tumors operable and allows more patients to undergo breast-conserving surgery instead of mastectomy.1–3 In addition to tumor control, NAC enables the in-vivo assessment of the efficacy of standard and investigational therapeutic agents and could expedite the clinical development and approval of targeted treatments for early breast cancer.4,5 The heterogeneity of breast cancer, however, results in variable responses to NAC.6,7 Pathologic complete response (pCR), which has been used as a surrogate endpoint that can predict long-term clinical outcome,8–10 is achieved in less than 30% of patients treated with NAC.11 On the other hand, patients who have residual disease at surgical pathology represent a high-risk population due to the poorer outcomes compared with patients who achieve pCR.8–10 For these patients, further investigational therapy can be considered according to the residual tumor burden. Although surgical pathologic information has been used as a prognostic indicator or a guide for further treatment after surgery, this cannot be assessed before the surgical removal of the tumor. Pretreatment prediction of the pathologic responses or long-term outcomes of patients scheduled to receive NAC could enable the development of personalized treatment protocols, adjusting NAC regimens or surgical procedures, thus improving the long-term outcomes of individual patients.
Imaging techniques have been used to identify imaging biomarkers that predict the development and prognosis of breast cancers. Mammographic breast density is well known to correlate with the risk of developing breast cancer,12 although it is not related to the survival of breast cancer.13 Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a commonly used imaging technique in breast cancer research that allows assessment of the functional behavior of the tumor and parenchyma.14 It is well established that normal fibroglandular tissue can show enhancement on DCE-MRI, which is termed background parenchymal enhancement (BPE). Analogous to the mammographic breast density, recent studies have shown that a higher BPE on screening MRI is associated with a higher probability of developing breast cancer.15,16 Many previous studies have demonstrated DCE-MRI parameters obtained prior to NAC and/or early (after the 1st or 2nd NAC cycles) during NAC and survival outcomes.17–20 However, these studies have focused on the tumors themselves or on tumor-induced changes in the tissue surrounding breast tumors. It remains unknown whether BPE on preoperative DCE-MRI, performed prior to and/or post NAC treatment, is associated with the therapeutic outcome in patients treated with NAC.
Therefore, we hypothesized that BPE of the contralateral healthy breast is comparable to that of the ipsilateral breast before cancer development.21,22 The purpose of this study was to retrospectively investigate whether BPE of the contralateral normal breast on preoperative DCE-MRI is associated with the therapeutic outcome in patients with unilateral invasive breast cancer who undergo NAC.
MATERIALS AND METHODS
Patients and Treatments
The institutional review board approved this retrospective study, and informed consent was waived. Between June 2009 and July 2011, 203 consecutive women with invasive breast cancers underwent NAC and subsequent curative breast surgery at our institution. We excluded 4 women with bilateral breast cancers and 34 women who did not undergo MRI prior to NAC. It is well known that BPE in premenopausal woman is affected by the menstrual cycle.23 To minimize the hormonal effects, we further excluded 51 premenopausal women who underwent pre-NAC MRI during the 1st or 4th week of the menstrual cycle and 21 premenopausal women who did not have information about the menstrual cycle on the medical record. Finally, 93 women (mean age 47.2 years, range 25–69 years) with unilateral invasive breast cancers (89 invasive ductal carcinomas, 2 invasive lobular carcinomas, 1 metaplastic carcinoma, and 1 mucinous carcinoma) were included in this study.
The NAC regimens of the 93 study patients were as follows: adriamycin with cyclophosphamide plus docetaxel (n = 51), adriamycin with cyclophosphamide (n = 12), adriamycin with docetaxel (n = 9), and human epidermal growth factor receptor 2 (HER2) targeted agent-based regimens (n = 21). The HER2 targeted agent-based regimens included adriamycin with cyclophosphamide plus docetaxel with trastuzumab, docetaxel with either trastuzumab or pertuzumab, and trastuzumab with pertuzumab. The median number of NAC cycles was 8 (range, 4–8 cycles). All patients underwent both pre-NAC and post-NAC MRI, performed after completion of NAC, as well as curative surgery for breast cancer (63 patients received breast-conserving surgery, 30 received total mastectomy). The median interval between post-NAC MRI and surgery was 15 days (range, 3–36 days). All patients who underwent breast-conserving surgery were treated with adjuvant radiation therapy. Adjuvant endocrine therapy had been recommended for patients with hormone receptor-positive cancers.
