Abstract
HIV and malaria overlap geographically, but the full impact of different antiretrovirals (ARVs) on malaria remains poorly understood. We examined the antimalarial activity of the HIV protease inhibitors (PI) lopinavir and saquinavir and the non-nucleoside reverse transcriptase inhibitor (NNRTIs) nevirapine on Plasmodium falciparum liver stages. Our results demonstrate that the HIV PI lopinavir inhibits liver stage parasites at clinically relevant concentrations, that is, at drug levels achieved in HIV-infected patients on standard dosing regimens. Because drugs that inhibit liver stages target parasites when they are present in lower numbers, these results might have implications for eradication efforts.
Keywords: HIV, malaria, antiretroviral therapy, liver stages, eradication
Introduction
HIV and malaria co-occur in many parts of the world, and evidence suggests that HIV and Plasmodium falciparum malaria, both commonly found in sub-Saharan Africa in epidemic proportion, exert co-pathogenic effects [1].
Previous work shows HIV protease inhibitors (PIs), but not non-nucleoside reverse transcriptase inhibitors (NNRTIs), kill P. falciparum asexual blood stages [2], and clinical studies are beginning to examine the antimalarial effects of PIs vs. NNRTIs in the context of antiretroviral therapy (ARV) on malaria [3, 4]. We have previously shown in rodent models of malaria that HIV PIs also appear to have more potent effect against Plasmodium compared to NNRTIs [5, 6]. However, antiretroviral effect on P.falciparum liver stages has not been reported. Currently, the World Health Organization (WHO) recommends HIV management with combination antiretroviral therapy (ARV), with first line regimens including an NNRTI (in children, nevirapine) and 2 nucleoside reverse transcriptase inhibitors (NRTIs) with few exceptions, and second line regimens including an HIV PI (in children, lopinavir-ritonavir) and 2 NRTIs [7, 8]. Since young children are at highest risk for severe malaria, we set out to investigate the effects of the HIV PI lopinavir and the NNRTI nevirapine on liver stage Plasmodium falciparum. Saquinavir was also investigated given prior results indicated it was comparably potent in vitro against liver stage rodent malaria [5]. Because these drugs are used in HIV-infected patients in malaria-endemic areas, and because there is evidence that indicates PIs may affect liver stage Plasmodium differently than NNRTIs [5, 6], the effects of various ARV components on Plasmodium falciparum requires further investigation.
Malaria infection begins with the bite of an infected female Anopheles mosquito, releasing sporozoites, the infective form of the parasite. Sporozoites travel to the liver and invade hepatocytes, developing into liver stages, or exoerythrocytic forms (EEFs). During these events, the host is asymptomatic, and parasite numbers are low [9]. Infected hepatocytes release merozoites, which invade erythrocytes, initiating the phase of infection responsible for all clinical symptoms of malaria. Most antimalarials target this symptomatic asexual blood stage. However, preventing malaria blood stage infection with drugs targeting liver stages might impact transmission and might contribute to malaria eradication efforts. Here, we describe our investigations of ARV effects on liver stages of P. falciparum malaria parasites at and above clinically relevant concentrations, or drug levels achieved in HIV-treated patients on standard dosing regimens [2, 5, 6, 10–12] (for a summary list of clinically relevant concentrations, please see reference [2]).
Methods
Lopinavir, saquinavir, and nevirapine were tested. Drugs were obtained from the NIH AIDS Research and Reference Reagent Program. Stocks were diluted to 5 mM with dimethyl sulfoxide (DMSO), and were tested at a range at and above clinically relevant concentrations [2, 5, 6, 10–12]. In vitro P. falciparum liver stage development assays were performed as previously described, with few modifications [13, 14]. Briefly, P. falciparum sporozoites were isolated from mosquitoes day 14 after the infective blood meal using the Ozaki method [15]. Then, 7.5 × 104 sporozoites per well were seeded onto monolayers of HC04 cells grown in standard 8-well Nunc ® Lab-Tek® chamber slides (well surface area, 0.8 cm2) and incubated in the presence of lopinavir, saquinavir, or nevirapine prepared in DMSO in DMEM culture medium, with media and drug and replenished daily. Control wells had culture medium only or DMSO and culture medium. On day 4, EEFs were fixed and stained with a monoclonal antibody specific for Plasmodium HSP70 [14, 16], followed by a fluorophore-conjugated secondary antibody. Samples were mounted with Vecta Shield (Vector Laboratories, Burlingame, CA) with DAPI nuclear stain (Sigma-Aldrich, St. Louis, MO) EEFs were counted with an epifluorescence microscope (model Olympus BX50 or BX51). Each developing EEF visualized was counted. Experiments were run in duplicates per condition, and 2 independent experiments were performed. Statistics were performed using Kruskal-Wallis with Dunn’s post-test on combined experiments values to identify statistically significant differences between treated and control. All values were normalized to DMSO control, and no significant differences were detected between DMSO control and infectivity controls. Statistical analyses were performed using GraphPad Prism software (version 5).
