Abstract
Glucocorticoids (GCs) are hormones released during the stress response that are well known for their immunosuppressive and anti-inflammatory properties; however, recent advances have uncovered situations wherein they have effects in the opposite direction. The CNS is a particularly interesting example, both because of its unique immune environment, and because GCs affect immune responses differently in different brain regions. In this perspective we discuss the contexts wherein GCs increase CNS inflammation and point out directions for future investigation.
We are born with the ability to respond to stress, and this is fortuitous because the act of being born is the first of many stressors that we will experience. As anyone who has come down with a cold during a stressful period knows, stress can impair immune function. In this perspective we review recent advances and remaining gaps in our understanding of how stress hormones affect inflammation, with a particular focus on the CNS. As the key point, a class of stress hormones renowned for their capacity to suppress inflammation often fails to do so, and can even worsen inflammation in the injured CNS.
An Introduction to Glucocorticoids and the Stress Response
Our understanding of the stress response aptly began during the American Great Depression. In 1935, Walter Cannon described the extraordinary flexibility of the body in its ability to respond to stress, or “accidents of existence” (Cannon, 1935). Cannon called the stress-induced increases in cardiac output the “fight or flight” response, and he had realized the importance of adrenal hormones in this response as early as 1924. In 1936, Hans Selye described the “general adaptation syndrome,” activated by an organism in order to overcome various challenges. The first observation that the stress response might have effects on immunity came when Selye noted that chronic stress atrophied the thymus (Selye, 1936).
The canonical physiological stress response begins when the brain detects a homeostatic challenge and activates the sympathetic nervous system (SNS), which releases the catecholamines epinephrine (E) and norepinephrine (NE). This is followed by the slower activation of the hypothalamic-pituitary-adrenal (HPA)-axis: hypothalamic secretion of corticotropin-releasing hormone (CRH) into the pituitary portal circulation triggers pituitary secretion of adrenocorticotropic hormone (ACTH), which then stimulates the secretion of glucocorticoids (GCs) by the adrenals (the endogenous GC is cortisol in primates and corticosterone in most rodents, hereafter abbreviated as CORT). While this review focuses on GCs, a number of other stress-responsive hormones affect immune function, so the effects of GCs are not always identical to the effects of stress. Moreover, the functioning of the HPA axis, and, indeed, all the facets of the stress response, show tremendous individual variability, a fact that helps to explain the considerable individual differences in vulnerability to stress-related disease (including psychiatric disorders).
Across all species, CORT secretion into the bloodstream peaks just prior to waking, with a 5-fold variation in levels across the circadian cycle. In response to substantial stressors, CORT secretion increases approximately an order of magnitude. In the literature, the type and duration of stressor used varies considerably. In this perspective, we define “acute stress” as a stressor of a few hours. If such stress is repeated daily for several days we will refer to it as “subacute stress,” and if it persists for weeks to months, then it is termed “chronic stress.”
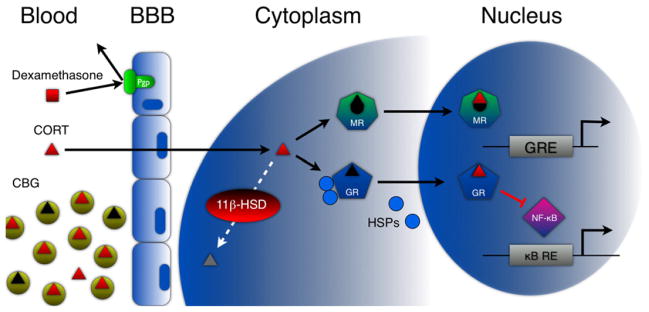
Once secreted, CORT is regulated at many stages before binding to a receptor in a target cell (Figure 1). Beginning in the bloodstream, CORT is normally 90% bound to corticosterone-binding globulin (CBG), and only unbound CORT readily crosses the blood-brain barrier (BBB) and cell membranes. Once in the cytoplasm, it can bind to two different receptors, the mineralocorticoid receptor (MR) or the glucocorticoid receptor (GR). When unoccupied, these receptors are bound to heat shock proteins (HSPs). On binding CORT, they homodimerize, shed their HSP chaperones, and translocate to the nucleus where they regulate gene transcription. The affinity of CORT for MR is ~10-fold higher than for GR, with MR heavily occupied by basal CORT levels and GR only heavily occupied during moderate to severe stress. Because MR and GR signaling can have different transcriptional effects, basal and high-stress CORT levels can have divergent, even opposite effects. In combination, this can produce an “inverse-U” pattern, where basal CORT levels produce a particular effect (mediated by heavy MR occupancy), and where the opposite effect occurs with either below normal (and insufficient MR occupancy) or elevated CORT levels (and heavy GR occupancy). This is often observed in the nervous system. For example, while basal to low stress levels of CORT enhance cerebral perfusion rate, glucose utilization, hippocampal synaptic excitability, and hippocampal-dependent learning, higher physiological levels of CORT do the opposite in all of these realms (Sapolsky, 2004). These complexities and MR and GR function are also pertinent to understanding GC effects in the immune system. For example, different immune cell populations and tissues express these two receptors heterogeneously and will thus respond differently to CORT (McEwen et al., 1997).