MRI Evaluation
All preoperative MRIs were performed using a 1.5-T Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a dedicated phased array breast coil, with the patient in the prone position. The routine MRI protocol included turbo spin-echo T1- and T2-weighted sequences and a 3-dimensional DCE sequence. All images were acquired with bilateral axial views. DCE-MRI included 1 precontrast and 6 postcontrast series. A 0.1-mmol/kg bolus of gadobutrol (Gadovist; Bayer Healthcare, Berlin, Germany) was injected into an antecubital vein, followed by a 20-mL saline flush. Postcontrast images were obtained at 30, 90, 150, 210, 270, and 330 seconds after the injection of contrast material. The DCE timing was the center of the k-space acquisition, and the acquisition time of each postcontrast series was 63 seconds. Early subtraction images and 3-dimensional maximum-intensity projection images were generated. The parameters for DCE-MRI were as follows: repetition time/echo time (msec), 6.5/2.5; slice thickness, 1.5 mm; flip angle, 10°; matrix size, 376 × 374; and field of view, 32 × 32 cm.
All pre-NAC and post-NAC MR images were retrospectively assessed by 2 radiologists in consensus (with 6 and 9 years of experience in breast MRI, respectively). To minimize bias, the radiologists were blinded to the clinicopathologic information, including tumor recurrence during follow-up, menopausal status, phase of the menstrual cycle, and hormone receptor status, although they were made aware that the patients had a diagnosis of invasive breast cancer. Tumor size, corresponding to the maximal diameter of the tumor, was measured on early postcontrast images (90 seconds after contrast material injection). Reduction of the tumor size on post-NAC MRI was calculated as follows:
(tumor sizepre-NAC − tumor sizepost-NAC) × 100/tumor sizepre-NAC
To assess tumor kinetics, all of the DCE-MRI series were used. The type of tumor kinetics was categorized as persistent, plateau, or washout based on the images obtained in the delayed enhancement phase.24 When there were multiple tumors on pre-NAC MRI, the radiologists evaluated the MRI features of the largest tumor. Qualitative BPE was assessed according to the Breast Imaging-Reporting and Data System (BI-RADS) categories as follows: minimal, mild, moderate, or marked.24 BPE was determined on the basis of both the volume and intensity of enhancement by using a combination of precontrast and early postcontrast DCE images (90 seconds after contrast injection), subtraction images, and maximum-intensity projection images.16,25,26 BPE categories were determined for pre-NAC and post-NAC MR images, respectively.
Histopathologic Analysis
Final histopathological results of surgical or core needle biopsy specimens were reviewed to determine the number and size of invasive and carcinoma in situ components of tumors, as well as histologic grade, presence of lymphovascular invasion, and molecular subtype determined by the immunohistochemical status (estrogen receptor [ER], progesterone receptor [PR], and HER2). In our study, we defined pCR as the absence of invasive cancer, irrespective of ductal carcinoma in situ, in the breast and axillary nodes. In cases of pCR, the expression status of ER, PR, and HER2 was determined based on the core needle biopsy specimens obtained before NAC. ER and PR positivity were determined using a cutoff value of >1% positively stained nuclei.27 HER2 staging was scored as 0, 1+, 2+, and 3+, according to the guidelines of the American Society of Clinical Oncology/College of American Pathologists.28 Tumors scored as 2+ required further examination using silver-enhanced in situ hybridization to measure HER2 amplification. An HER2 gene/chromosome 17 ratio of greater than 2.2 was considered HER2-positive by silver-enhanced in situ hybridization.
Data and Statistical Analysis
Clinicopathologic data (age, menopausal status, mammographic density, clinical tumor and nodal stages, pathologic response to NAC, immunohistochemical subtype, histologic grade, surgical margin status, and adjuvant therapy) were collected through review of medical records. MRI findings (BPE of the contralateral breast, size and number of tumors on pre-NAC MRI/BPE of the contralateral breast, and tumor size, presence of a washout kinetic component within the tumor on post-NAC MRI) and clinicopathologic variables were compared using the Mann–Whitney U-test, the Chi-square test, or Fisher exact test, according to the breast cancer recurrence event of the patients.
The therapeutic outcomes were recurrence-free survival (RFS) and overall survival (OS), which were assessed by the Kaplan–Meier method and compared by the log-rank test. Breast cancer recurrence included local recurrence (limited to the ipsilateral breast or chest wall and/or axillary, infraclavicular, or supraclavicular lymph nodes), contralateral cancer, and distant metastasis to the other parts of the body. OS was defined from the date of breast cancer diagnosis to the date of death. RFS was calculated from the date of NAC initiation to the date of breast cancer recurrence, the date of death, or the date on which the patient was last known to have no evidence of breast cancer recurrence.29 Patients without recurrence were censored at the date of the most recent follow-up, regardless of whether they were scheduled for future follow-up or whether they had been lost to follow-up.
