Abstract
Little is known about injury of the arcuate fasciculus (AF) in patients with mild traumatic brain injury (TBI). We investigated injury of the AF in the dominant hemisphere in patients with mild TBI, using diffusion tensor tractography (DTT).
We recruited 25 patients with injury of the left AF among 64 right-handed consecutive patients with mild TBI and 20 normal control subjects. DTTs of the left AF were reconstructed, and fractional anisotropy (FA), apparent diffusion coefficient (ADC), and fiber number of the AF were measured.
Among 64 consecutive patients, 25 (39%) patients showed injury of the left AF. The patient group showed lower FA value and fiber number with higher ADC value than the control group (P < 0.05). On K-WAB evaluation, aphasia quotient and language quotient were 95.9 ± 4.1 (range 85–100) and 95.0 ± 5.4 (range 80–100), respectively. However, 23 (92.0%) of 25 patients complained of language-related symptoms after TBI; paraphasia in 12 (48.0%) patients, deficits of comprehension in 4 (16.0%) patients, deficits of speech production in 1 (4.0%) patient, and >2 language symptoms in 6 (24.0%) patients.
We found that a significant number (39%) of patients with mild TBI had injury of the AF in the dominant hemisphere and these patients had mild language deficit. These results suggest that DTT could provide useful information in detecting injury of the AF and evaluation of the AF using DTT would be necessary even in the case of a patient with mild TBI who complains of mild language deficit.
INTRODUCTION
Traumatic brain injury (TBI) is a major cause of neurological disability in adults worldwide.1 TBI is classified as mild, moderate, and severe according to the severity, and mild TBI has been reported in 75% to 85% of cases of TBI.2–5 Patients with mild TBI frequently experience various neurological symptoms derived from neural injury.6–9 Previous studies have reported that 80% to 100% of patients with TBI had some forms of language deficit.10,11 However, little is known about the incidence and the pathogenetic mechanisms of language deficits in mild TBI.12 In addition, patients with mild TBI rarely show significant abnormality on standardized language assessment tools.13 As a result, language deficits in patients with mild TBI have been overlooked so far.
The arcuate fasciculus (AF) is an important neural tract for language with the inferior fronto-occipital fasciculus.14–16,20 Various language deficits including conduction aphasia can be caused by injury of the AF.17,20 Therefore, precise estimation of the state of the AF in patients with language deficits following mild TBI is clinically important because it would be useful for clinicians in setting precise rehabilitative strategy and predicting prognosis of language deficits.17,18,20
Recent development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled 3-dimensional reconstruction of the architecture and integrity of the neural tracts at the subcortical level.19 Injury of the neural tracts in patients with mild TBI, which is not detected on conventional MRI, has been demonstrated in many studies using DTT.6,8,9,20 However, so far, little is known about injury of the AF.12
In the present study, we attempted to investigate injury of the AF in the dominant hemisphere in patients with mild TBI using DTT.
METHOD
Subjects
Twenty-five right-handed patients with mild TBI (9 men, 16 women; mean age 43.4 ± 14.8, range 19–64) and 20 age- and sex-matched right-handed healthy control subjects (10 men, 10 women; mean age 40.25 ± 13.6, range 21–63) were recruited for the study. Patients were recruited among 64 right-handed consecutive patients with mild TBI who were admitted to the rehabilitation department of a university hospital, according to the following inclusion criteria: (1) loss of consciousness (LOC) for <30 min, post-traumatic amnesia (PTA) for ≤24 hours, and initial Glasgow Coma Scale score of 13 to 15,2,4 (2) no brain lesion on conventional magnetic resonance imaging, (3) the time of head trauma: >18 years old, (4) left AF injury which was defined in terms of DTT parameters (the fiber number or FA value of the left AF deviated >2 SDs from the value of the control group) or DTT configuration (the integrity of the left AF was discontinued or the left AF was not reconstructed), (5) no symptoms related to injury of the corticobulbar tract, dysarthria, (6) no cognitive impairment to rule out the effect of cognition on the result of language evaluation as assessed by the Mini-Mental State Examination (MMSE ≥ 25),21 (7) no previous history of neurologic or psychiatric disease, and (8) no oromotor dysfunction or language disorder before the head trauma. The study was conducted retrospectively, and the Institutional Review Board of the Yeungnam University Hospital approved the study protocol.
