Supplemental Digital Content is available in the text
Abstract
Ovarian clear cell carcinoma (CCC) is a distinct histologic subtype with relatively poor survival. No prognostic or predictive molecular marker is currently available. Recent studies have shown that AT-rich interactive domain 1A (ARID1A) and phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) mutations are common genetic changes in ovarian CCC. Hepatocyte nuclear factor-1β (HNF-1β) expression has been proven to be highly sensitive and specific for clear cell histology. However, the correlations between these biomarkers and clinicopathologic variables and survival outcomes are controversial.
The immunohistochemical analysis for HNF-1β, ARID1A, and PIK3CA was performed on a tissue microarray (TMA) consisting of 130 cases of ovarian CCC (237 tissue blocks) linked with clinical information. The immunostaining results were interpreted in a manner consistent with previous publications. The associations between biomarker expression and clinical and prognostic features were examined. All statistical analyses were conducted using 2-sided tests, and a value of P < 0.05 was considered significant.
HNF-1β was expressed in 92.8% of all primary ovarian tumors, while the loss of ARID1A and PIK3CA was noted in 56.2% and 45.0%, respectively. Early-stage tumors tended to have high levels of HNF-1β immunoreactivity and expression of ARID1A (P = 0.02 and P = 0.03). Most patients (76.9%, 20/26) with concurrent endometriosis stained negative for ARID1A (P = 0.02). No relation was found between PIK3CA expression and clinical features. Low-level HNF-1β expression and loss of ARID1A were more commonly observed in patients with tumor recurrence (P = 0.02 and P < 0.001). Antibody expression was not associated with platinum-based chemotherapy response. Patients with negative ARID1A expression had worse survival outcome in terms of both overall survival (OS) and progression-free survival (PFS) (P = 0.03 and P = 0.01, respectively). On the contrary, patients with high-level HNF-1β were associated with good prognosis (P = 0.02 for OS and P = 0.01 for PFS). PIK3CA expression had no impact on survival. For univariate and multivariate analyses, only HNF-1β expression seemed to be a prognostic factor for favorable OS (P = 0.04).
The loss of ARID1A was correlated with late-stage and endometriosis-associated tumors. The measurement of ARID1A expression might be a method to predict the risk of recurrence. Among the 3 biomarkers, only high-level HNF-1β expression proved to be a positive predictor for OS.
INTRODUCTION
The histologic subtypes of ovarian carcinoma are distinct diseases, each with different clinical and molecular features.1,2 Ovarian clear cell carcinoma (CCC) tends to be associated with endometriosis3 and is often diagnosed at early stages.4 Despite this, patients with ovarian CCC usually have lower rates of survival when compared with the more common serous counterpart.2,4 Therefore, the diagnosis and management of ovarian CCC is deemed as a great challenge and an area of unmet medical need. The absence of prognostic or predictive molecular markers might be central to this problem.
Hepatocyte nuclear factor-1β (HNF-1β) plays a role in the anti-apoptosis of ovarian CCC and is essential for tumor cell survival.5 The immunohistochemical expression of HNF-1β has been shown to be highly sensitive and specific for clear cell histology.6 Recently, AT-rich interactive domain 1A (ARID1A) was established as a tumor suppressor gene in a broad spectrum of cancers, including ovarian CCC.7 In addition to ARID1A, an activating mutation in phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) is a common genetic alteration in clear cell histology.8 Recent publications demonstrate that ARID1A and PIK3CA mutations might cooperate to promote tumor growth.9,10 However, studies are conflicting on the question of its prognostic significance in ovarian CCC.11–14 Currently, it is still unclear whether the molecular alterations of ARID1A are associated with clinicopathologic variables and survival outcome in ovarian CCC.
Most published studies focusing on ovarian CCC contain small sample sizes due to the rarity of the disease, especially in the western population. Our previous publications,3,4,15,16 based on our institutional database, illustrated the clinicopathologic features and treatment of ovarian CCC. To investigate the possible biomarkers underlying these distinct features, we constructed a CCC tissue microarray (TMA) for further analysis.
The present study had 2 main purposes. First, it aimed to evaluate the immunohistochemical expression of HNF-1β, AIRD1A, and PIK3CA in a cohort of ovarian CCC. Secondly, the possible association with clinicopathologic characteristics and survival outcomes were investigated.