Cox proportional hazards modeling was used to determine the associations between RFS and the variables. For continuous variables, the proportional hazard assumption and linear assumption were checked using restricted cubic splines. For categorical variables, the proportional hazard assumption was tested using the Cox proportional hazards model with time-varying coefficients. BPE results on pre-NAC MRI were grouped according to the category (low [minimal or mild] or high [moderate or marked]) for univariate and multivariate Cox regression analyses of RFS. Variables of BPE on post-NAC MRI and adjuvant endocrine therapy were not included in the univariate Cox regression analysis because all patients in both the recurrence and the recurrence-free groups showed a low BPE (90 minimal and 3 mild) on post-NAC MRI, and our study population included hormone-receptor-negative cancers, respectively. The variables with P values less than 0.05 in univariate analysis were included in a multivariate model. The stepwise method was applied to control collinearity and determine the final model.
All statistical analyses were performed with SPSS version 20.0 (IBM Corp., Armonk, NY). Statistical significance was determined with a P value less than 0.05.
RESULTS
Patients and Survival Outcomes
The median RFS was 49 months (interquartile range [IQR], 39–55 months), and the median OS was 51 months (IQR, 45–57 months) during the follow-up period (range, 9–68 months). There were 23 recurrences (15 distant/8 local [2 ipsilateral breast, 6 regional]) at a mean time-to-recurrence of 21.8 months. Among patients with recurrence, 2 died during management of their tumor recurrence, with OS values of 37 and 43 months. The clinicopathologic characteristics and MRI findings of the study population are shown in Table 1 . The proportions of BPE on pre-NAC MRI were significantly different between the recurrence group and the recurrence-free group. All patients with high BPE (4 marked and 13 moderate) and most patients with mild BPE (88%, 22/25) on pre-NAC MRI showed a reduction of BPE identical to the minimal category on post-NAC MRI in this study. All patients with minimal BPE and 3 patients with mild BPE on pre-NAC MRI showed no change of BPE category on post-NAC MRI. Consequently, all patients in both the recurrence and recurrence-free groups showed low BPE (90 minimal and 3 mild) on post-NAC MRI. The proportions of BPE on post-NAC MRI were not significantly different between the recurrence group and the recurrence-free group.
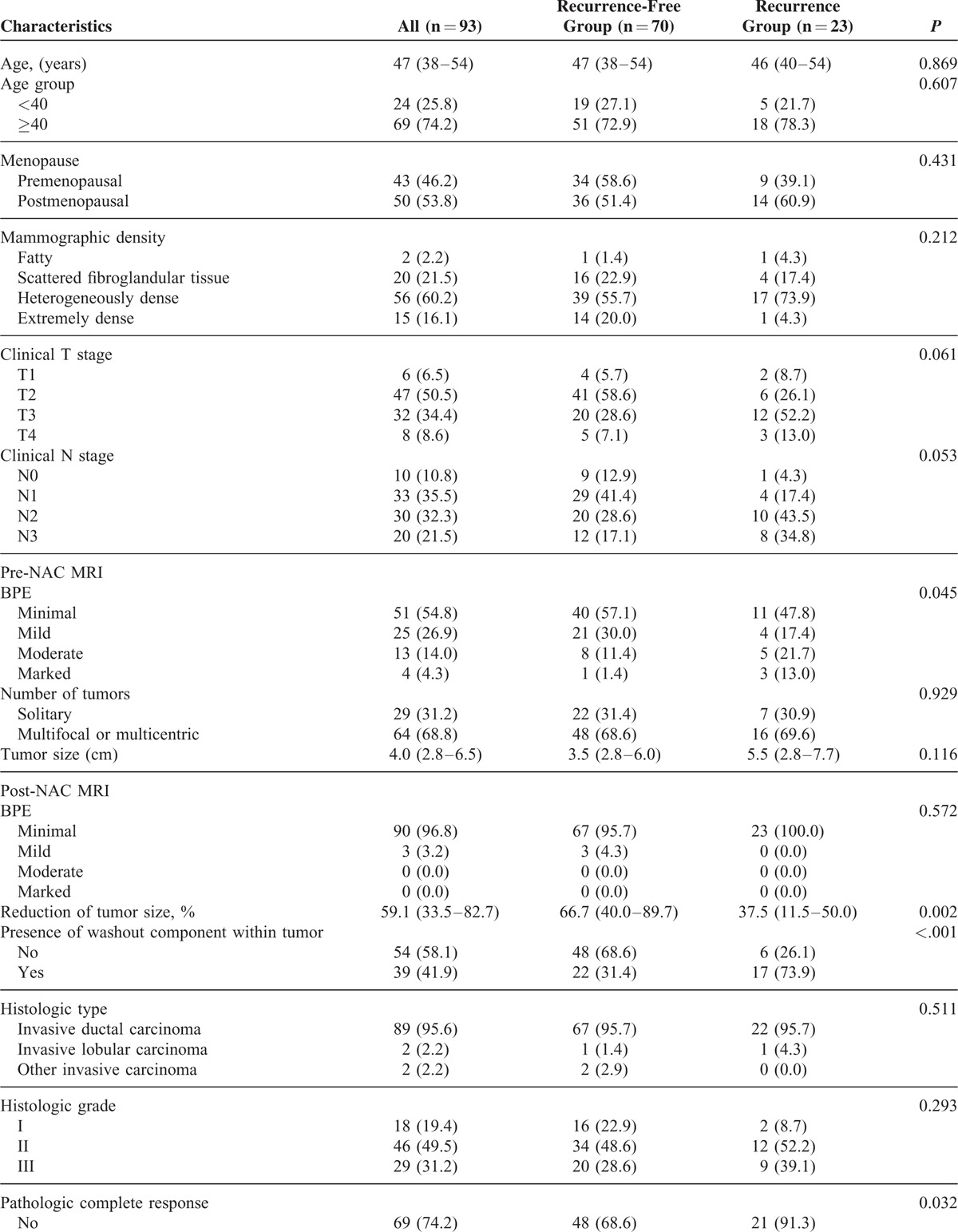
TABLE 1.