Results
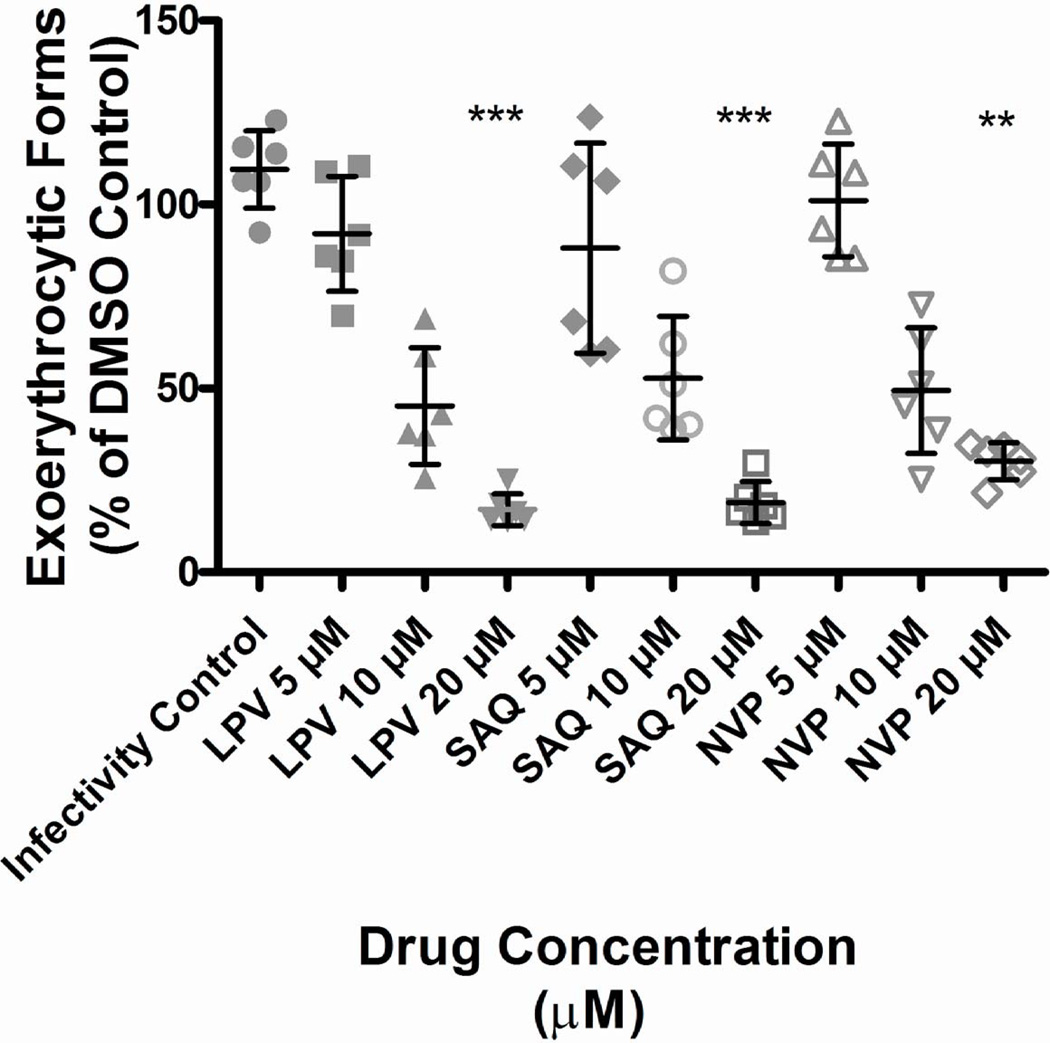
The HIV PI lopinavir reduced P. falciparum developing EEFs at clinically relevant concentrations that would be achieved with ritonavir boosting (p<0.001). Saquinavir and nevirapine also reduced P. falciparum developing EEFs (p<0.01), but only at concentrations exceeding clinical relevance (Fig.1).