METHODS
Study Population and Case Selection
This study was approved by the Institutional Review Board at Peking Union Medical College Hospital. By searching the Ovarian Clear Cell Carcinoma Database, we included all cases with retrievable pathology archives in our institution. Formalin-fixed, paraffin-embedded tissue specimens were obtained,17 including primary ovarian tumors, abdominal disseminated lesions, lymph node metastases, and recurrent tumors.
Data collection included age at diagnosis, International Federation of Gynecology and Obstetrics (FIGO) stage, serum cancer antigen 125 (CA 125) level, residual disease (optimal debulking ≤1 cm), adjuvant chemotherapy, date of disease progression or recurrence, and tumor status at the date of last contact. All patients with stage I and II ovarian CCC underwent complete staging surgery and patients with stage III and IV ovarian CCC received cytoreductive surgery. Adjuvant chemotherapy was routinely administered after primary surgery with few exceptions. The vast majority of the patients received platinum-based chemotherapy regimens, with the number of cycles ranging from 6 to 9. Progression-free survival (PFS) was defined as the time interval from the date of primary surgery to the date of disease progression or recurrence. Overall survival (OS) was defined as the time interval from the date of the primary surgery to the date of death or last contact. All patients provided informed consent before their inclusion in the study.
TMA Construction
Microscopic slides (hematoxylin and eosin, H&E) of selected cases were reviewed by the same gynecology-dedicated pathologist with profound clinical experience. A second senior pathologist was responsible for confirming the diagnosis, as well as marking the targeted areas on the H&E sections and the corresponding paraffin blocks. One-millimeter tissue cores taken from each donor block were transferred to the recipient block using a tissue arrayer (MiniCore, Alphelys, Plaisir, France). Two cores were obtained per block, providing a representation of 2 to 8 TMA spots per case.
A total of 130 cases of ovarian CCC were obtained from the archives of 2000 to 2012 in the Department of Pathology, yielding 237 tissue blocks in all. These included 114 cases of primary tumors (130 blocks), 56 cases of abdominal disseminated lesions (60 blocks), 26 cases of lymph node metastasis (28 blocks), and 17 cases of recurrent tumors (19 blocks). We chose 10 cases with normal ovarian tissue resected for benign reasons (fibroid) as controls.
Immunohistochemistry
Four-micrometer-thick sections of TMA were cut and stained within 2 weeks after sectioning. TMA sections were deparaffinized and rehydrated. For antigen retrieval, slides were microwave-treated in 10 mM citrate buffer (pH = 6.0) for 20 min. After cooling for 20 min, slides were quenched with 3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. Nonspecific binding was inhibited with 10% normal rabbit serum for 10 min. The TMA slides were then incubated with 3 antibodies (see Table, Supplemental Content, which illustrates the details of antibodies and immunostaining procedures) for 1 h at 37°C in a moist chamber, which were followed by a 30-min secondary incubation with prediluted anti-rabbit HRP (Maixin Biological Technology Development, Fuzhou, China). Immunohistochemical reactions were developed with diaminobenzidine as the chromogenic peroxidase substrate, and the slides were counterstained with hematoxylin.
Slides for all samples were independently assessed with a light microscope by the 2 above-mentioned pathologists, who were blind to the clinical information. We excluded cases in which immunostaining could not be evaluated for technical reasons, such as failure of the tissue cores to stick to the slides and no representative tissue in either core. One positive core in a duplicate was sufficient to count the case as positive; the higher score was accepted for the cases in which discrepancy existed in tumor staining. The ARID1A immunoreactivities were divided into undetectable or positive for nuclear staining, in line with previous publications.8,18,19 Concerning HNF-1β staining, the results were scored on the basis of the percentage of positive nuclei: 0, no positive cells; 1, 1–10%; 2, 11–30%; 3, 31–60%; 4, >61% positive cells).20 For statistical analysis, final scores were further dichotomized into low expression (0–2) and high expression (3–4). For PIK3CA expression, the scoring results were assigned into the following 3 categories: 1, decreased or no trace of staining; 2, equivalent staining intensity to the corresponding normal tissue; and 3, increased staining intensity.21
In the final slides with immunohistochemistry staining, some spots were missing due to different reasons, including sectioning and staining problems. Therefore, the patients’ number did not necessarily add up to the total number of case (e.g., 114 cases of primary tumor).