Characteristics of the Study Population
Association Between BPE and Survival Outcomes
In univariate analysis, high (moderate or marked) BPE on pre-NAC MRI was significantly associated with worse RFS in comparison to low (minimal or mild) BPE (hazard ratio [HR] = 2.769, P = 0.020). Reduction of tumor size, the presence of washout component within a tumor on post-NAC MRI, achievement of pCR, and the triple-negative cancer subtype also showed significant associations with RFS (P < 0.05). There were no significant associations between RFS and age, menopausal status, mammographic density, clinical T/N stage, size and number of the tumors on pre-NAC MRI, histologic grade, and surgical margin status (P > 0.05) (Table 2).
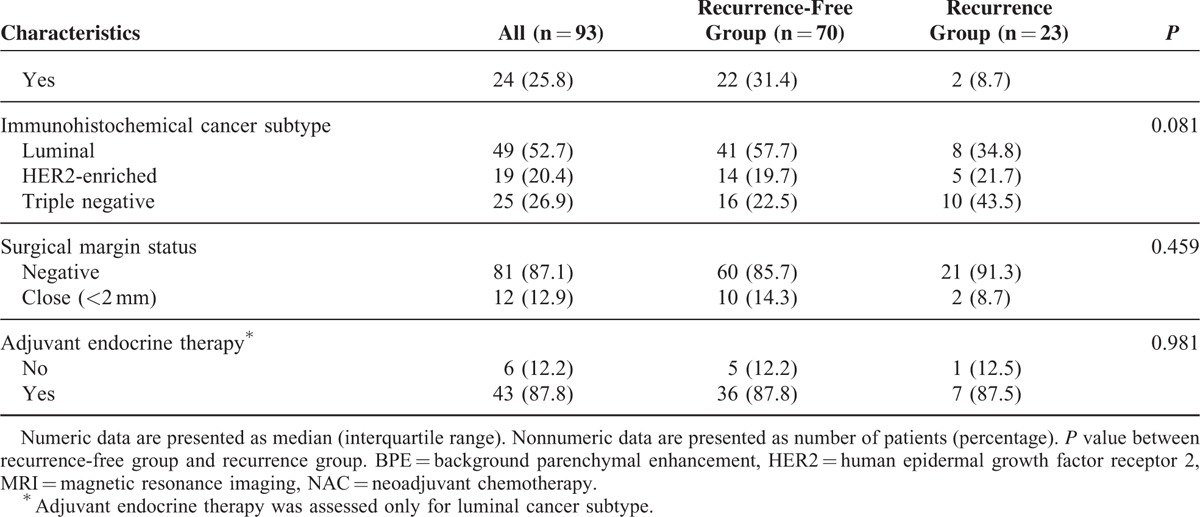
TABLE 1 (Continued).
Characteristics of the Study Population
Variables showing a significant association (P < 0.05) with RFS in univariate analyses were entered as input variables for the multivariate analysis (Table 3). In the multivariate analysis, high BPE (HR = 3.851, P = 0.006) was an independent variable associated with worse RFS compared with low BPE (Figure 1). In addition, the triple-negative cancer subtype (HR = 3.192, P = 0.026) was significantly associated with worse RFS, whereas a greater percentile reduction of tumor size was associated with better RFS (HR = 0.984, P = 0.021).
TABLE 2.