Figure 1. HIV PIs and NNRTIs inhibit Plasmodium falciparum liver stage parasites (exoerythrocytic forms/EEFs) at the high end of clinically relevant concentrations.
P. falciparum sporozoites were seeded onto monolayers of HC04 cells and incubated in the presence of indicated ARTs (lopinavir, LPV; saquinavir, SAQ; nevirapine, NVP) at the indicated concentrations. On day 4, cells were fixed and stained with a monoclonal antibody specific for Plasmodium HSP70 and counted on an epifluorescence microscope. Shown are the combined results from 2 independent experiments, with horizontal lines indicating the mean. Statistics were performed using Kruskal-Wallis with Dunn’s post-test on combined experiments values to identify statistically significant differences between treated and control. All values were normalized to DMSO control and expressed as percent of DMSO control. No significant differences were detected between DMSO control and infectivity controls (cell medium only). Three stars indicate p< .001, two stars indicate p<0.01. For EEF number, significant differences were found between treated and control groups at the highest doses only for each ART tested (lopinavir and saquinavir each compared to control, p<0.001; nevirapine, p<0.01). Only lopinavir significantly inhibited EEF development at clinically relevant concentrations.
Conclusion/Discussion
These results show that the PI lopinavir has anti-liver stage specific activity at clinically relevant concentrations. Saquinavir and nevirapine demonstrate anti-liver stage activity, but only at concentrations exceeding clinical relevance. The results are consistent with prior data which demonstrates that lopinavir kills rodent malaria liver stage parasites [5], and Plasmodium falciparum asexual blood stage [2, 17–20] and transmission stage parasites ([21, 22]) at concentrations that or at or are below clinically relevant concentrations. We, and others have also shown that NNRTIs have little to no effect on malaria parasites in liver [6], asexual blood [2], and transmission stage parasites [22], and any effect that is seen only occurs at concentrations exceeding clinical relevance. HIV PIs were originally tested against P. falciparum blood stages as these drugs inhibit the aspartyl protease of HIV [19, 23], and it was hypothesized similar inhibition may occur with Plasmodium aspartyl proteases, although this requires further investigation. For the NNRTIs, anti-Plasmodium activity observed at concentrations exceeding clinical relevance may come from increased oxidative stress on the parasite [6].
A possible limitation of these experiments may be different drug metabolism within in vitro vs. in vivo systems. Reassuringly, the human-derived HC04 cell lines used in this study were shown in a series of previous investigations to express a variety of Phase 1 and 2 drug metabolizing enzymes, including those involved in lopinavir [11], saquinavir [12], and nevirapine [10] biotransformation [13, 24]; these primarily include cytochrome P450 3A4 (CYP3A4) (lopinavir, saquinavir, and nevirapine) and CYP2B6 (nevirapine). Thus, the in vitro systems should reflect in vivo metabolic activity. However, subtle drug metabolism differences may exist. Field studies are required to determine whether PIs like lopinavir offer benefit in reducing malaria burden through an anti-liver stage effect.
Acknowledgements
We thank the NIH AIDS Research and Reference Reagent Program for provision of drugs.
This work was funded by the NIH, NIAID, Division of Intramural Research and by the by the US Army and Materiel Command.
For the colleagues from WRAIR, the following statement has been requested also: The views of the authors do not purport to reflect the position of the Department of the Army or the Department of Defense
Footnotes
Authors’ contributions:
Each author contributed to the experiments or writing of this paper.
None of the authors have any conflicts of interest to declare.