Statistical Analyses
Package for Social Sciences (SPSS) statistical software (Version 17.0, SPSS, Inc., Chicago, IL) was used for the statistical analysis. GraphPad Prism (Version 5.0, GraphPad Software, Inc., La Jolla, CA) was applied in survival curve illustration. Chi-squared tests were employed for categorical variables while parametric Student tests or Mann–Whitney U tests for continuous counterpart as appropriate. We used Kaplan–Meier model and Cox regression for PFS/OS estimation and multivariate analysis, respectively. Multivariate analysis included variables that were significant in univariate analysis. All tests were 2-sided, and the level of statistical significance was set at P value < 0.05.
RESULTS
Immunostaining of 3 Biomarkers in Ovarian CCC
Table 1 lists the immunohistochemistry results, and Figure 1 shows representative tissue spots. It is clear that HNF-1β was expressed in 92.8% of all the primary ovarian tumors, while the loss of ARID1A and the PIK3CA expression rate were 56.2% (63/112) and 45.0% (50/111), respectively. Further comparison of immunoreactivity in different types of tumors revealed no significant differences (P = 0.22 for HNF-1β, P = 0.56 for ARID1A, and P = 0.810 for PIK3CA; Chi-squared tests).
TABLE 1.
Immunohistochemical Staining of 3 Markers in Different Tumor Lesions

FIGURE 1.

Representative immunostains for HNF-1β, AIRD1A, and PIK3CA (magnification ×50). Primary: primary ovarian tumors; abdominal: abdominal disseminated lesions; recurrent: recurrent tumors. ARID1A = AT-rich interactive domain 1A, BAF 250a = protein of ARID1A gene, HNF-1β = hepatocyte nuclear factor-1β, PIK3CA = phosphatidylinositol 3-kinase catalytic subunit alpha.
Relationship Between Protein Expression and Clinicopathologic Parameters
For all 130 patients, early-stage disease accounted for 47.7% (62/130). A total of 30 patients (23.1%) had concurrent endometriosis in the same pathologic specimen according to original pathologic reports. As mentioned earlier, only 114 patients had archived tissue blocks for primary ovarian tumors. The following analysis is focused on the immunohistochemistry results of these patients.
We looked for a possible association between protein expression and clinicopathologic features. The results in Table 2 show that patients with early-stage disease were more likely to have high-level HNF-1β immunoreactivity and expression of ARID1A (P = 0.02 and P = 0.03, respectively; Chi-squared tests). In other words, the loss of immunostain was more prominent in advanced diseases. Similarly, patients with concurrent endometriosis tended to have higher expression of HNF-1β (P = 0.016, Chi-squared tests). It is worth noting that most (20/26, 76.9%) endometriosis-associated patients stained negative for ARID1A (P = 0.02, Chi-squared tests). As clearly observed in the table, PIK3CA expression did not correlate with any variable involved.
TABLE 2.
Association Between Immunostaining and Clinicopathologic Parameters in Ovarian Clear Cell Carcinoma Patients

Effect of Protein Expression on Chemotherapy Response and Survival Outcomes
Among 130 patients, 4 were lost to follow-up after adjuvant chemotherapy and were thus excluded from survival analysis. For the 126 cases, 62 patients (55.9%) had tumor recurrence in the study period. A total of 111 patients had both primary tumor immunohistochemistry results (at least 1 antibody marker) and follow-up information (refer to Table 3). Low levels of HNF-1β expression and loss of ARID1A were more commonly observed in patients with tumor recurrence (P = 0.02 and P < 0.001, respectively; Chi-squared tests).
TABLE 3.