Univariate Analysis Between Variables and Recurrence-Free Survival of Breast Cancer Patients Receiving NAC
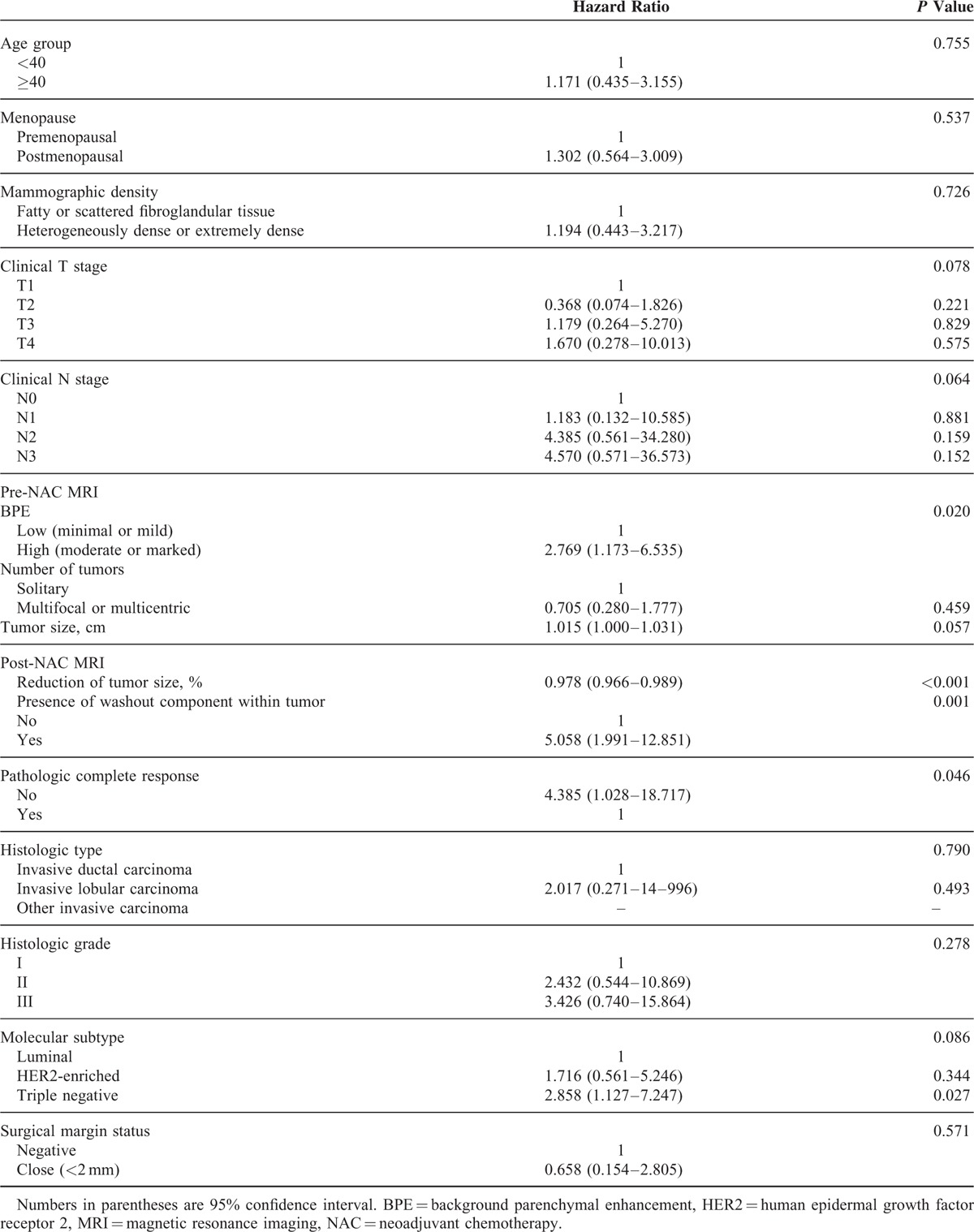
FIGURE 1.
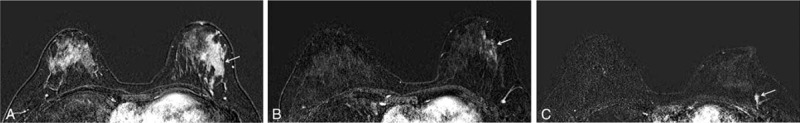
MR images of a 37-year-old woman with invasive ductal carcinoma whose recurrence occurred in the ipsilateral portion of the breast 17 months after NAC initiation. (A) Pre-NAC MR image acquired in the subtracted early postcontrast phase shows a marked BPE and an 8.7-cm nonmass enhancement involving the left lower outer breast (arrow). (B) Post-NAC MR image acquired in the subtracted early postcontrast phase shows that the BPE decreased to the minimal grade and that the nonmass enhancement also decreased in size and volume (arrow). Initial surgical histopathology findings yielded an HER2-enriched type of invasive ductal carcinoma 0.2 cm in size. Seventeen months later, a 1.2-cm linear nonmass enhancement was detected on a subtraction image of a screening MRI, (C) and it was confirmed to be a 0.8-cm ductal carcinoma in situ upon subsequent breast surgery. BPE = background parenchymal enhancement, MR = magnetic resonance, MRI = magnetic resonance imaging, NAC = neoadjuvant chemotherapy, HER2 = human epidermal growth factor receptor 2.