References
- 1.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–556. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- 2.Nsanzabana C, Rosenthal PJ. In vitro activity against Plasmodium falciparum of antiretroviral drugs. Antimicrob Agents Chemother. 2011 doi: 10.1128/AAC.05130-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanzabana C, et al. Antiretroviral Agents and Prevention of Malaria in HIV-Infected Ugandan Children. N Engl J Med. 2012;367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikilezi G, Achan J, Kakuru A, Ruel T, Charlebois E, Clark TD, et al. Prevalence of Asymptomatic Parasitemia and Gametocytemia among HIV-Infected Ugandan Children Randomized to Receive Different Antiretroviral Therapies. Am J Trop Med Hyg. 2013 doi: 10.4269/ajtmh.12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobbs CV, Voza T, Coppi A, Kirmse B, Marsh K, Borkowsky W, et al. HIV protease inhibitors inhibit the development of preerythrocytic-stage plasmodium parasites. J Infect Dis. 2009;199:134–141. doi: 10.1086/594369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobbs CV, Voza T, De La Vega P, Vanvliet J, Conteh S, Penzak SR, et al. HIV Nonnucleoside Reverse Transcriptase Inhibitors and Trimethoprim-Sulfamethoxazole Inhibit Plasmodium Liver Stages. J Infect Dis. 2012;206:1706–1714. doi: 10.1093/infdis/jis602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach and Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access, Recommendations for a Public Health Approach. 2010 < http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf and http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf>.
- 8.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access, Recommendations for a Public Health Approach. 2010 < http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf>. [PubMed] [Google Scholar]
- 9.Sinnis P, Coppi A. A long and winding road: the Plasmodium sporozoite's journey in the mammalian host. Parasitol Int. 2007;56:171–178. doi: 10.1016/j.parint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. aidsinfo.gov. AIDSinfo Drug Database, Nevirapine. < http://aidsinfo.nih.gov/drugs/116/nevirapine/0/professional>. [Google Scholar]
- 11. aidsinfo.gov. AIDSinfo Drug Database, Lopinavir. < http://aidsinfo.nih.gov/drugs/316/lopinavir---ritonavir/0/professional>. [Google Scholar]
- 12. aidsinfo.gov. AIDSinfo Drug database, Saquinavir. < http://aidsinfo.nih.gov/drugs/164/saquinavir/0/professional>. [Google Scholar]
- 13.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- 14.VanBuskirk KM, O'Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci U S A. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozaki LS, Gwadz RW, Godson GN. Simple centrifugation method for rapid separation of sporozoites from mosquitoes. J Parasitol. 1984;70:831–833. [PubMed] [Google Scholar]
- 16.Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994;80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]
- 17.Andrews KT, Fairlie DP, Madala PK, Ray J, Wyatt DM, Hilton PM, et al. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob Agents Chemother. 2006;50:639–648. doi: 10.1128/AAC.50.2.639-648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins TM, Domingos A, Berry C, Wyatt DM. The activity and inhibition of the food vacuole plasmepsin from the rodent malaria parasite Plasmodium chabaudi. Acta Trop. 2006;97:212–218. doi: 10.1016/j.actatropica.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 2005;49:2983–2985. doi: 10.1128/AAC.49.7.2983-2985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner-Adams TS, McCarthy JS, Gardiner DL, Hilton PM, Andrews KT. Antiretrovirals as antimalarial agents. J Infect Dis. 2004;190:1998–2000. doi: 10.1086/425584. [DOI] [PubMed] [Google Scholar]
- 21.Peatey CL, Andrews KT, Eickel N, MacDonald T, Butterworth AS, Trenholme KR, et al. Antimalarial asexual stage-specific and gametocytocidal activities of HIV protease inhibitors. Antimicrob Agents Chemother. 2010;54:1334–1337. doi: 10.1128/AAC.01512-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobbs CV, Tanaka TQ, Muratova O, Van Vliet J, Borkowsky W, Williamson KC, et al. HIV Treatments have Malaria Gametocyte Killing and Transmission Blocking Activity. J Infect Dis. 2013 doi: 10.1093/infdis/jit132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh S, Liu J, Sijwali P, Gut J, Goldberg DE, Rosenthal PJ. Antimalarial effects of human immunodeficiency virus type 1 protease inhibitors differ from those of the aspartic protease inhibitor pepstatin. Antimicrob Agents Chemother. 2006;50:2207–2209. doi: 10.1128/AAC.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim PL, Tan W, Latchoumycandane C, Mok WC, Khoo YM, Lee HS, et al. Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro. 2007;21:1390–1401. doi: 10.1016/j.tiv.2007.05.003. [DOI] [PubMed] [Google Scholar]