Comparison of Immunostaining Between Recurrent and Disease-Free Patients

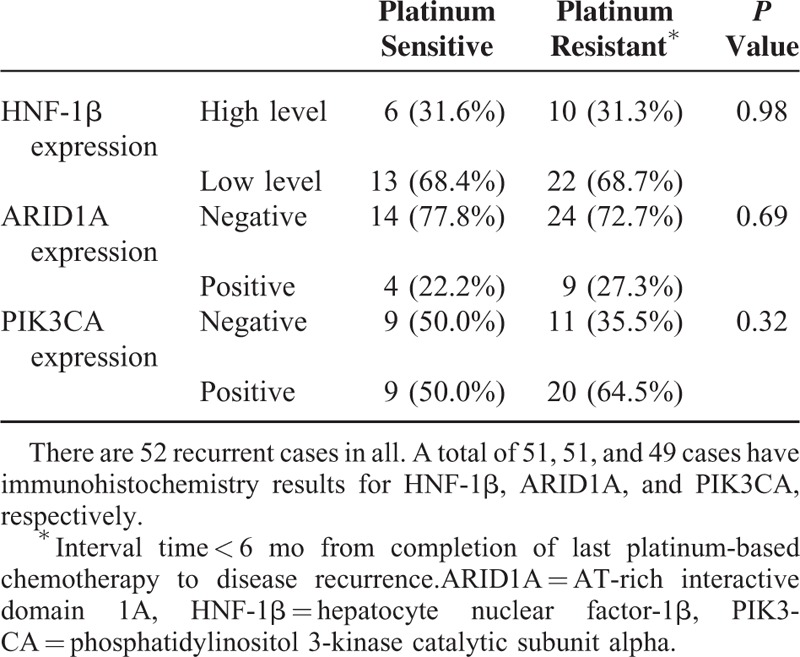
The stratification of the recurrent cases in Table 3 based on the response to platinum-based chemotherapy is shown in Table 4. No statistically significant difference existed between platinum-sensitive and platinum-resistant tumors.
TABLE 4.
Association Between Immunoreactivity and Platinum-Based Chemotherapeutic Response
We looked for the possible prognostic impacts of protein expression on survival outcomes. Figure 2 shows the estimated PFS/OS based on protein expression. Patients with loss of ARID1A expression had worse survival outcome in terms of both PFS and OS with statistical significance (Figure 2A and B). High-level HNF-1β expression was associated with good prognosis (Figure 2C and D). We were not able to find any effect of PIK3CA expression on survival (Figure 2E and F).
FIGURE 2.

Kaplan–Meier survival curves based on immunohistochemistry results. A/B shows that negative ARID1A expression is associated with a shorter overall survival and progression-free survival (P = 0.03 and P = 0.01; log-rank test). C/D illustrates that patients with high levels of HNF-1β expression have better survival outcomes (P = 0.02 for overall survival and P = 0.01 for progression-free survival, respectively; log-rank test). E/F shows that no significant correlation was found between PIK3CA immunoreactivity and survival. (B) Tissue microarray blocks after sectioning; a total of 237 tumor tissue blocks and 10 cases of normal ovarian tissue blocks generated 494 duplicate cores. ARID1A = AT-rich interactive domain 1A, HNF-1β = hepatocyte nuclear factor-1β, PIK3CA = phosphatidylinositol 3-kinase catalytic subunit alpha.
On univariate and multivariate analyses (results shown in Table 5), patients with loss of ARID1A (ARID1A [−]) and ARID1A expression (ARID1A [+]) tumors displayed no statistically significant difference in either PFS or OS. On the contrary, high-level HNF-1β expression seemed to be a prognostic factor for favorable OS.
TABLE 5.
Univariate and Multivariate Analyses

DISCUSSION
In this study, we evaluated the significance of HNF-1β, ARID1A, and PIK3CA expression, as assessed by immunohistochemistry, in a group of ovarian CCC patients. A total of 130 cases and 237 tissue blocks were retrieved to generate a TMA, including primary ovarian tumors, disseminated abdominal lesions, lymph node metastasis, and recurrent tumors. To the best of our knowledge, this is the first such study from China. Comparable immunoreactivity results were found in 4 types of tumors. Thus, we focused on cases with primary tumor immunostaining for further analysis. It was worth noting that late-stage tumors were more likely to be HNF-1β (low level) and ARID1A (−), while tumors with coexisting endometriosis tended to be HNF-1β (high level) and ARID1A (−). Moreover, HNF-1β (low level) and ARID1A (−) correlated with tumor recurrence, but not chemosensitivity. We failed to find any relationship between PIK3CA immunoreactivity and clinicopathologic parameters. Concerning survival, only HNF-1β (high level) and optimal debulking remained positive predictors for OS.