Figure 1. Multiple Levels of GC Signaling and Regulation.
Blood: Beginning in the bloodstream, approximately 90% of CORT is bound to CBG, whereas DEX and predisone do not bind CBG. BBB: Only unbound CORT is able to cross the BBB. DEX and prednisone are both extruded by the multi-drug resistance transporters. Cytoplasm: Once CORT has entered the cell, it can bind to MR, GR, or both, or it can be degraded by the enzyme 11β-HSD. Nucleus: Both GR and MR are normally bound to HSPs and other cofactors in the cytoplasm. On binding GCs they undergo a conformational change that releases these HSPs. Activated receptors then homodimerize and enter the nucleus where they mediate changes in gene transcription either via binding to GC response elements upstream of target genes or physically interacting with other transcription factors (e.g., NF-κB).
It has become clear that steroid hormones can have rapid effects that are independent of classical transcription-dependent receptor signaling, implying that GCs are interacting with other proteins, receptors, or membrane channels. For CORT, these effects are important in the control of motor activity, memory, and HPA regulation (Dallman, 2005). There is some evidence that membrane-bound MR is responsible for some of these fast-acting CORT effects (Karst et al., 2005).
Synthetic versions of CORT (e.g., prednisone and dexamethasone [DEX]) were created to harness its immunosuppressive properties. The distinction between synthetic GCs and CORT is critical because they have different receptor binding affinities (e.g., DEX is a selective GR agonist in vivo). Furthermore, synthetic GCs do not bind CBG, and thus have much stronger effects than CORT (McEwen et al., 1997). In the CNS the picture is more complex because synthetic GCs are excluded from crossing the BBB by multidrug resistance transporters (De Kloet et al., 1998). Thus, in vivo studies using synthetic GCs must utilize high enough doses to overwhelm these transporters if central effects are desired.
The 1950 Nobel Prize was awarded for identifying the anti-inflammatory properties of GCs; in this perspective we trace how this view has been refined in the decades since. In the last decade, increasingly reductive studies have revealed situations where GCs actually increase inflammation, including in the injured CNS. Because of the obvious clinical implications of these findings, it is imperative to determine how GCs affect CNS inflammation, particularly when they make it worse. We begin with the well-studied anti-inflammatory effects of GCs as a prelude to considering the situations where GCs make inflammation worse.
When GCs Are Immunosuppressive
We begin outside the CNS, where the anti-inflammatory properties of GCs have been most studied (for more detail, see De Bosscher et al., 2003). The context of GC exposure is critical in determining how GCs will affect inflammation. Important details to consider include (1) whether the duration of exposure is acute, subacute, or chronic; (2) whether GC levels are basal, in the stress range, or supraphysiological; (3) the timing of GC exposure relative to immune activation; and (4) which type of GC is used. Additional relevant factors include: species, strain, gender, age, time of day, the immune challenge used and outcome measured, and tissue differences in receptor expression.