Language Evaluation
The aphasia quotient (AQ) and language quotient (LQ) of K-WAB, the Korean version of the Western Aphasia Battery, were used for assessment of language deficit (range 0–100th percentile, severity criteria; mild: 80–99, mild to moderate: 60–79, moderate: 40–59, moderate to severe: 20–39, severe: 0–1922) at mean 5.57 ± 6.57 months after the trauma. Both the reliability and validity of the K-WAB are well established.23 In addition, patients were also asked to describe any language symptoms which have been started since the onset of the trauma.
Diffusion Tensor Imaging and Tractography
DTI scanning was performed at an average of 5.49 ± 6.57 months after head trauma using a 6-channel head coil on a 1.5 T Philips GyroscanIntera (Philips Healthcare, Best, The Netherlands) with single-shot, spin echo-planar imaging. Sixty-seven contiguous sections were acquired for each of the 32 noncollinear diffusion-sensitizing gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to 192 × 192; field of view = 240 mm × 240 mm; TR = 10,726 ms; TE = 76 ms; parallel imaging reduction factor (SENSE factor) = 2; EPI factor = 59; b = 1000 s/mm2; NEX = 1; slice gap = 0 mm and a slice thickness = 2.5 mm.
Eddy current-induced image distortions were removed using affine multiscale 2-dimensional registration at the Oxford Centre for FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fls).24 DTI studio software (CMRM, Johns Hopkins Medical Institute, Baltimore, MD) was used for evaluation of the left AF,25 based on the fiber assignment continuous tracking (FACT) algorithm and the multiple-ROIs approach. Using the method of Nucifora et al and Vernooij et al,26,27 2 ROIs were selected for tracking of the AF (i.e., the seed region of interest was located in the deep white matter of the posterior parietal area of the superior longitudinal fascicle and the target region of interest was located in the posterior temporal lobe) and fiber tracts passing through both ROIs were designated as the final tracts of interest. Termination criteria used for fiber tracking were FA < 0.2 and angle <60. The values of fiber number, fractional anisotropy (FA), and apparent diffusion coefficient (ADC) of the reconstructed left AF were measured. The patients were classified according to 3 groups based on the severity of the left AF injury. (1) The AF was not reconstructed. The nonreconstruction of the AF was confirmed by lowering the FA value to 0.1 and placing only 1 region of interest along the AF pathway. (2) The AF was discontinued between Broca's and Wernicke's area. (3) The integrity of the AF was preserved but severely narrowed (Figure 1).
FIGURE 1.
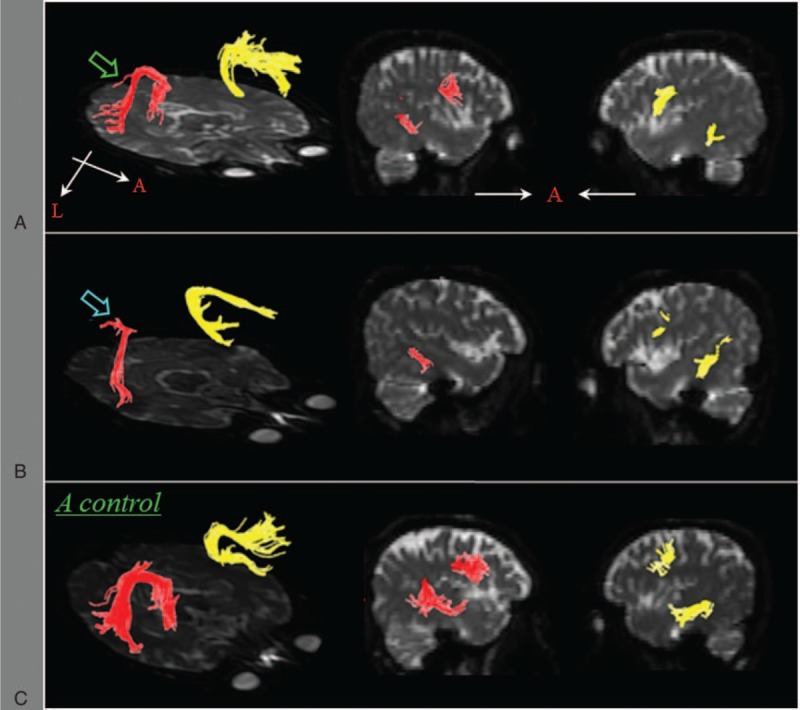
Diffusion tensor tractography for the arcuate fasciculus (right AF: yellow color, left AF: red color)—(A) a patient with decreased fiber number of the left AF (green arrow); (B) a patient with a discontinued left AF (blue arrow); (C) a normal subject. AF = arcuate fasciculus.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS, Version 17.0; Chicago). The Mann–Whitney test was used for comparison of differences in DTT parameters (fiber number, FA, and ADC) between the patient group and healthy group. The level of statistical significance was set at P < 0.05.