Tsuchiya et al5 from Japan first demonstrated the up-regulation of HNF-1β in ovarian CCC at both the mRNA and protein levels. Further evidence supports that HNF-1β is a good marker for differentiating ovarian CCC from other epithelial ovarian cancers.6 Our study presented that the majority of the ovarian CCC stained positive for HNF-1β; so did the abdominal disseminated lesions, lymph node metastasis, and recurrent tumors. We speculated that HNF-1β might be applied in the differential diagnosis of carcinoma of unknown primary origin. High-level HNF-1β was related to endometriosis-associated CCC, which was quite consistent with previous publications.20 Interestingly, we found that patients with high-level HNF-1β had better survival, and high-level HNF-1β proved to be an independent predicator for favorable OS. More large studies are required to further test the prognostic role of HNF-1β expression in ovarian CCC.
Known as a tumor suppressor gene, ARID1A is mutated in approximately 50% of ovarian clear cells and 30% of ovarian endometrioid carcinomas.19,22 Given the significant association between the loss of ARID1A immunostaining and ARID1A gene mutation,19 immunohistochemical analysis of ARID1A might be a useful and feasible strategy for mutational analysis in the clinical setting. The clinical and prognostic implications of ARID1A expression in ovarian CCC were inconsistent in different studies. Itamochi et al conducted a literature review and found that loss of ARID1A expression did not affect survival,23 which was in line with our study. Katagiri et al12 performed whole tissue immunohistochemistry in 60 cases of ovarian CCC, arriving at the conclusion that the loss of ARID1A expression was significantly correlated with advanced stage, chemoresistance, and shorter survival (both PFS and OS). In our series, negative staining of ARID1A was related to endometriosis-associated tumors, late disease, and tumor recurrence. No statistically significant impact of ARID1A on survival was found. However, the loss of ARID1A immunostaining might have the potential to be used alone or in combination with other markers (e.g., low-level HNF-1β expression) to identify susceptibility to tumor recurrence. Further studies with larger sample sizes and uniform immunohistochemical methods are needed to further establish the role of ARID1A in prognosis.
As expected, PI3KCA expression was not associated with any clinical parameter and prognosis. In 1 Japanese study, immunohistochemical analysis of PIK3CA was performed in 62 ovarian CCC tumors, and PIK3CA over-expression was correlated with early-stage disease, the absence of residual tumors at the initial surgery and improved OS.21 Whether the results could be repeated requires further tests.
Possible explanations for the discordance of different studies are listed as follows. First, the archival and tumor bank cohorts are fundamentally different with regard to tissue handling and fixation. In addition, different degrees of antigen degradation due to tissue aging might lead to reduced immunostaining. Furthermore, the application of TMA versus whole tissue sectioning might present different results concerning intra-tumor heterogeneity. It is true that TMA has the advantage of high efficiency and the same experimental conditions, but it might not truly represent the whole picture of the tumor, which could be regarded as a limitation of our study. Last but not least, immunohistochemistry methods vary from lab to lab. Standardized procedures and interpretations would help researchers yield more subjective and consistent findings, which is quite necessary given the feasibility, availability, and cost-effectiveness of immunohistochemistry.
CONCLUSION
Loss of ARID1A expression was associated with advanced stage and endometriosis-associated tumors. The measurement of ARID1A expression could be applied to predict the risk of recurrence. Among the 3 biomarkers, only high-level HNF-1β expression proved to be a positive predictor for OS.
Supplementary Material
Footnotes
Abbreviations: ARID1A (−) = Loss of ARID1A expression, ARID1A (+) = Expression of ARID1A, ARID1A = AT-Rich Interactive Domain 1A, CA 125 = Cancer Antigen 125, CCC = Clear Cell Carcinoma, FIGO = International Federation of Gynecology and Obstetrics, HNF-1β = Hepatocyte Nuclear Factor-1β, OS = Overall Survival, PFS = Progression-Free Survival, PIK3CA = Phosphatidylinositol 3-Kinase Catalytic Subunit Alpha, TMA = Tissue Microarray.