With these contexts in mind, we now review GC and stress effects on immunity and inflammation in the periphery. The stress-induced thymic involution first noted by Selye was subsequently shown to be caused by CORT-induced, GR-mediated apoptosis in immature T cells (Tarcic et al., 1998). Moreover, chronic exposure to GCs (particularly synthetic GCs at supra-physiological levels) inhibits both innate and adaptive immunity. For example, chronic GC exposure reduces circulating leukocyte counts and decreases the production of a large variety of proinflammatory cytokines including IL-1β and TNF-α (De Bosscher et al., 2003).
The transcription factors nuclear factor (NF)-κB and activator protein-1 (AP-1) play central roles in promoting inflammation, and chronic GC signaling through the GR counters their actions in multiple ways. GR signaling can increase the expression of the inhibitor of NF-κB (IκB); however, blocking GR transcriptional activity does not inhibit all of the anti-inflammatory properties of GCs (Reichardt et al., 2001). GCs also decrease immune activation via protein-protein interactions between GR and NF-κB in the nucleus (Figure 1) (De Bosscher et al., 2003).
During a stressful crisis, it seems logical that CORT suppresses nonessential activities like reproduction, growth, and digestion. But an immunological challenge is itself a stressor, and other stressful events can be accompanied by immunological challenges; thus, decreased immune activity at such times seems maladaptive. Insights into this puzzle came with advances in methods for assaying CORT levels and immune activation. Subsequent studies showed that prior to the release of CORT, immune function is actually augmented early in the stress response. This led to the theory that CORT secretion during stress actually mediates recovery from this initial immune activation (Munck et al., 1984). This theory predicts that if CORT release is impaired, an organism might suffer from disorders of excessive immune activation such as autoimmunity; indeed, impaired CORT secretion is implicated in several autoimmune disorders (Wick et al., 1993).
Basal GC Concentrations Are Required for Immune Activation
The initial stress-induced increases in immune activity are predominantly mediated by catecholamine release. Supporting this conclusion, adrenoceptor antagonists inhibit stress-induced increases in CNS cytokine production (Johnson et al., 2005). CORT plays a role in this immune activation as well because basal levels of CORT are required for the synthesis of E in the adrenals (Wurtman and Axelrod, 1966) and for proper NE signaling (Joels and de Kloet, 1989). This is an example of a common situation in endocrinology, where basal levels of a hormone exert “permissive” effects (i.e., are required for normal functioning of other systems). One can readily appreciate the adaptive benefits of immune activation early in the stress response and recovery from such activation at later stages.
CORT Released during Acute Stress Stimulates the Cellular Immune Response
Despite this teleology, acute stress still induces the high CORT concentrations that lead to decreased blood leukocyte counts, and this does not fit with the view that stress adaptively activates the immune response. On closer examination, however, CORT-induced decreases in blood leukocyte counts are not due to apoptosis as long thought. Instead, acute stress directs these cells to leave circulation and relocate to the tissues where they might be needed, a redeployment from the “barracks” to the “battle stations” (McEwen et al., 1997). Acute physiological exposure to CORT and GR agonists mimics this response (Dhabhar et al., 1996). By contrast, chronic exposure to CORT or synthetic GCs decreases the response, in line with the immunosuppressive properties previously described (Dhabhar and McEwen, 1997) (however, see Bowers et al., 2008 for a demonstration that, in some circumstances, chronic GC exposure can augment cellular immunity).
The nature of the switch from acute-activating to chronic-suppressive GC effects remains unknown. The fact that the duration of GC exposure is so important to the outcome suggests that the timing of the inflammatory challenge relative to GC exposure is also important.
Acute CORT Exposure Primes a Subsequent Inflammatory Response
If stress occurs prior to immune activation, it can have a “priming” effect that makes the subsequent response greater. For example, acute stress 24 hr prior to lipopolysaccharide (LPS) treatment augments LPS-induced increases in plasma levels of IL-1β, TNF-α, and IL-6 (Johnson et al., 2002). By contrast, acute stress post-LPS decreases the production of these same cytokines (Goujon et al., 1995). Similarly, in cultured macrophages, CORT treatment 24 or 12 hr prior to LPS increases LPS-induced TNF-α, IL-6, and nitrite levels, while CORT treatment at 6, 3, or 0 hr prior to LPS does not (Smyth et al., 2004). In humans, plasma levels of TNF-α and IL-6 are higher if cortisol is given 12 hr prior to, but not concurrent with, LPS (Barber et al., 1993).