RESULTS
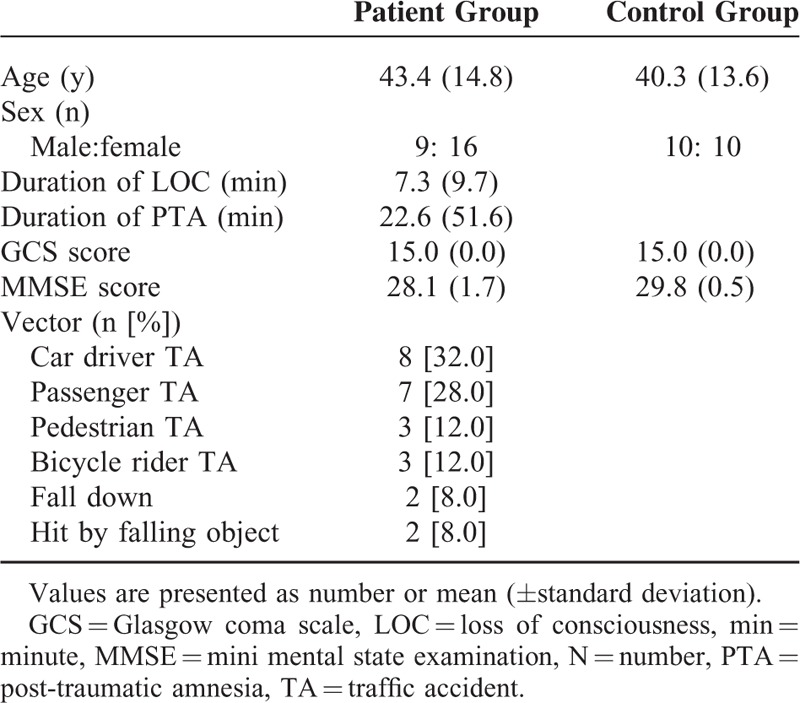
Among 64 consecutive patients with the same inclusion criteria, 25 (39%) patients with injury of the left AF were recruited. The demographic data of the patients are summarized in Table 1. The causes of TBI were as follows: motor vehicle accidents (21 patients), falls (2 patients), and hit by falling objects (2 patients).
TABLE 1.
Demographic Data of Patient and Control Group
The patient group showed a significantly lower FA value and fiber number compared with the control group (P < 0.05). By contrast, a significantly higher ADC value was observed in the patient group compared with the control group (P < 0.05) (Table 2). A summary of the AQ and LQ of K-WAB and language symptoms is shown in Table 3. On K-WAB evaluation, AQ and LQ were 95.9 ± 4.1 (range 85–100) and 95.0 ± 5.4 (range 80–100), respectively. Fourteen (56.0%) of 25 patients showed an abnormal score in terms of AQ and LQ of K-WAB (severity criteria—mild: 80–99, mild to moderate: 60–79, moderate: 40–59, moderate to severe: 20–39, severe: 0–1922) and all 14 patients corresponded to mild language impairment (80–99%ile) on K-WAB. According to DTT, among 25 patients, 10 patients showed discontinuation of the left AF, and 15 patients showed narrowing of the left AF. In the patients with the discontinued left AF, the mean AQ and LQ were 96.46 ± 4.75 and 96.16 ± 4.50, respectively, and in the patients with the narrowed AF, the mean AQ and LQ were 96.09 ± 3.65 and 95.81 ± 5.98, respectively.
TABLE 2.