TABLE 3.
Multivariate Analysis Between Variables and Recurrence-Free Survival of Breast Cancer Patients Receiving NAC
Patients with high BPE on pre-NAC MRI had significantly worse 5-year RFS compared with patients with low BPE (high BPE 52.9% vs low BPE 79.6%, P = 0.015) (Figure 2). Survival analysis for OS was not performed because there were only 2 death events in our study population.
FIGURE 2.
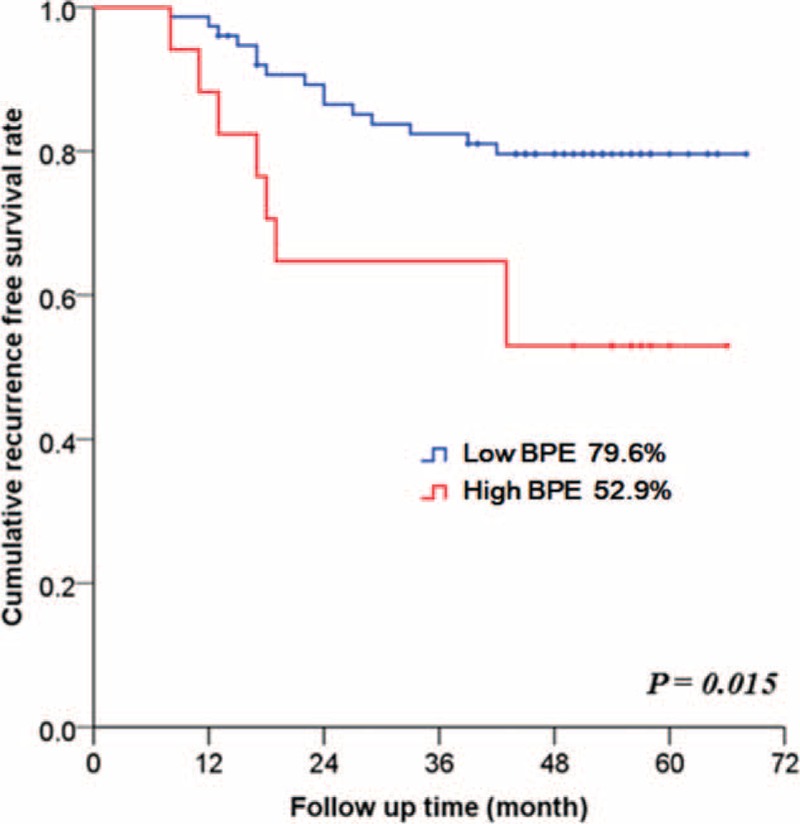
Five-year recurrence-free survival in patients who underwent NAC according to the BPE on pre-NAC magnetic resonance imaging (P = 0.015): high BPE (moderate or marked) versus low BPE (minimal or mild). The log-rank test was used to determine a P value. BPE = background parenchymal enhancement, NAC = neoadjuvant chemotherapy.
DISCUSSION
In an NAC setting, several other studies have found that parenchymal enhancement of normal-appearing tissue surrounding breast cancer was significantly associated with the response to NAC and the survival outcome.17,30 However, measurement of the parenchymal enhancement of the tissue surrounding breast cancer is strongly dependent on the selection of a representative image and the placement of the region of interest. On the other hand, the BPE of the contralateral normal breast can be measured if DCE-MRI is performed bilaterally on breast cancer patients. Compared to the BPE of the diseased breast, the BPE measured from the contralateral normal breast may be a more unbiased method that can reflect the relationship between normal tissue enhancement and treatment outcomes after NAC on the basis of breast symmetry.21,22 To our knowledge, this study is the first to report on the association between the BPE of healthy parenchyma and survival outcomes of breast cancer patients treated with NAC. We found that patients with a high BPE on pre-NAC MRI (HR = 3.851, P = 0.006) were significantly more likely to develop tumor recurrence after NAC than were patients with low BPE. Our findings indicate that high BPE of normal breast parenchyma may have the potential to predict a patient's long-term outcome after NAC treatment, as well as a woman's individual risk of developing breast cancer.15,16
BPE is related to the vascular supply and ovarian function, which is affected by endogenous hormone levels.23 To minimize the hormonal effect due to the menstrual cycle, we excluded premenopausal women who underwent pre-NAC MRI during the 1st or 4th week of the menstrual cycle from our study population, although recent studies on the association between BPE and the outcomes of breast cancer patients did not correct for the effects of the menstrual cycle.17,20–22,25 Even after adjustment for menstrual cycle effects on BPE, we found a significant association between high BPE on pre-NAC MRI and worse RFS. One possible explanation for this finding is that higher blood perfusion into the breast, expressed as higher BPE, may lead to a proangiogenic environment for tumor growth.31 Another possible explanation is that higher BPE may represent a larger amount of physiologically active breast tissue that is more vulnerable to malignant transformation, such as areas of increased inflammation that can aid in tumor progression and metastasis.32 In order to elucidate the precise mechanism contributing to the relationship between high BPE and worse survival outcomes, additional studies are needed.