These studies provide exceptions to the traditional view that GCs are uniformly anti-inflammatory. First, early in the stress response, basal levels of CORT permit the immune-activating actions of catecholamines. Second, stress-induced rises in CORT levels facilitate immune cell mobilization to injured tissues. Third, acute stress or CORT exposure can augment the response to a subsequent inflammatory challenge. We now consider how these novel GC effects apply to the CNS.
Stress and the Uninjured CNS
CORT has numerous effects in the uninjured brain, particularly in regions with high levels of GR and MR (i.e., hippocampus and cortex). For example, basal or acutely elevated CORT levels increase synaptic plasticity and facilitate hippocampal-dependent cognition. By contrast, chronically elevated CORT levels impair synaptic plasticity and cognition, decrease neurogenesis and spine density, and cause dendritic atrophy (McEwen and Magarinos, 2001).
In the absence of injury, acute stress can activate inflammatory mediators in the CNS. Acute stress increases NF-κB activity (Madrigal et al., 2001), intracellular TNF-α-convertase activity, extracellular TNF-α (Madrigal et al., 2002), and prostaglandin E2 and COX-2 levels (Madrigal et al., 2003) in the cortex. At least some of these stress effects are GC dependent and GR mediated (Garcia-Bueno et al., 2008). Moreover, animals with higher basal CORT levels have a greater accumulation of proinflammatory mediators (Perez-Nievas et al., 2007).
Acute CORT Exposure Has Priming Effects on Inflammation in the CNS
As in the periphery, the timing of GC exposure affects CNS inflammatory responses. CORT given prior to LPS challenge increases IL-1β and TNF-α expression in the hippocampus (Frank et al., 2009). This does not depend on whether CORT is administered 24 hr prior to LPS (which would allow CORT levels to return to baseline by the time LPS is given) or 2 hr before (which would be insufficient time). By contrast, CORT diminishes the expression of these same cytokines when given 1 hr post LPS. Microglia are important in this response because when they are extracted from CORT pretreated rats and challenged ex vivo with LPS, they upregulate MHCII and toll-like receptor-4 (TLR-4) and produce more IL-1β and TNF-α than microglia extracted from control rats do (Frank et al., 2009).
Brain Regional Differences in GC Effects
There are also brain regional differences in how GCs modulate the inflammatory response to LPS. For example, GR activation during chronic stress increases LPS-induced NF-κB activation and TNF-α, IL-1β, and iNOS expression in the hippocampus and frontal cortex, but has opposite effects in the hypothalamus (Munhoz et al., 2006). Moreover, TNF-α expression is increased by chronic stress after intracortical LPS administration, also in a GR-dependent manner (de Pablos et al., 2006). Supporting these results, in the frontal cortex, GR signaling is essential for chronic stress to increase LPS-induced activation of several components of the MAPK signaling pathway involved in activating NF-κB (de Pablos et al., 2006). While chronic GC exposure can decrease GR protein levels, these studies used GR inhibitors to demonstrate that GR signaling is required during chronic stress to enhance inflammatory signaling in the fore-brain.
The mechanisms leading to opposite responses in the hypothalamus and forebrain are unknown. The mechanism cannot be the same as what distinguishes between acute and chronic GC exposure because all brain regions received an equivalent duration of GC exposure. The mechanism also must be distinct from what leads to GC priming because priming occurs in a variety of regions including the cortex, hippocampus, hypothalamus, pituitary, and cerebellum (Johnson et al., 2002). We now consider several mechanistic factors that could contribute to these varied GC effects, beginning with those at the cellular level, moving to intracellular signaling pathways, and ending in the nucleus with gene transcription.
Putative Mechanisms underlying the Inflammation-Enhancing Effects of GCs
GCs have cell-type-specific effects that could influence how different tissues respond to their signaling. For example, even after CORT enters the cell it is possible for it to be inactivated by 11β-HSD (Figure 1), which can also vary in its expression. The transcriptional effects of GCs may also be cell type specific, as is the case with increased NF-κB signaling, which in neurons is protective (Camandola and Mattson, 2007) and in microglia is damaging (Kreutzberg, 1996). GC effects on microglia are particularly relevant, and indeed subacute stress induces GR-dependent microglia proliferation (Nair and Bonneau, 2006) and prior CORT exposure primes cytokine release from microglia ex vivo (Frank et al., 2006).