Comparison of Diffusion Tensor Tractography Parameters of Left Arcuate Fasciculus Between the Patient and Control Group
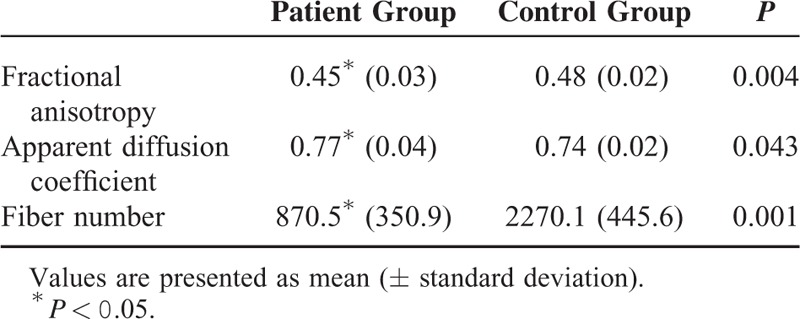
TABLE 3.
Scores on the Korean Version of the Western Aphasia Battery and Language Symptoms of Patients
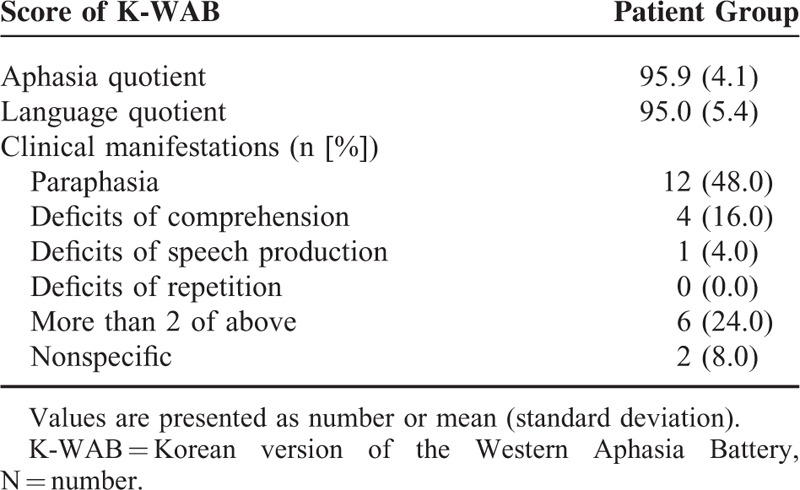
Twenty-three (92.0%) of 25 patients complained of language-related symptoms after the trauma. The most common symptom was paraphasia in 12 (48.0%) patients. They confuse the words or replace 1 word with another real word or feel difficulty in word-finding. Four (16.0%) patients complained of deficits of comprehension when they communicate, and 1 (4.0%) patients felt difficulty in making sentences while speaking. Six (24.0%) patients complained of >2 language symptoms (paraphasia and deficits of comprehension: 4 [16.0%]; deficits of comprehension and repetition: 1 [4.0%]; paraphasia and deficits of speech production: 1 [4.0%]).
DISCUSSION
In this study, we investigated injury of the AF in the dominant hemisphere on DTT in patients with mild TBI. AF injury was defined in terms of DTT parameters (fiber number or FA value of the left AF deviated >2 SDs from the value of the control group) or DTT configuration (the integrity of the left AF was disrupted or the left AF was not reconstructed). The results were as follows: (1) among 64 consecutive patients with the same inclusion criteria, 25 (39%) patients showed injury of the left AF, (2) in the group analysis, the patient group showed lower FA value and fiber number with higher ADC value than the control group, and (3) 14 (56%) of 25 patients showed an abnormal score on K-WAB and all 14 patients corresponded to the mild language impairment (80–99%ile) on K-WAB.
In the individual analysis, among 64 consecutive right-handed patients with mild TBI, injury of the left AF was demonstrated on DTT in 25 (39%) patients. Results of the group analysis showed that the FA value and the fiber number were decreased and the ADC value was increased in the patient group compared with the control group. FA, ADC, and fiber number have been frequently used for evaluation of the state of a neural pathway in patients with brain injury.19,28,29 The FA value indicates the degree of directionality of water diffusion, therefore, indicates the white matter organization.19,28,29 The ADC value means the degree of water diffusion, which increases in some pathology such as vasogenic edema or axonal damage.19,28,29 By contrast, the fiber number indicates the existing number of voxels in a neural tract.30 Therefore, the decreased FA value or fiber number with the increased ADC value confirms injury of the AF. Consequently, it appears that the results of the group analysis on DTT parameters were consistent with the results of the individual analysis.