Conception and design: SY, JY, DC, HH, MW, JL, KS; collection and assembly of data: SY, JY, YY, DC, HH, MW, JC, JL, KS; data analysis and interpretation: all authors; manuscript writing: SY, JY, DC, HH, MW, JL, KS; and final approval of manuscript: all authors.
Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. National High Technology Research Development Program of China (863 program, Grant 2012 AA02A507) and National Natural Science Foundation of China (Grant 81172482 and Grant 81372780). The funding bodies have no role in the design of the study, the collection of data, or the manuscript preparation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 2008; 5:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anglesio MS, Carey MS, Kobel M, et al. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol. 2011;121:407–415. [DOI] [PubMed] [Google Scholar]
- 3.Ye S, Yang J, You Y, et al. Comparative study of ovarian clear cell carcinoma with and without endometriosis in People's Republic of China. Fertil Steril 2014; 102:1656–1662. [DOI] [PubMed] [Google Scholar]
- 4.Ye S, Yang J, You Y, et al. Comparison of clinical characteristic and prognosis between ovarian clear cell carcinoma and serous carcinoma: a 10-year cohort study of Chinese patients. PLoS ONE 2015; 10:e0133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchiya A, Sakamoto M, Yasuda J, et al. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol 2003; 163:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao YC, Lin MC, Lin WC, et al. Utility of hepatocyte nuclear factor-1beta as a diagnostic marker in ovarian carcinomas with clear cells. Histopathology 2012; 61:760–768. [DOI] [PubMed] [Google Scholar]
- 7.Luchini C, Veronese N, Solmi M, et al. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: a systematic review and meta-analysis. Oncotarget 2015; 6:39088–39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang HN, Lin MC, Huang WC, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations and ZNF217 amplification in ovarian clear cell carcinoma. Mod Pathol 2014; 27:983–990. [DOI] [PubMed] [Google Scholar]
- 9.Chandler RL, Damrauer JS, Raab JR, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun 2015; 6:6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bitler BG, Fatkhutdinov N, Zhang R. Potential therapeutic targets in ARID1A-mutated cancers. Expert Opin Ther Targets 2015; 19:1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S, Tsuda H, Takano M, et al. PIK3CA mutations and loss of ARID1A protein expression are early events in the development of cystic ovarian clear cell adenocarcinoma. Virchows Arch 2012; 460:77–87. [DOI] [PubMed] [Google Scholar]
- 12.Katagiri A, Nakayama K, Rahman MT, et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol 2012; 25:282–288. [DOI] [PubMed] [Google Scholar]
- 13.Maeda D, Mao TL, Fukayama M, et al. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci 2010; 11:5120–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowery WJ, Schildkraut JM, Akushevich L, et al. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer 2012; 22:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye S, Yang J, Cao D, et al. Characteristic and prognostic implication of venous thromboembolism in ovarian clear cell carcinoma: a 12-year retrospective study. PLoS ONE 2015; 10:e0121818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai H, Sha G, Cao D, et al. Salvage chemotherapy for patients with recurrent or persistent ovarian clear cell carcinoma: a retrospective study of 164 cases. Medicine (Baltimore) 2015; 94:e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui T, Bai H, Zeng J, et al. Tumor heterogeneity in the recurrence of epithelial ovarian cancer demonstrated by polycomb group proteins. Onco Targets Ther 2014; 7:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegand KC, Lee AF, Al-Agha OM, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 2011; 224:328–333. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010; 363:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol 2006; 19:83–89. [DOI] [PubMed] [Google Scholar]
- 21.Abe A, Minaguchi T, Ochi H, et al. PIK3CA overexpression is a possible prognostic factor for favorable survival in ovarian clear cell carcinoma. Hum Pathol 2013; 44:199–207. [DOI] [PubMed] [Google Scholar]
- 22.Jones S, Wang TL, Shih Ie M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010; 330:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itamochi H, Oumi N, Oishi T, et al. Loss of ARID1A expression is associated with poor prognosis in patients with stage I/II clear cell carcinoma of the ovary. Int J Clin Oncol 2015; 20:967–973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