Traditional endocrine and pharmacologic approaches are limited by the fact that different cell types express variable amounts of MR and GR, can be exposed to variable hormone levels due to regulation, and even with similar receptor activation might have completely different effects on neuronal survival during an injury (Figure 2). This can be addressed in vitro, or by genetic approaches using cell-specific manipulations of regulatory proteins. We next consider some of these important signaling molecules.
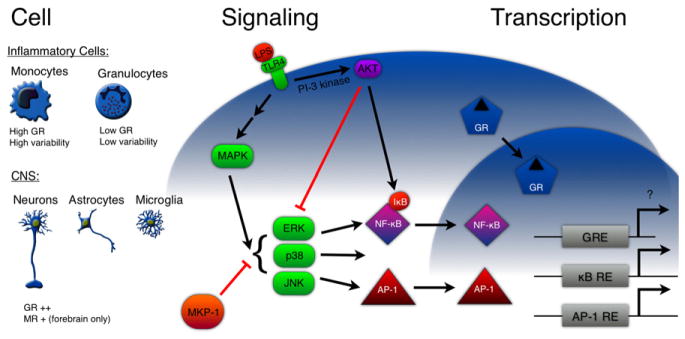
Figure 2. Perspectives.
Cell: GCs may have cell-type-specific effects for a number of reasons. Monocytes and granulocytes, for example, differ greatly in their level of GR expression and the variability in that expression between cells. In the CNS, GR is ubiquitously expressed at relatively high levels in neurons, astrocytes, and microglia, whereas MR expression is restricted to the forebrain. Thus, in the CNS it is not variation in receptor expression, but rather cell-specific responses to GCs that might contribute to functional differences. Signaling: LPS, a stereotypical inflammatory challenge, binds to TLR-4 at the plasma membrane, leading to activation of the MAPK and PI-3 kinase signaling pathways. MAPK signaling activates NF-κB and AP-1 and is antagonized by MKP-1 and AKT. GCs could regulate inflammatory activation by inducing or suppressing proteins involved in these pathways. Transcription: We need a better understanding of what genes are altered by GC signaling in different contexts, and whether the changes are produced by protein-protein interactions with other transcription factors or direct activation of gene transcription.
Proinflammatory signals activate NF-κB by binding to cell-membrane receptors and activating intracellular signaling pathways (Figure 2). For example, LPS binds to TLR-4 and activates the MAPK pathway, which activates NF-κB. The MAPK superfamily includes extracellular-regulated kinases (ERKs), c-Jun-N-terminal kinases (JNKs), stress-activated protein kinases (SAPK), and p38-MAPK (Karin, 1998). The activity of these MAP kinases is antagonized by the PI-3 kinase/AKT pathway, which inhibits TLR-4 signaling in macrophages (Zhang and Daynes, 2007).
GCs not only act genomically, but also via crosstalk with other signaling pathways and transcription factors (De Bosscher et al., 2003). Basal CORT levels induce SHIP1, which increases NF-κB activation by inhibiting AKT (Zhang and Daynes, 2007). This could explain the permissive effects of basal GCs. Higher levels of CORT antagonize MAPK signaling by inducing expression of MAPK phosphatase-1 (MKP-1), which reduces NF-κB activation. MKP-1 is a dual specificity phosphatase induced by cellular stress, serum, and growth factors that dephosphorylates and inactivates MAP kinases such as JNK, p38, and ERK1/2 (Clark and Lasa, 2003). Acute GC exposure induces sustained expression of MKP-1 in a variety of cells including macrophages (Roger et al., 2005). Proinflammatory stimuli such as LPS also induce MKP-1 expression, creating a negative feedback loop that downregulates production of TNF-α, IL-1β, and IL-6 (Zhao et al., 2005). Reciprocally, MAPK (ERK and JNK) can decrease GR nuclear transport and affinity to cofactors via phosphorylation (Rogatsky et al., 1998).