In the current study, all patients of both recurrence and recurrence-free groups showed low BPE (90 minimal and 3 mild) on post-NAC MRI. Among these, all patients with a high BPE (4 marked and 13 moderate) and most patients with a mild BPE (88%, 22/25) on pre-NAC MRI showed an identical reduction of BPE to the minimal category on post-NAC MRI. Consequently, after NAC treatment, there was a greater reduction of the BPE in patients with a high BPE on pre-NAC MRI than in those with a low BPE. These findings are consistent with the results of a previous study in which a higher baseline BPE of pre/perimenopausal women was associated with a greater reduction of BPE on follow-up MRI after NAC.33 A reduction of BPE after NAC may be attributed to the effects of chemotherapy agents, which can induce ovarian suppression resulting in a loss of tissue proliferation and can directly damage the vessels in normal tissue.21,33–35 Recent studies have reported that the degree of BPE reduction after NAC seemed to correlate with the tumor response to NAC.21,36 As these studies reported, the BPE reduction may represent the effects of chemotherapy agents, which may be associated with the short-term outcome, such as pCR, in patients who received NAC. However, BPE reduction after NAC may be the natural consequence of a high BPE on pre-NAC MRI rather than a prognostic indicator, considering our finding that a high BPE on pre-NAC MRI was associated with a greater reduction of the BPE on post-NAC MRI than in those with a low BPE. Therefore, we think that future studies will be needed to verify the association between the BPE reduction after NAC and the survival outcome.
As for tumor characteristics, triple-negative cancer was significantly associated with a worse RFS in a multivariate analysis of this study (P = 0.026), compared to luminal type cancer. It is well known that RFS and OS in triple-negative cancers are significantly worse than in cancers of other molecular subtypes.37 On the other hand, achievement of pCR has been proposed as a surrogate endpoint that can predict the survival outcomes of patients treated with NAC.10 However, achievement of pCR was associated with better RFS in a univariate analysis but did not retain significance in a multivariate analysis in this study. In fact, some studies have failed to show that pCR predicts long-term clinical outcomes,10,38 although other studies have shown that pCR is associated with significant improvement in survival outcomes.8–10 This discrepancy can be explained by the different definitions of pCR used in different studies. Although a recent study showed that patients who attain pCR defined as “absence of invasive cancer in the breast and axillary nodes, irrespective of in situ residuals” had better disease-free survival than patients with pCR defined as tumor eradication from the breast alone,38 further study is needed to validate the utility of pCR in predicting the long-term outcomes of patients treated with NAC.
This study had several limitations. First, our study population size is relatively small, and our population included only a small number of patients with marked BPE on pre-NAC MRI (n = 4). Therefore, we combined the patients with moderate and marked BPE into 1 group for statistical analysis. In order to clarify the association between BPE and survival outcomes, further studies with a larger number of patients will be needed. Second, we did not perform a quantitative measurement of the BPE to determine objective BPE levels. However, published studies that have quantitatively measured BPE used different MRI hardware and parameters between institutions, which restricts the generalized use of quantitative methods in clinical practice. In contrast, we performed qualitative measurement of the BPE based on 4 categories, and therefore minimal differences in the BPE may not be reflected in our results. Nonetheless, we found a significant association between qualitative measurements of BPE on pre-NAC MRI and RFS after NAC. On the other hand, qualitative measurement of the BPE may be prone to interobserver variability. However, there was no significant discrepancy between the 2 reviewers for determination of BPE category in our study, because they are accustomed to assess BPE category according to BI-RADS in routine clinical practice. Considering our results, qualitative measurement of BPE may be applicable to decide the follow-up interval or imaging modality for postoperative surveillance of patients who undergo NAC. Finally, this study was performed in a single institution and included only Asian population. Further studies including other populations need to be conducted.
In conclusion, a high BPE of the contralateral breast is significantly associated with worse RFS in patients with unilateral invasive breast cancer who underwent NAC. This study suggests that BPE on pre-NAC DCE-MRI may have potential as a predictor for long-term outcomes in breast cancer patients who undergo NAC.