Only 14 (56.0%) of 25 patients who had injury of the left AF showed an abnormal score on K-WAB (cut off value: <99%ile).22 In addition, the language abnormality on K-WAB was mild: the mean AQ and LQ were 95.9%ile (range 0–100%ile) and 95.0%ile (range 0–100%ile), respectively.22 Especially, in particular, even the mean AQ of K-WAB results of the patients who showed discontinuation of the left AF were 96.46%ile. In comparison with previous studies of stroke patients, the mean values of AQ in the group of stroke patients with discontinued AF in the dominant hemisphere were 52.43 ± 25.75%ile31 and 41.60 ± 24.50%ile,32 respectively,. We can confirm that the language deficit in patients with injury of the left AF following mild TBI was much milder than stroke patients. On the other hand, these results suggest that more detailed language evaluation tools might be necessary for patients with mild TBI although K-WAB is a commonly used language evaluation tool.33
Since the introduction of DTI, many studies have reported on the usefulness of DTT for evaluation of the AF in patients with brain injury; however, most studies focused on stroke.12,18,31,32,34–38 Regarding TBI, to the best of our knowledge, only 2 studies have reported on injury of the AF.12,36 In 2009, Wesson reported on a patient with mild TBI who showed conduction aphasia due to narrowing of the left AF on DTT following a blast injury.12 Recently, Liegeois et al36 investigated language function and DTT parameters of the AF, uncinate fasciculus, and the corpus callosum in 32 patients with mild to moderate-severe TBI and found that long-term outcome regarding poor language following TBI could be predicted by changes in tractography-derived properties of the 3 language neural tracts described above. As a result, this is the first original study to investigate injury of the AF in patients with mild TBI. However, several limitations of this study should be considered. First, this study included a relatively small number of patients and did not include long-term follow up DTI data. Therefore, further long-term follow-up studies including a larger number of patients and DTI data to elucidate natural course and prognosis should be encouraged. Second, we recruited patients who had visited the rehabilitation department of a university hospital. Therefore, it is possible that among all patients with mild TBI, patients with severe clinical manifestations might be included in this study. Third, although DTI is a powerful anatomic imaging tool which can visualize the gross fiber architecture, because regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture by DTT, it may underestimate or overestimate the fiber tracts.39,40 Finally, the fact that we did not analyze the other neural tracts which are involved in language function is another limitation in this study.
In conclusion, we investigated injury of the AF in the dominant hemisphere on DTT in patients with mild TBI and found that a significant number (39%) of patients with mild TBI had injury of the AF in the dominant hemisphere and these patients had mild language deficit. These results suggest that DTT could provide useful information in detecting injury of the AF, which could not be detected on conventional brain MRI in patients with mild TBI. Furthermore, evaluation of the AF using DTT would be necessary even in the case of a patient with mild TBI who complains of mild language deficit. Further studies on the other various neural tracts should be encouraged.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, AF = arcuate fasciculus, AQ = aphasia quotient, DTT = diffusion tensor tractography, FA = fractional anisotropy, GCS = Glasgow coma scale, K-WAB = Korean version of the Western Aphasia Battery, LOC = loss of consciousness, LQ = language quotient, MMSE = mini-mental status examination, PTA = posttraumatic amnesia, ROI = region of interest, TBI = traumatic brain injury.
Author contributions: SHJ—study concept and design, manuscript development and writing; AYL—acquisition and analysis of data; SMS—study concept and design, acquisition and analysis of data, manuscript authorization.
Disclosures: The authors report no disclosures relevant to the manuscript.