In addition to GR phosphorylation, the balance between NF-κB and GR signaling is important in predicting GC actions. The duration over which NF-κB remains active in the nucleus may determine which genes it will activate (Hoffmann et al., 2002). Normally, NF-κB induces the IκB family of genes to negatively inhibit itself over time. GR can also inactivate NF-κB by inducing IκBα expression, but this is only one protein from a family of NF-κB inhibitors.
The effects of different GC exposure conditions on GR, NF-κB, and AP-1 transcriptional activation are minimally understood. In one study, microarray profiling demonstrates that while DEX can decrease human monocyte expression of many anti-inflammatory genes, it simultaneously increases the expression of a number of proinflammatory chemokines, complement proteins, and cytokines (Galon et al., 2002).
An essential component of GR signaling is its interaction with other transcription factors. As discussed, GR activation is required for chronic-stress-augmented NF-κB activation, but it is not known whether GR also affects AP-1. This role may not be the same as that for NF-κB because different GR domains are responsible for NF-κB and AP-1 interactions (Bladh et al., 2005).
Future research must therefore explore what changes in gene transcription occur under different GC conditions. Chromatin immunoprecipitation (ChIP) could be used to identify altered or novel transcription factor binding targets. Combined with quantitative PCR, this could also shed light on the degree of target induction. ChIP could also reveal a role for coactivators, corepressors, or chromatin-modifying enzymes in different GC exposure contexts.
Functional Implications: GCs Endanger Injured Neurons
Until now we have primarily considered situations wheren inflammation occurs in the absence of neuronal death (i.e., after LPS administration). Numerous studies demonstrate that GC signaling via GR makes neurons (most notably in the hippocampus) less capable of surviving a variety of insults. For example, subacute CORT treatment worsens CA1 hippocampal neuron loss caused by global ischemia (Sapolsky and Pulsinelli, 1985) and CA3 neuron loss caused by excitotoxins (Dinkel et al., 2003).
One mechanism to explain these endangering effects of CORT is that the hormone decreases glucose transport into neurons and glia in the hippocampus. As a result of this decreased energy availability, following an excitotoxic insult, GC-treated neurons are less capable of removing glutamate from the synapse, extruding intracellular calcium, and quenching oxygen radicals (Sapolsky, 1999).
GC-augmented inflammation may also contribute to this endangerment. As evidence, prior acute stress worsens stroke damage by increasing IL-1β levels in the postischemic cortex (Caso et al., 2007). Moreover, subacute stress induces an increase in TNF-α levels in the cortex, and blocking this increase lessens stroke damage (Caso et al., 2006). Subacute CORT treatment increases inflammatory cell recruitment and cytokine production at an excitotoxic injury site (Dinkel et al., 2003). Finally, chronic stress increases both neuronal death and astrocytic death in a GR-dependent manner after direct LPS injection into the cortex (de Pablos et al., 2006). Importantly, in this study, chronic stress occurred after the injury, but still increased LPS-induced TNF-α expression and microglia activation.
These observations raise the question of whether GC-augmented inflammation contributes to, or is solely a consequence of, GC-augmented neuronal death. The former conclusion is likely because GCs can augment LPS-induced CNS inflammation in the absence of neuronal death. Moreover, if such augmentation adds to the ability of GCs to worsen neuronal death, the augmentation must precede the death. This was seen in one study where GCs increased inflammatory cell infiltration prior to the emergence of neuronal death (Dinkel et al., 2003).
Conclusions
There are several points from this Perspective that we would like to emphasize:
GCs, while renowned for their anti-inflammatory actions, are not uniformly anti-inflammatory, including in the context of CNS inflammation. Both GCs and stress (in a GC-and GR-dependent manner) can augment aspects of inflammation in the CNS, and these GC actions can exacerbate injury-induced neuronal death.
Whether GCs increase or decrease CNS inflammation depends on the dose, timing, duration of GC exposure, and the type of GC, since these steroids are a heterogeneous group of compounds.
While there is widespread use of GCs in clinical neurology to control CNS inflammation in general and poststroke edema in particular, such interventions are often ineffective and can even worsen clinical outcome (Gomes et al., 2005). The findings reviewed in this Perspective further support the notion that GCs should be used cautiously, if at all, in many settings of clinical neurology.