Footnotes
Abbreviations: BPE = background parenchymal enhancement, DCE-MRI = dynamic contrast-enhanced magnetic resonance imaging, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, NAC = neoadjuvant chemotherapy, OS = overall survival, pCR = pathologic complete response, PR = progesterone receptor, RFS = recurrence-free survival.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998; 16:93–100. [DOI] [PubMed] [Google Scholar]
- 2.Calais G, Berger C, Descamps P, et al. Conservative treatment feasibility with induction chemotherapy, surgery, and radiotherapy for patients with breast carcinoma larger than 3 cm. Cancer 1994; 74:1283–1288. [DOI] [PubMed] [Google Scholar]
- 3.Powles TJ, Hickish TF, Makris A, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol 1995; 13:547–552. [DOI] [PubMed] [Google Scholar]
- 4.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 2012; 366:2438–2441. [DOI] [PubMed] [Google Scholar]
- 5.Wolff AC, Berry D, Carey LA, et al. Research issues affecting preoperative systemic therapy for operable breast cancer. J Clin Oncol 2008; 26:806–813. [DOI] [PubMed] [Google Scholar]
- 6.Abrial SC, Penault-Llorca F, Delva R, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat 2005; 94:255–263. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998; 16:2672–2685. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008; 26:778–785. [DOI] [PubMed] [Google Scholar]
- 10.Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015; 66:31–48. [DOI] [PubMed] [Google Scholar]
- 11.Jones RL, Smith IE. Neoadjuvant treatment for early-stage breast cancer: opportunities to assess tumour response. Lancet Oncol 2006; 7:869–874. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Martin LJ, Bronskill M, et al. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 2010; 102:1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst 2012; 104:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha S, Sinha U. Recent advances in breast MRI and MRS. NMR Biomed 2009; 22:3–16. [DOI] [PubMed] [Google Scholar]
- 15.Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 2015; 276:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011; 260:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones EF, Sinha SP, Newitt DC, et al. MRI enhancement in stromal tissue surrounding breast tumors: association with recurrence free survival following neoadjuvant chemotherapy. PLoS One 2013; 8:e61969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SP, Makris A, Beresford MJ, et al. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology 2011; 260:68–78. [DOI] [PubMed] [Google Scholar]
- 19.Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184:1774–1781. [DOI] [PubMed] [Google Scholar]
- 20.Yi A, Cho N, Im SA, et al. Survival outcomes of breast cancer patients who receive neoadjuvant chemotherapy: association with dynamic contrast-enhanced MR imaging with computer-aided evaluation. Radiology 2013; 268:662–672. [DOI] [PubMed] [Google Scholar]
- 21.Chen JH, Yu HJ, Hsu C, et al. Background parenchymal enhancement of the contralateral normal breast: association with tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Transl Oncol 2015; 8:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Velden BH, Dmitriev I, Loo CE, et al. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology 2015; 276:675–685. [DOI] [PubMed] [Google Scholar]
- 23.Giess CS, Yeh ED, Raza S, et al. Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 2014; 34:234–247. [DOI] [PubMed] [Google Scholar]
- 24.D'Orsi C, Sickles E, Mendelson E, Morris E. ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston, VA: Am Coll Radiol 2013. [Google Scholar]
- 25.Kim SA, Cho N, Ryu EB, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014; 270:699–707. [DOI] [PubMed] [Google Scholar]
- 26.Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am 2007; 45:863–880.vii. [DOI] [PubMed] [Google Scholar]
- 27.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25:118–145. [DOI] [PubMed] [Google Scholar]
- 29.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25:2127–2132. [DOI] [PubMed] [Google Scholar]
- 30.Hattangadi J, Park C, Rembert J, et al. Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. Am J Roentgenol 2008; 190:1630–1636. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333:1757–1763. [DOI] [PubMed] [Google Scholar]
- 32.Bhatelia K, Singh K, Singh R. TLRs: linking inflammation and breast cancer. Cell Signal 2014; 26:2350–2357. [DOI] [PubMed] [Google Scholar]
- 33.Chen JH, Yu H, Lin M, et al. Background parenchymal enhancement in the contralateral normal breast of patients undergoing neoadjuvant chemotherapy measured by DCE-MRI. Magn Reson Imaging 2013; 31:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller KD, Sweeney CJ, Sledge GW. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol 2001; 19:1195–1206. [DOI] [PubMed] [Google Scholar]
- 35.Swain S, Jeong J, Geyer C, et al. NSABP B-30: definitive analysis of patient outcome from a randomized trial evaluating different schedules and combinations of adjuvant therapy containing doxorubicin, docetaxel and cyclophosphamide in women with operable, node-positive breast cancer. Cancer Res 2009; 69 (2 Suppl):75.19117989 [Google Scholar]
- 36.Preibsch H, Wanner L, Bahrs S, et al. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: correlation with tumour response. Eur Radiol 2015; 1–7. [DOI] [PubMed] [Google Scholar]
- 37.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 38.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384:164–172. [DOI] [PubMed] [Google Scholar]