Funding: this work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP) (2015R1A2A2A01004073).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Maas Al SN, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008; 7:728–741. [DOI] [PubMed] [Google Scholar]
- 2.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995; 45:1253–1260. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehab Med 2004; (43 suppl):28–60. [DOI] [PubMed] [Google Scholar]
- 4.De Kruijk JR, Twijnstra A, Leffers P. Diagnostic criteria and differential diagnosis of mild traumatic brain injury. Brain Injury 2001; 15:99–106. [DOI] [PubMed] [Google Scholar]
- 5.Styrke J, Stalnacke BM, Sojka P, et al. Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J Neurotrauma 2007; 24:1425–1436. [DOI] [PubMed] [Google Scholar]
- 6.Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007; 24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HG, Jang SH. Delayed gait disturbance due to injury of the corticoreticular pathway in a patient with mild traumatic brain injury. Brain Injury 2014; 28:511–514. [DOI] [PubMed] [Google Scholar]
- 8.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma 2008; 25:1335–1342. [DOI] [PubMed] [Google Scholar]
- 9.Rutgers DR, Toulgoat F, Cazejust J, et al. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am J Neuroradiol 2008; 29:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin N Am 2014; 37:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarno MT. The nature of verbal impairment after closed head injury. J Nerv Ment Dis 1980; 168:685–692. [DOI] [PubMed] [Google Scholar]
- 12.Wesson Ashford J, Yu Zhang AR, Wang Han, et al. Mild traumatic brain injury and conduction aphasia from a close proximity blast resulting in arcuate fasciculus damage diagnosed on DTI tractography. Mil Med 2009; 174:v–vi. [PubMed] [Google Scholar]
- 13.Bernstein DM. Recovery from mild head injury. Brain Injury 1999; 13:151–172. [DOI] [PubMed] [Google Scholar]
- 14.Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain 2012; 135 (pt 12):3529–3550. [DOI] [PubMed] [Google Scholar]
- 15.Geschwind N. The organization of language and the brain. Science 1970; 170:940–944. [DOI] [PubMed] [Google Scholar]
- 16.Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 2009; 213:343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernal B, Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain 2009; 132 (pt 9):2309–2316. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Lee DG, You H, et al. The clinical application of the arcuate fasciculus for stroke patients with aphasia: a diffusion tensor tractography study. Neuro Rehab 2011; 29:305–310. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45:265–269. [DOI] [PubMed] [Google Scholar]
- 20.Jang SH. Diffusion tensor imaging studies on arcuate fasciculus in stroke patients: a review. Front Hum Neurosci 2013; 7:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 22.Kim HH, Na DL. Revised Korean-version of Western Aphasia Battery (K-WAB). Seoul: Paradise Welfare Foundation; 2012. [Google Scholar]
- 23.Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB). J Speech Hear Disord 1980; 45:308–324. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuro Image 2004; 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006; 81:106–116. [DOI] [PubMed] [Google Scholar]
- 26.Nucifora PG, Verma R, Melhem ER, et al. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 2005; 16:791–794. [DOI] [PubMed] [Google Scholar]
- 27.Vernooij MW, Smits M, Wielopolski PA, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuro Image 2007; 35:1064–1076. [DOI] [PubMed] [Google Scholar]
- 28.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34:51–61. [DOI] [PubMed] [Google Scholar]
- 29.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging 2008; 27:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Pagani E, Agosta F, Rocca MA, et al. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuro Image 2008; 41:657–667. [DOI] [PubMed] [Google Scholar]
- 31.Kim SHJS. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. Am J Neuroradiol 2013; 34:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tak HJ, Jang SH. Relation between aphasia and arcuate fasciculus in chronic stroke patients. BMC Neurol 2014; 14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin YI, Yoon LJ, Kim JA, et al. KL. The survey outcome meausres in department of rehabilitation medicine in hospitals of Korea. Brain Neurorehab 2013; 6:17–25. [Google Scholar]
- 34.Breier JI, Hasan KM, Zhang W, et al. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. Am J Neuroradiol 2008; 29:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosomi A, Nagakane Y, Yamada K, et al. Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Neuroradiology 2009; 51:549–555. [DOI] [PubMed] [Google Scholar]
- 36.Liegeois FJ, Mahony K, Connelly A, et al. Pediatric traumatic brain injury: language outcomes and their relationship to the arcuate fasciculus. Brain Lang 2013; 127:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci 2009; 1169:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Wang C, Zhao X, et al. Diffusion tensor imaging depicting damage to the arcuate fasciculus in patients with conduction aphasia: a study of the Wernicke–Geschwind model. Neurol Res 2010; 32:775–778. [DOI] [PubMed] [Google Scholar]
- 39.Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005; 25:53–65. [DOI] [PubMed] [Google Scholar]
- 40.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 2005; 360:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]