Given these clinical implications, we would like to emphasize the need for continued basic investigations to understand the unexpected capacity of GCs to augment aspects of CNS inflammation. Future research ought to be directed at determining how GCs act on different cell types and brain regions to produce such profoundly different immunomodulatory outcomes. Insights will require work at the systems, cellular, and molecular levels.
Acknowledgments
Manuscript assistance was provided by Marta P. Pereira, Morgan K. Theis, Nathan C. Manley, and Trevor R. Sorrells; funding for some of the studies described was provided by the Adler Foundation. S.F.S. is supported by NIH NRSA predoctoral fellowship F31 NS063491-01A1. J.R.C. is a recipient of a MICINN (I-D+I 2008-2011) postdoctoral fellowship.
References
- Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- Bladh LG, Liden J, Dahlman-Wright K, Reimers M, Nilsson S, Okret S. Identification of endogenous glucocorticoid repressed genes differentially regulated by a glucocorticoid receptor mutant able to separate between nuclear factor-kappaB and activator protein-1 repression. Mol Pharmacol. 2005;67:815–826. doi: 10.1124/mol.104.005801. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Stresses and strains of homeostasis. Am J Med Sci. 1935;189:1–14. [Google Scholar]
- Caso JR, Lizasoain I, Lorenzo P, Moro MA, Leza JC. The role of tumor necrosis factor-alpha in stress-induced worsening of cerebral ischemia in rats. Neuroscience. 2006;142:59–69. doi: 10.1016/j.neuroscience.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Caso JR, Moro MA, Lorenzo P, Lizasoain I, Leza JC. Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. Eur Neuropsychopharmacol. 2007;17:600–607. doi: 10.1016/j.euroneuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Clark AR, Lasa M. Crosstalk between glucocorticoids and mitogen-activated protein kinase signalling pathways. Curr Opin Pharmacol. 2003;3:404–411. doi: 10.1016/s1471-4892(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS proinflammatory cytokine responses. Brain Behav Immun. 2006;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.07.008. in press Published online July 30 2009. [DOI] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O’Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Madrigal JL, Perez-Nievas BG, Leza JC. Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology. 2008;149:1969–1978. doi: 10.1210/en.2007-0482. [DOI] [PubMed] [Google Scholar]
- Gomes JA, Stevens RD, Lewin JJ, 3rd, Mirski MA, Bhardwaj A. Glucocorticoid therapy in neurologic critical care. Crit Care Med. 2005;33:1214–1224. doi: 10.1097/01.ccm.0000166389.85273.38. [DOI] [PubMed] [Google Scholar]
- Goujon E, Parnet P, Laye S, Combe C, Kelley KW, Dantzer R. Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav Immun. 1995;9:292–303. doi: 10.1006/brbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989;245:1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann N Y Acad Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem. 2001;76:532–538. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, Bosca L, Leza JC. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Lima LS, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Perez-Nievas BG, Garcia-Bueno B, Caso JR, Menchen L, Leza JC. Corticosteroneas a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology. 2007;32:703–711. doi: 10.1016/j.psyneuen.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBOJ. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Logan SK, Garabedian MJ. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci USA. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–3413. doi: 10.1002/eji.200535413. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and cognition. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, Massachusetts: MIT Press; 2004. p. 1031. [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Selye H. Thymus and adrenals in the response of the organism to injuries and intoxications. Br J Exp Pathol. 1936;17:234–248. [Google Scholar]
- Smyth GP, Stapleton PP, Freeman TA, Concannon EM, Mestre JR, Duff M, Maddali S, Daly JM. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappaB activation in macrophages. J Surg Res. 2004;116:253–261. doi: 10.1016/S0022-4804(03)00300-7. [DOI] [PubMed] [Google Scholar]
- Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82:40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993;14:539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Axelrod J. Control of Enzymatic Synthesis of Adrenaline in Adrenal Medulla by Adrenal Cortical Steroids. J Biol Chem. 1966;241:2301. [PubMed] [Google Scholar]
- Zhang TY, Daynes RA. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3-kinase-Akt pathway. J Immunol. 2007;178:2517–2526. doi: 10.4049/jimmunol.178.4.2517. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280:8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]